Full Length Research Paper
ABSTRACT
In order to determine the diet of Nannothrissa stewarti in Lake Mai Ndombe, 667 specimens with total length between 9.0 and 49.84 mm were studied. These fish were sampled by active fishing during the 24 h cycle between September 2018 and October 2021. The vacuity (%) and intestinal coefficients were 38.71% and 0.69 ± 0.03, respectively, classifying N. stewarti as invertivorous. The calculated feeding indices showed that zooplankton are the essential prey (%Ffi = 88.8; %IP = 90.0; and %IRI = 90.8) while phytoplankton are the incidential prey. Larvae feed on cyclopoid, copepods, and nauplius while adults consume cladoceras, copepods, rotifers and phytoplankton. This fish begins its feeding activity early in the morning (5:00 am), his stomach fills up around 1:00 pm and empties completely around 1:00 am. Thus, N. stewarti in various steps growing ages both males and females are zooplanktons feeders.
Key words: Lake Mai-Ndombe, Nannothrissa stewarti, food indices, diurnal.
INTRODUCTION
Food is the only source of energy acquisition that the animal uses to accomplish its different functions (Lévêque et al., 1994; Lévêque and Paugy, 2006). The study of the diet of fish in the natural environment is an essential approach to the knowledge of their biology and ecology. Not only on the presence, abundance and availability of food potential in the natural environment, but also, it allows us to understand the relationships between fish and prey, as well as interspecific relationships (Rosecchi and Nouaze, 1987; Froese and Pauly, 1999; Kouamélan, 1999; Kouamé et al., 2006, Pwema et al., 2015; Thumithol et al., 2016). Stomach content analysis is one of the possibilities to know the feeding habits of fish (Kouamélan, 1999; Thumithol et al., 2016).
Work on fish diets should also precede the implementation of conservation or management policies for ichthyological populations (Ouattara et al., 2014).
In Lake Mai-Ndombe, Nannothrissa stewarti (Clupeidae) constitutes 90% of all fish species landed in the shore seine fishery. It contributes to the animal protein supply of the population in this area (Micha et al., 2020). The concern would be on how its exploitation is done without respect for ecological principles and the maintenance of essential processes of conservation of critical habitats and other systems on which they depend, which could lead to the rarefaction or even extinction of species (Inogwabini et al., 2009; De Keyzer et al., 2020).
This work aims to determine the qualitative and quantitative diet of N. stewarti in Lake Mai-Ndombe based on food indices.
MATERIALS AND METHODS
Study environment
Lake Mai-Ndombe (Figure 1) is located at 1° 32' - 2° 43' South latitude and 18° 03' - 18° 36' East longitude. It is 146 km long, 18 km wide and covers 2300 km2 (Ebengo, 2022).

Climatic data
According to Bultot and Griffits (1971), the Lake Mai-Ndombe region has an Af-type climate according to the Köppen classification. Analysis of 41 years of data revealed that the monthly and annual diurnal air temperature varies, respectively from 25.96 to 27.25°C, the average of 26.4 ± 0.49°C and from 25.69 to 27.3°C, the average of 26.5 ± 0.44°C. Consequently, the monthly and annual rainfall amounts are, respectively 69.91 to 153.61 mm while the mean is 115.42 ± 28.16 mm and from 1000.9 to 1740.7 mm, the mean is 1376.22 ± 184.34 mm.
Umbrothermal diagram for the Lake Mai-Ndombe region is as shown in Figure 2. The umbrothermal curve of Lake Mai-Ndombe indicates that the Lake Mai-Ndombe region does not experience the marked dry season, but a decrease in rainfall is observed in the months of June and July.

The diet of N. stewarti was determined from 667 fish caught from September 2018 to October 2021 at Lake Mai-Ndombe.
Sample collection
Fish were sampled using shore seines in February and September on a 24-h cycle from 2018 to 2021 (8:00 am, 12:00 pm, 4:00 pm, 8:00 pm, 24:00 am and 4:00 am) using two types of monofilament nets with 0.1 and 2.5 cm knots, 500 m length and 2 m drop.
Analysis of the samples
Method of studying the diet
Captured fish were measured to the nearest millimeter total length (TL) and standard length (SL) using a 200 mm long digital caliper (accuracy 0.1 mm). The fish were then weighed to the nearest gram using the "Digital pocket scale" model 200, precision 0.001 g, and then preserved in jars containing 4% formalin before the study of the stomach contents. In the laboratory, the fish were dissected and the digestive tubes preserved in pillboxes containing 4% formalin for adults. The stomach contents thus obtained were placed on a 25 × 75 mm slide, then diluted with 2 to 5 drops equivalent to 0.08 to 0.2 ml of distilled water to visualize the prey. As for the larvae they were crushed and immediately observed under microscopy because of their very tiny size. Observations of the digestive contents were made under a MOTIC (swift line) light microscope at 100x magnification. Prey were identified using the keys established by Dussart (1967a, 1967b, 1982), Bourrelly (1972), Harding and Smith (1974), and Descy et al. (2016).
For this purpose, we used the method of estimating the volume ingested and the relative proportions of each type of prey contained in the stomach or point (volumetric) method. In this method, each food item contained in the stomach is assigned a number of points based on its volume. A total of 16 points are assigned to the highest volume of the diet item and all other items are 16, 8, 4, 2, 1, and 0 points based on volume relative to the component with the highest volume (Manko, 2016).
Expression of results
Determination of fish size classes
Size classes of sampled specimens were determined from Sturge's rule (Scherrer, 1984):
NC = 1+ (3.3 log10 N)
Where NC is the number of classes; N: total number of individuals for the considered sample. The class interval is determined by the following relationship:
CI = Maximum size - minimum size / Total number of classes
Intestinal coefficient (IC)
The intestinal coefficient gives an indication of the diet of a fish. It was obtained according to the following relationship:
CI = Li / Ls
Where Li is the length of the intestine (mm) and Ls is standard length of the fish (mm).
Coefficient of vacuity (CV)
The vacuity coefficient is the number of empty stomachs in relation to the total number of stomachs examined. Its formula is:
CV = (Number of empty stomachs / Number of stomachs analyzed) × 100
Numerical index (N)
The numerical index is the percentage of the number of individuals of a prey category for the whole sample compared to the total number of prey. Its formula is:
N = (Total number of individuals of the prey (i) / Total number of prey inventoried) × 100
Occurrence index (Ffi)
The occurrence determines the number of stomachs in which a prey or a category of prey is present. It is expressed as a percentage of the total number of stomachs. Empty stomachs havebeen set aside because it can significantly change the results. It is calculated by the following relationship (Rosecchi and Nouaze, 1987; Manko, 2016; Zacharia, 2016; Mahesh et al., 2019):
%Ffi = (Nfi / Nf) × 100
Where %Ffi is the frequency of occurrence of a given item i, Nfi is the number of stomachs in which an item i is found, and Nf is the total number of stomachs examined.
Rate of feeding activity
The filling state (replenishment) has been defined as follows: stage 0, empty stomach; stage 1, 25% full stomach; stage 2, 50% full stomach; stage 3, 75% full stomach; and stage 4, 100% full stomach (Garrido et al., 2008).
Volumetric index (Point method)
The percentages of volumes in each subsample were calculated as follows (Bertran and Calvez, 1988; Manko, 2016; Mahesh et al., 2019):
α = (Number of points assigned to the α component / Total points allocated to the subsample) × 100
Where α is the volume percentage of the prey component α.
Preponderance Index
The preponderance index developed by Natarajan and Jhingran (1961) gives a unique value for each attribute. It is based on the frequency of occurrence and volume of different foods. It is calculated using the following formula (Lauzanne, 1976; Baker et al., 2014; Mahesh et al., 2019):
IPi = (Vi Ffi / ΣVi Ffi) × 100
Where Vi is the percentage of volume of food item i, Ffi is the percentage of occurrence of a given food i.
By comparing the values obtained, the foods are ranked in order of dominance. This index varies from 0 to 100. Thus, prey can be classified into 4 categories according to the value of their food indices: IA< 10: prey of secondary importance; 10< IA< 25: important prey; 25< IA< 50 essential prey; IA> 50: largely dominant prey.
Relative importance index (IRI)
The IRI is used to describe fish diets and determine the relative importance of common food categories (Diaha et al., 2018). The IRI is calculated as follows:
IRI = (% Ni + % Vi) % Ffi; and % IRIi = (IRIi / ΣIRIi) × 100
Where % Ni is the percentage of the specific food category by number, % Vi is the percentage by volume, % Ffi is the frequency of occurrence, % IRIi is the percentage of relative importance index, IRIi is the relative importance index for each prey category, ΣIRIi is the sum of relative importance index for each prey category.
Statistical analyses
Multi-variate and correlation statistical analyses were performed using Past 4.03 and Statistica 7.1 software, respectively (Scherrer, 1984). Trophic similarities were assessed by subjecting these data to a cluster analysis in order to eventually group size classes with similar diets.
Spearman's rank correlation coefficient (rs) analysis was used to indicate the degree of relationship between male and female diets. If rs = 1, the diets are strictly identical. If rs = -1, the diets are strictly inverse. Finally, if rs = 0, the diets are independent (Scherrer, 1984).
RESULTS
Size frequencies
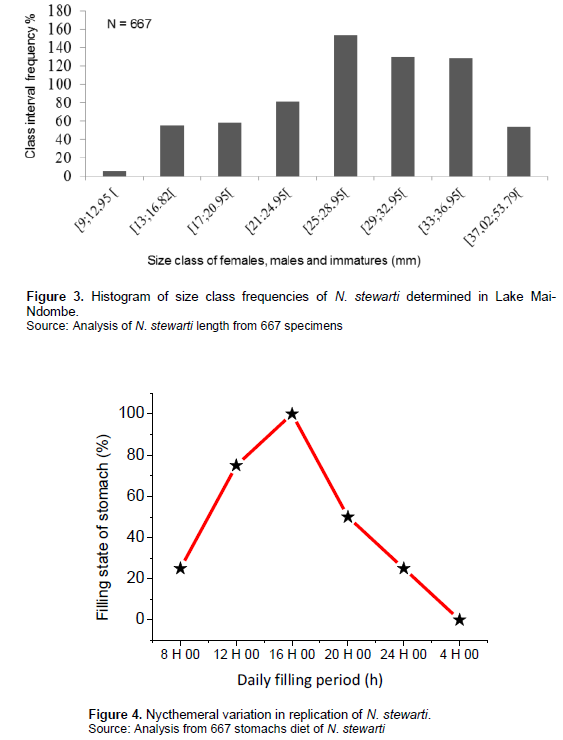
The size frequencies of N. stewarti specimens were determined from Sturge's rule as shown in Figure 4.
Ten size classes of N. stewarti were determined with 3.95 mm interval based on Sturge's rule. Size classes 8, 9 and 10 were merged into one class because of the low number of specimens in each. Thus, 8 size classes were established (Figure 3).
Class interval frequency
Diet of N. stewarti
The aspects of diet addressed in this work were the
vacuity coefficient (CV), feeding activity rate, intestinal coefficient (IC), numerical index (% IN), occurrence (or frequency) index (%Ffi), volumetric index (% IV), preponderance index (% IP), and relative importance index (% IRI).
Coefficient of vacuity (CV)
For all individuals examined, the calculated vacuity coefficient was 38.71%.
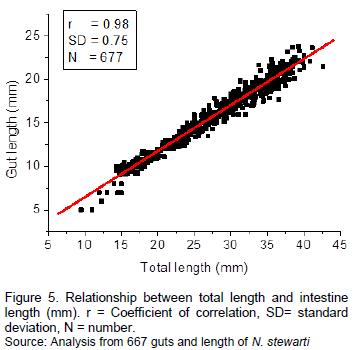
Feeding activity rhythm
Figure 4 visualizes the evolution of the degree of replenishment of N. stewarti in Lake Mai-Ndombe. N. stewarti had begun its trophic activity early in the morning at 5:00 am where the stomach begins to fill to peak at 1:00 pm and begins to empty to cancel at 1:00 am.
Intestinal coefficient (IC)
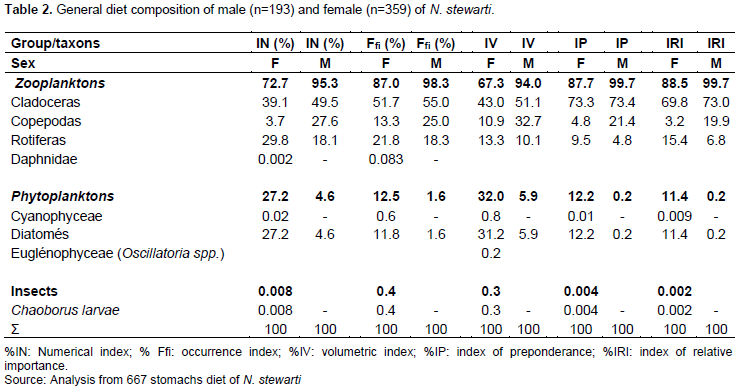
The calculated intestinal coefficient for 667 specimens of N. stewarti ranged from 0.5 to 1.0 (mean 0.69 ± 0.03). The relationship between total fish length and gut length is as shown in Figure 5.
Gut length ranged from 0.7 to 24.22 mm with an average of 15 ± 3.78 mm. The relationship between total length and fish gut length demonstrated the simultaneous increase between the two variables (r = 0.98; SD = 0.73; N = 667).
General diet composition of N. stewarti
Table 1 presents the general diet of N. stewarti in Lake Mai-Ndombe. Qualitative analysis of 667 stomachs containing prey items identified 19 food types, grouped into 4 categories: zooplankton, phytoplankton, insects, and worms. Zooplankton were the most consumed prey (%IRI=90.8). Phytoplankton (%IRI = 9.18), insects (%IRI = 0.003) and worms (%IRI = 0.000) are less observed or sometimes absent in the food bowl of this fish.

The preponderance index thus calculated had allowed us to classify the food according to the following order: zooplankton (PI > 50) is the dominant prey, phytoplankton 10< PI< 25: important prey, the remains (insect and worms) (PI < 10) are secondary prey.
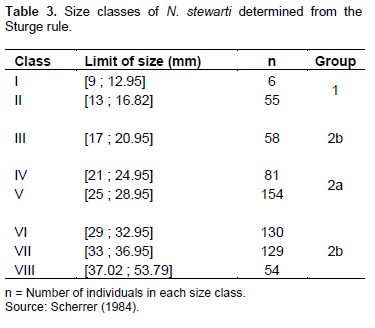
Diet by sex of individuals
Table 2 presents the diet of N. stewarti according to sex except immatures. Table 2 indicates that female and male N. stewarti had consumed the same prey categories, so there is no significant difference in diet with respect to sex (t = 0.42566, p value = 0.68157 at the 0.05 significance level). Spearman's rank correlation coefficient (rs), calculated on the basis of the index percentages of food consumed by females and males, shows a significant correlation (N = 552; rs = 0.83; p = 0.01).
Diet as a function of individual size
The size classes determined by Sturge's rule are presented in Table 3. The diet of individuals in each size class is presented in (Figures 6a, b, c, d, e, f, g, h). Individuals in all size classes feed on plankton and composed of Cladocerans, Copepods, Rotifers, Daphnia, Diatoms, and Euglenophyceae.
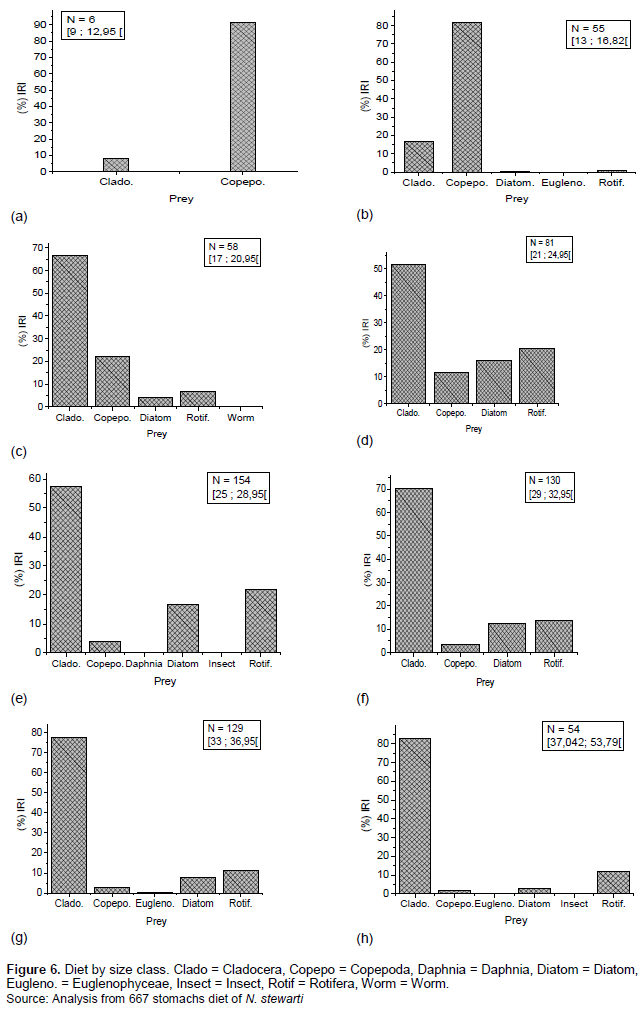
Individuals in the size class [9; 12.95], consisting mainly of larvae, feed on copepods (Nauplius), with the relative importance index (%RI = 91.66).
Individuals belonging to the size class [13; 16.82[, consisting of juveniles, feed at the expense of zooplankton (Copepods (% IRI = 81.92) and Cladoceras (% IRI = 16.76)).
Fish belonging to the size class [17; 20.95], especially sub-adults feed on Cladocerans including (% IRI = 66.56), Copepods (% IRI = 22.35), Rotifers (% IRI = 6.89) and Diatoms (% IRI = 4.15)
Fish in the size classes [21; 24.95] and [37.042; 53.79] were considered as adults feed mainly on Cladocerans (% IRI 51.76 and 83.03%), Rotifers (% IRI 11.47 and 21.93%), Diatoms (% IRI 2.71 and 16.64) and Copepods (% IRI 1.73 and 11.62).
Dietary similarity between individuals belonging to the different size classes
The dendrogram (Figure 7) visualizes the grouping of size classes of N. stewarti based on prey consumed.
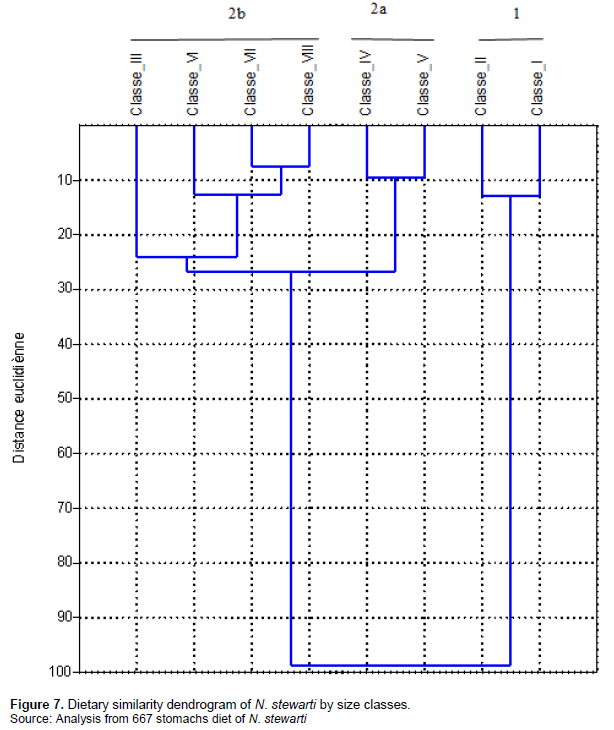
The food similarity dendrogram (correlation coefficient 0.97) based on the food items consumed by individuals of each size class of N. stewarti established from the Relative Importance Index (RI) highlighted two main trophic groups. The first group (1) is distant from the second by 73. The first one, made up of individuals belonging to classes I and II. These individuals feed at the expense of copepods (IRI = 81.92 - 91.66%) and cladocerans (8.33 - 16.76%) composed of Nauplius and Diaphonosoma species, respectively. Other prey such as Daphinia, Eglenophyceae and Rotifera are consumed in very small quantities (%IRI = 0.004 and 0.89) (Figure 7). The second group consisted of classes III, IV, V, VI, VII and VIII consuming cladocerans (IRI = 51.76 - 83.03), copepods (IRI = 1.73 - 22.35), rotifers (IRI = 6.89 - 21.93), and Diatoms (%IRI = 6.89 - 21.93). The rest of the preys are very weakly represented. This second group is subdivided into two subgroups; the first subgroup includes classes IV and V which had consumed more Cladocerans (%IRI = 57.26 - 70.27), Copepods (%IRI = 3.46 - 4.14), Rotifers (%IRI = 13.78 - 21.93) and Diatoms (%IRI = 12.47 - 16.64). The second subgroup includes classes III, VI, VII and VIII that feed on Cladocerans (%IRI = 66.56 and 83.0), Copepods (%IRI = 1.73 and 22.35), Diatoms (%IRI = 2.71 to 12.47) and Rotifers (%IRI = 6.89 to 13.78). Subgroup 2 broadens its food spectrum on prey on Euglenophyceae, insects and worms.
DISCUSSION
The average vacuity coefficient determined in the N. stewarti fish specimens studied was 38.71%. These results are similar to those obtained by Ouattara et al. (2014) (CV = 42.22% in flood season) and (CV = 15.03% in flood periods) in Engraulis encrasicolus (Linneaus, 1758) from the Ivory Coast. The trophic activity of N. stewarti starts early at 5 am where the stomach starts to fill up to reach the peak around 1 pm and starts to empty to cancel at 1 am. Similar results were obtained by Kaningini (2003) in Lake Kivu who observed empty stomachs in Limnothrissa miodon (Boulanger, 1906) larvae fished between 22:00 and 06:00 h.
In many vertebrates, a positive relationship has been demonstrated between the length of the gut and the nature of the food they consume (Grassé and Devillers, 1965; Kramer and Bryant, 1965; Paugy, 1994). Thus, the intestine appears to be longer in herbivores, shorter in carnivores and of intermediate length in omnivores. Paugy (1994) had classified fish as follows: ichthyophagous (CI) less than 0.85, invertivorous, CI between 0.32 and 2.18, omnivorous, CI between 0.8 and 3.01 and phytophagous, CI between 4.71 and 6.78.
During the present investigation, the intestinal coefficient of N. stewarti specimens ranged from 0.5 to 1.0 with an average of 0.69 ± 0.03, which classifies it as an invertivore species.
In Lake Mai-Ndombe, N. sterwarti feeds on plankton (zooplankton and phytoplankton), insects, and worms. Of the 19 food items inventoried, we find copepods (Calanoides, Tropodiaptomus species, Cyclopoide, Thermocyclops species, Nauplis), Cladocerans (Bosminopsis, Bosmina, Diaphanosoma, Alona, and Daphnia species), Rotifera (Keratela cochlearis, Keratela serulata, Keratela tecta, Trichocera marina), Phytoplankton (Diatoms, Phacus, Pinnularia, Plectonema species), (Oscillatoria species, Euglenophyceae), insects (Grasshopper, Chaoborus larva), and worms (Nemathelminthe). Indeed, Paulsen (1993), Mandima (1999), Isumbisho et al. (2004), Isumbisho et al. (2006), and Mandima (2017) reported that in the stomach contents of clupeidae a microplankton slurry based on copepods and insects is observed. Variations in air temperature, rainfall and the presence of mineral salts in the water are factors that cause the proliferation of plankton that are prey to this fish in Lake Mai-Ndombe. The present results are in line with those of Otobo (1977) and Kolding et al. (2019) who report that the clupeidae Stolothrissa tanganicae feeds mainly on Copepods zooplankton, Cladocerans and some phytoplankton for reproductive activities (Mulimbwa et al., 2022).
No qualitative diet differences were observed between male and female N. stewarti specimens studied in Lake Mai-Ndombe. Spearman's rank correlation coefficient (rs) indicates that there is similarity in diet between the two sexes (rs = 0.83; p = 0.01).
Cladocerans and copepods were the preferred foods of individuals regardless of size. Larvae and juveniles feed on Copepods (Nauplius) and some Cladocerans while sub-adults add Rotifers, Diatoms and worms to their food bowl (Števove and Ková?, 2016).
According to Matthes (1968), Otobo (1977) and Muvengwi et al. (2012), a number of genera of Clupeidae have adapted to African fresh waters and some species can really be considered pelagic. This is the case for example of Stolothrissa tanganicae and Limnothrissa miodon from Lake Tanganyika. S. tanganicae is a planktivorous species feeding on tiny pelagic shrimps (Limnocaridina), copepods, cladocerans and some phytoplankton. L. miodon also consumes shrimps and zooplankton but also young stages of Stolothrissa. Some genera like Pellonula, Cynothrissa, and Sierrathrissa are fluviatile forms whose diet is mainly formed by insects (aquatic and terrestrial). However, they seem to adapt very well in dam lakes such as Lake Volta or Lake Kainji where they find an abundant food supply based on insects (Ephemera, Chaoborids) and zooplankton. The present results did not reveal the presence of shrimps.
CONCLUSION
The diet of the fish N. stewarti from Lake Mai-Ndombe was determined from the food indices calculated on 667 specimens fished according to a 24 h cycle.
This fish starts its feeding activity at dawn from 5 am where its stomach starts to fill and reaches the maximum around 1 pm to empty at 1 am. Its diet consists of plankton composed of Cladocerans, Copepods, Rotifers, Daphnia, Diatoms and Euglenophyceae.
No difference in qualitative diet was observed according to sex. Larvae consume especially copepods (Nauplius) while adults are both zooplanktonophagous (cladophagous, copepophagous, rotiphagous) and phytophagous. Insects are an accessory food.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The authors thank the political and administrative authorities of the Mai-Ndombe province for various authorizations. They are very grateful to Messrs, Freddy and Mandela for their contribution to the sampling of data on the Lake. They are also grateful to Willy Lusasi, Santos Kavumu, Clément Munganga and Miriam Modimo, all researchers at the Laboratoire de Limnologie, Hydrobiologie et Aquaculture of the University of Kinshasa for their scientific assistance.
REFERENCES
|
Baker R, Buckland A, Sheaves M (2014). Fish gut content analysis: robust measures of diet composition. Fish 15(1):170-177. |
|
|
Bertran R, Calvez JC (1988). Contenus stomacaux de jeunes homards Européens capturés en pêche à pieds à Blainville (Ouest-Contentin), ICES, Museum National d'histoire naturelle, France, Laboratoire maritime 13 p. |
|
|
Bourrelly P (1972). Les algues d'eau douce. Initiation à la systématique Tome I : Les algues vertes. Place Saint-Andre-Des-Arts, Paris VI. Editions N. BOUBEE & Cie 3. |
|
|
Bultot F, Griffits JF (1971). The equatorial wet zone. In: Griffits JF (ed.) Climates of Africa, World Survey of Climatology. Elsevier publishing company, Amsterdam-London-New York 10:451-456. |
|
|
Descy JP, Darchambeau F, Lambert T, Stoyneva-Gaertner MP, Bouillon S, Borges AV (2016). Phytoplankton dynamics in the Congo River. Freshwater Biology 62(1):87-101. |
|
|
Diaha NC, Amande MJ, Agnissan AR (2018). Relation régime alimentaire-stade de maturité sexuelle chez le listao (Katsuwonus pelamis Linnaeus, 1 758) débarqué au port de pêche d'Abidjan en Côte d'Ivoire. Tropicultura 36(4):705-712. |
|
|
Dussart B (1967a). Les copépodes des eaux continentales d'Europe occidentale. I. Calanoïdes et Harpacticoïdes. Boubée and Cie, Paris 500p. |
|
|
Dussart B (1967b). Les copépodes des eaux continentales d'Europe occidentale. II. Cyclopoïdes et Biologie. Paris, Boubée and Cie 292p. |
|
|
Dussart B (1982). Faune de Madagascar: crustacés copépodes des eaux intérieures. Paris, ORSTOM-CNRS 146 pp. |
|
|
Ebengo (2022). Opérateur de systèmes d'informations géographiques, Laboratoire de cartographie numérique. Faculté des Sciences agronomiques, Université de Kinshasa 1P. |
|
|
De Keyzer EL, Mulungula PM, Lufungula GA, Manala CA, Muniali AA, Cibuhira P B, Van Steenberge M (2020). Local perceptions on the state of the pelagic fisheries and fisheries management in Uvira, Lake Tanganyika, DR Congo. Journal of Great Lakes Research 46(6):1740-1753. |
|
|
Froese R, Pauly D (1999). FishBase. Concepts, structures et sources des données. ICLARM, Manille, Philippines, 324p. |
|
|
Garrido S, Murta AG, Moreira A, Ferreira MJ, Angélico MM (2008). Horse mackerel (Trachurus trachurus) stomach fullness off Portugal: index calibration and spatio-temporal variations in feeding intensity. ICES Journal of Marine Science: Journal du Conseil 65(9):1662-1669. |
|
|
Grassé PP, Devillers C (1965). Précis des sciences biologiques. Zoologie. Tome 2: Vertébrés. Paris, Masson et Cie 1130 p. |
|
|
Harding JP, Smith WA (1974). A key to the British freshwater cyclopoid and calanoid copepod with ecological notes. Freshwater Biological Association 18:54. |
|
|
Inogwabini BI, Mputu D, Zanga L (2009). The use of breeding sites of Tilapia congica (Thys & van Audenaerde 1960) to delineate conservation sites in the Lake Tumba, Democratic Republic of Congo: Toward the conservation of the lake ecosystem. Journal: African Journal of Ecology 48(3):800-806. |
|
|
Kaningini M (2003). Moment d'alimentation et régime alimentaire des larves de Limnothrissa miodon (Blgr, 1906) dans la partie sud du lac Kivu (Bassin de Bukavu) 16 p. |
|
|
Isumbisho M, Kaningini M, Descy J, Baras E (2004). Seasonal and diel variations in diet of the young stages of the fish Limnothrissa miodon in Lake Kivu, Eastern Africa. Journal of Tropical Ecology 20(1):73-83. |
|
|
Isumbisho M, Sarmento, Kaningini B, Micha JC, Descy JP (2006). Zooplankton of Lake Kivu, East Africa, half a century after the Tanganyika sardine introduction. Journal of Plankton Research 28(11):971-989. |
|
|
Kolding J, van Zwieten P, Marttin F, Funge-Smith S, Poulain F (2019). Freshwater small pelagic fish and fisheries in major African lakes and reservoirs in relation to food security and nutrition. FAO Fisheries and Aquaculture Technical Paper No. 642. Rome, FAO. 124 p. CC BY-NC-SA 3.0 IGO. |
|
|
Kouamé MK, Ouattara A, Dietoa MY, Gourène G (2006). Alimentation du Clupeidae Pellonula leonensis dans le lac de barrage de Buyo (Côte d'Ivoire) Cybium 30(2):145-150. |
|
|
Kouamélan EP (1999). L'effet du lac de barrage Ayamé (Côte d'Ivoire) sur la distribution et l'écologie alimentaire des poissons Mormyridae (Teleostei, Ostéoglossiformes). Thèse de doctorat, Katholieke Universteit Leuven, Belgique, 221 p. Labeo senegalensis Valenciennes 1842 (Teleostei: Cyprinidae) in the Ouémé basin, Benin. African Journal of Aquatic Science 35(1):81-88. |
|
|
Kramer DL, Bryant MJ (1995). Intestine length in the fishes of tropical stream: 1. Ontogenic allometry. Environmental Biology of Fishes 42:115-127. |
|
|
Lauzanne L (1976). Régimes alimentaires et relations trophiques des poissons du lac Tchad. Cah. Orstom, sér. Hydrobiology 10:267-310. |
|
|
Lévêque C, Paugy D (2006). Les poissons des eaux continentales Africaines Diversité, écologie, utilisation par l'homme. IRD (Institut de Recherche Pour le Développement) 2e Éditions Paris, ISBN: 2-7099 -1589 - 8. P 573. |
|
|
Lévêque C, Bruton MN, Ssentongo GW (1994). Biology and ecology of African freshwater fishes. Institut Francais de recherche scientifique pour le développement en coopération. ORSTOM France. 490 P. |
|
|
Mahesh V, Ambarish PG, Rekha JN (2019). Stomach Content Analysis Techniques in Fishes. Demersal Fisheries Division. ICAR-Central Marine Fisheries Research Institute pp. 104-115. |
|
|
Mandima JJ (2017). The feeding biology of Limnothrissa miodon (Boulenger, 1906) in Lake Kariba. Joensuu, University of Eastern Finland (PhD thesis) 111 p. |
|
|
Mandima JJ (1999). The food and feeding behaviour of kapenta, Limnothrissa miodon (Boulenger, 1906) in Lake Kariba, Zimbabwe. Hydrobiologia 407:175-182. |
|
|
Manko P (2016). Stomach content analysis in freshwater fish feeding ecology. University of Prešov. Spain. ISBN 978-80-555-1613-4. |
|
|
Matthes H (1968). Preliminary investigation into the biology of Lake Tanganyika clupeidae, Zambia. Fisheries Research Bulletin 4:39-46. |
|
|
Micha JC, Nabwenge BLB, Ibofa R, Mumba F, Mutambwe S, Zanga N, Willem E, Svennsson JE, Wilander A (2020). Une ressource surexploitée, Nannothrissa stewarti, sardine endémique du lac Maï-Ndombe (RD Congo), résultat inattendu du Programme national de Lutte contre le Paludisme. Meded. Zitt. K. Acad. Overzeese Wet.Bull. Séanc. Academie Royale Des Sciences D'outre-mer 64(1):61-91. |
|
|
Mulimbwa N'sibula T, Milec LJM, Raeymaekers JAM, Sarvala J, Plisnier PD, Marwa B, Micha JC (2022). Spatial and seasonal variation in reproductive indices of the clupeids Limnothrissa miodon and Stolothrissa tanganicae in the Congolese waters of northern Lake Tanganyika. Belgian Journal of Zoology 152:13-31. |
|
|
Muvengwi J, Muposhi VK, Veremu K, Mbiba M, Nyenda T (2012). The diet of Limnothrissa miodon and zooplankton densities in Sanyati Basin, Lake Kariba. Indian Journal of Environmental Health 1(4):480-490. |
|
|
Natarajan AV, Jhingran AC (1961). Index of preponderance'-a method of grading the food elements in the stomach analysis of fishes. Indian Journal of Fisheries 8:54-59. |
|
|
Otobo FO (1977). The biology of clupeid fishes in Lake Kainji, Nigetia. Ph. D. thesis, University of Ife, Ile-Ife. Nigeria 272 p. |
|
|
Ouattara S, Ouattara N, Soro D, Fantodji A (2014). Régime alimentaire de Engraulis encrasicolus (Linneaus, 1758) du littoral de la Côte d'Ivoire. International Journal of Biological and Chemical Sciences 8:916-924. |
|
|
Paugy D (1994). Ecologie des poissons tropicaux d'un cours d'eau temporaire (Baoulé, haut bassin du Sénégal au Mali) : adaptation au milieu et plasticité du régime alimentaire. Revue d'Hydrobiologietropicale 27:157-172. |
|
|
Paulsen H (1993). Feeding biology of Kapenta, Limnothrissa miodon, in Lake Kariba. Project No. 30, Zambia/Zimbabwe SADC Fisheries Project. Kariba, Zimbabwe, Lake Kariba Fisheries Research Institute. |
|
|
Pwema VK, Mbomba NB, Takoy AL, Malekani JM, Micha JC (2015). Comparison of the diet of two species of Labeo (Cyprinidae): a rheophilic one, Labeo sorex and a limnophilic one, Labeo lineatus in the Malebo Pool (Congo River). Congo Sciences 3:1. |
|
|
Rosecchi E, Nouaze Y (1987). Comparaison de cinq indices utilisés dans l'analyse Des contenus stomacaux. Revue des travaux de l'Institut des Pêches Maritimes 49:111-123. |
|
|
Scherrer B (1984). Biostatistique. 850 p. Québec, Boucherville: Gaëtan Morin Éditions. |
|
|
Števove B, Ková? V (2016). Ontogenetic variations in the diet of two invasive gobies, Neogobius melanostomus (Pallas, 1814) and Ponticola kessleri (Günther, 1861), from the middle Danube (Slovakia) with notice on their potential impact on benthic invertebrate communities. Science of the Total Environment 557:510-519. |
|
|
Thumithol JPU, Mambo TB, Uromi CC, Ngab'u JC, Kankonda AB, Ulyel JA, Ngemale MG, Ngbolua KN (2016). Food ecology of Ichtyoborus besse congolensis (Giltay, 1930: Teleostei: Distichodontidae) from Biaro River and its tributary Yoko in Yoko Forest Reserve (DR Congo). International Journal of Innovation and Scientific Research 21(2):330-341. |
|
|
Zachari PU (2016). Trophodynamics and Review of methods for Stomach content analysis of fishes Demersal Fisheries Divisio. Central Marine Fisheries Research Institute, Kochi. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0