ABSTRACT
The pulp and paper industry is the sixth largest polluter discharging a variety of gaseous, liquid and solid wastes into the environment. This pollution mainly arises due to chemicals used during production, so this study compared these two chemicals to determine the better one for a cleaner production process. A dewatered kenaf stem was cooked in the 20, 60 and 90% concentrations of formic acid and sodium hydroxide at time intervals of 1, 2 and 3 h to compare the solids (total suspended solid, total dissolved solid and total solid) of their effluent for environmental friendliness. After examining the whole concentrations and the time intervals, 60% concentration of the acids at 2 h pulping gave better pulp on physical examination. When the solids of the effluents of the two chemicals were analyzed, formic acid effluent had a TSS of 5768 mg/L, TDS of 54088 mg/L and TS of 59855 mg/L, while that of sodium hydroxide was 6053 mg/L for TSS, 96628 mg/L for TDS and 102680 mg/L for TS. This study showed that pulping of kenaf stem with 60% formic acid for 2 h has effluent that is greener than the use of sodium hydroxide of the same concentration at the same duration.
Key words: Effluent, environmental quality, formic acid, kenaf stem, sodium hydroxide, total solids.
Generally, the pulp and paper industry has been considered to be a major consumer of natural resources (wood, water) and energy (fossil fuels, electricity) and a significant contributor of pollutants discharge to the environment (Hossain and Ismail, 2015). Environmental effects have been attributed to chemicals introduced during the manufacturing process, to natural compounds released from plant material used as mill furnish, to interactions of these compounds with each other and interactions with biota in mill effluent during waste water treatment (Hewitt et al., 2006). Accordingly, in both traditional and emerging paper and pulp producers (Chen et al., 2012) such as United States (Schneider, 2011), China (Zhu et al., 2012) and India (Afroz and Singh, 2014), pulp and paper mills are considered a major source of environmental pollutants. Pulping wood or agricultural residue using conventional methods releases a range of pollutants, including organic products that cause eutrophication in water, aluminium salts and sometimes, sulphur dioxide to the atmosphere. Polluted river due to pulp and paper effluent discharge has adversely affected the aquatic fauna as well as communities in the surrounding areas who economically depend on this river for fishing and agriculture purposes (Zuby and Ajay, 2014). Effluent quality is commonly judged on the basis of such aggregate characteristics as biochemical oxygen demand, chemical oxygen demand, total suspended solids (TSS), total solids (TS), turbidity, pH, color e.t.c. Total suspended solid (TSS) represents the solid particles mixed in water or effluent. Total dissolved solids (TDS) are measured as the mass of residue remaining when a measured volume of filtered water is evaporated. Total solids (TS) are the amount of solid present in dissolved and suspended form. The significant solid wastes such as lime mud, lime slaker grits, green liquor dregs, boiler and furnace ash, scrubber sludges, wood processing residuals and wastewater treatment sludges are generated from different mills. Disposal of these solid wastes cause environmental problems because of high organic content, partitioning of chlorinated organics, pathogens, ash and trace amount of heavy metal content (Monte et al., 2009).
Pulping procedure consists of a selective extraction of lignin from lignocellulosics like wood and nonwood materials without degradation of cellulose. It can be chemical pulping (e.g., kraft or soda chemical pulping), mechanical pulping, and semichemical pulping. Soda pulp is the original chemical pulp and is produced by cooking chips of (usually) deciduous woods in a solution of caustic soda under pressure. The pulping process affects the strength, appearance and intended use characteristics of the resultant paper product. Pulping processes are the major source of environmental impacts in the pulp and paper industry, each pulping process has its own set of process inputs, outputs and resultant environmental impacts. The story of the Nigerian paper and pulp manufacturing sub-sector of the economy, especially in the last three decades at best, resonates with the familiar take of the comatose operational state of the manufacturing sector in general. The few existing ones have resisted pressure to make their processes environmental benign. Pulp and paper industry is considered as one of the most polluting industry contributing 100 million kg of toxic pollutants that are being released every year in the environment (Dey et al., 2013). The introduction and development of organosolv pulping is aimed to reduce the environment pollution by improved pulping process. Some other organosolv processes include ASAM (Kordsachia and Patt, 1988; Kordsachia et al., 2002), MILOX (Ligero et al., 2010) e.t.c. Previous studies (Preeti, 2008; Ligero et al., 2010) have concentrated on some characteristics of effluent from pulping with formic acid and soda without considering the interaction between the chemical, time, and concentration. Chempolis belongs to Organosolv which is a chemical pulping carried out by using organic solvents and chemicals (Lora and Aziz, 2000; Lonnberg et al., 1987). Chempolis process is based on acidic delignification to remove lignin, a desired part of the hemicelluloses and nutrients. In Chempolis process (Rousu et al., 2002), pulping is carried out with formic acid at slightly elevated temperatures with a conventional liquor-to-straw ratio. Most studies in the field of organic pulping have only focused on the properties of the pulp while the waste is a growing public health concern worldwide. Hence, the goal of this paper is to find out if there exists a possibility for improving the effluent quality by exploring the interactions of the chemicals, concentration, and time which was not favored in prior attempts by previous researchers.
Kenaf stem was chopped into 1 to 4 cm long, washed with warm water to remove dirt and dust. The washed kenaf was dewatered to a solid content of 40% to 45%. Five grams of kenaf stem was taken in 400ml of pulping mixture in 1000 ml flask at atmospheric pressure and pulped at 20, 60 and 90% concentrations of formic acid and sodium hydroxide, cooking time was varied from 1, 2 h and 3 h at 95°C as shown in Figure.1. At the end of each period, the sample was filtered with a fine mesh sieve of size 0.027 mm to get the effluent used in the analyses. The tests were carried out in triplicate and each value is an average of three samples.
The effluent was analysed using the Standard Method for Examination of Water and Wastewater (APHA, 2005). The parameters determined were TSS, TDS and TS. The ANOVA tables on appendix 2 were used to test the hypothesis which states that duration and concentration of chemicals do not affect waste characterization during pulping.
After 1 h and 20% concentration of acids pulping, the residue was not well pulped. At 2 h and 60% concentration pulping, the residue was well pulped. At 3 h and 90% concentration, some were well pulped while others were over pulped. Table 1 shows the values of TSS, TDS and TS of the effluent when the stem was pulped with 20% concentration of formic acid and sodium hydroxide at 1, 2 and 3 h intervals. With sodium hydroxide (NaOH), the TSS values in the reaction was highest (9903 mg/L) after 1 h but reduced with increase in time with a value of 7385 mg/L at 3 h.
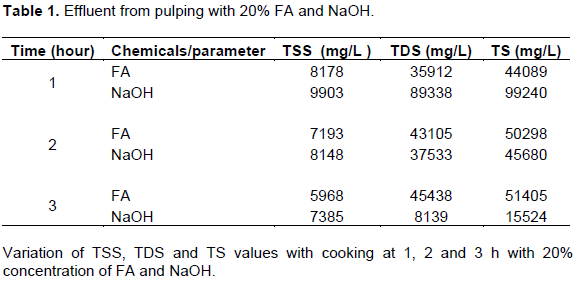
Formic acid had its lowest TSS value (5968 mg/L) at 3 h and highest at 1 h (8178 mg/L). The finding of the present study suggest that value of TSS in effluent from formic acid and sodium hydroxide pulping decrease with increase in pulping time which is in agreement with Preeti (2008) and Pooja et al. (2013). With NaOH, the values of TDS reduced with time from 89338 mg/L at 1 h to 8139 mg/L after 3 h while TDS increased from 35912 mg/L at 1 h to 45438 mg/L after 3 h with formic acid. This may be indicative that chemicals and raw materials react differently. This findings showed increase in TS with time in formic acid (44089 to 51405 mg/L) and decrease with time in NaOH (99240 to 15524 mg/L). This suggests that solids in the formic acid solution were not degraded much with time and concentration.
Table 2 provides the values of TSS, TDS and TS of effluent obtained when the stem was pulped with 60% concentration of FA and NaOH. From the data in the table, there is a clear trend of decreasing in the TSS values of FA and NaOH effluent which suggest that more organic matter was degraded with time. As illustrated in Table 2, TDS of effluent from FA pulping increased with increase in time while that from NaOH pulping decreased with increase in time which shows that NaOH was able to digest more organic matter. The TS content of FA pulped effluent showed increment with time while NaOH pulped effluent reduced with time.
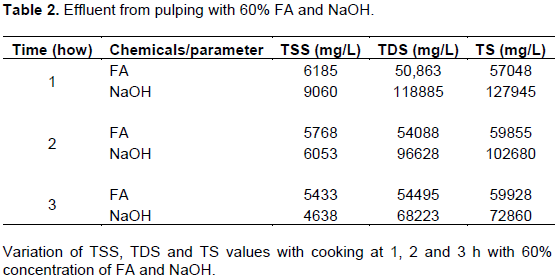
Table 3 presents the values of TSS, TDS and TS from effluent obtained from pulping kenaf stem with 90% concentration of FA and NaOH. As illustrated in Table 1 and 2, TSS from FA and NaOH pulping effluent decreased with time having highest and lowest as 5273/4973 and 7353/3165 mg/L. While the value of TDS from NaOH pulping followed the same pattern, while that from FA increased with increase in time and the same occurred with TS values. The effluent from NaOH pulping showed a high reduction in TS values from 1 to 3 h with 90% concentrations which may be as a result of reduction in the amount of solid particles in the solution with time as can be seen in Pooja et al. (2013).
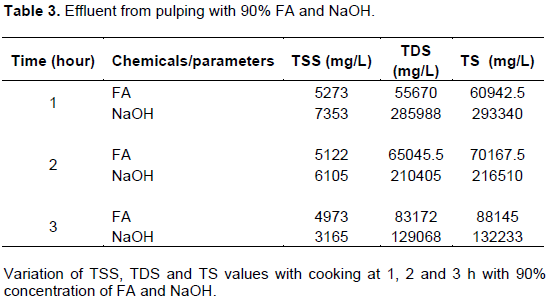
All the concentrations with the two chemicals have their maximum TSS after 1 h and minimum after 3 h which gave highest value at 20% for 1 h and lowest at 90% for 3 h (9903 and 3165 mg/L) The finding is consistent with findings of past studies by Preeti (2008) and Pooja et al. (2013), in which there were decreases in the TSS values. The effluent from NaOH pulping showed a high reduction in TS values from 1 to 3 hours in all the concentrations which may be as a result of reduction in the amount of solid particles in the solution as reflects in Pooja et al. (2013). With formic acid, TS value did not decrease rather it showed a minimal increase with time along the concentration lines. This showed that solids in the solution did not degraded much with time and concentration.
In this research, solids in the pulping effluent are considered with respect to environmental quality. High values of solids in effluent are detrimental to the environment. Federal Ministry of Environment has effluent standard for discharge into the environment. The maximum limit for total dissolved solid is 2000 mg/L, total suspended solid is 30 mg/L while total solid is 2030 mg/L (FEPA, 1991). From the two pulping processes, though formic acid effluent has values far above the limit, it is lower than that of sodium hydroxide.
Environmental qualities of solids from soda and formic acid pulping of kenaf stem were compared. This was done by pulping at three different concentrations of the chemicals during three time periods. Based on the information from the bench, it was found that pulping with formic acid and soda at 60% concentration for two hours has better environmental quality. This was selected from other conditions because it gave better pulp on physical examination as well as better environmental qualities on analyses. Though the results do not indicate that this option is the best in all parameters analyzed, it ranked the best in most of the parameters. Turning kenaf waste into resources is not only a good idea but also a proven one; it could have a very positive impact on people and the planet while building a profitable business. However, further research is needed to check other effluent parameters to know whether they toe the same line as the solids.
The authors have not declared any conflict of interests.
REFERENCES
|
Afroz Z, Singh A (2014). Impact of pulp and paper mill effluent on water quality of river Aami and its effect on aquatic life (fish). Glob. J. Pharmacol. 8:140-149.
|
|
|
|
APHA, AWWA, WEF (2005). Standard Methods for the Examination of and Wastewater 21st ed. American Public Health Association, Washington, D.C.
|
|
|
|
|
Chen H, Hsu C, Hong G (2012). The case study of energy flow analysis and strategy in pulp and paper industry. Energy Policy 43:448-455.
Crossref
|
|
|
|
|
Dey S, Choudhury M, Das S (2013). A review on toxicity of paper mill effluent on fish. Bull. Environ. Pharmacol. Life Sci. 2:17-23.
|
|
|
|
|
FEPA (1991). Guideline and Standard for Environmental Pollution Control in Federal Environmental Protection Agency Act (Cap 131 LFN), National Environmental Protection (Effluent Limitation) Regulation Official Gazatte.
|
|
|
|
|
Hewitt L, Parrott J, McMaster M (2006). A decade of research on the environmental impacts of pulp and paper mill effluents in Canada: Sources and characteristics of bioactive substances. J. Toxicol. Environ. Health Part B: Crit. Rev. 9:341-356.
Crossref
|
|
|
|
|
Hossain K, Ismail N (2015). Bioremediation and detoxification of pulp and paper mill effluent: A review. Res. J. Environ. Toxicol. 9:113-134.
Crossref
|
|
|
|
|
Kordsachia O, Patt R (1988). Deutsches Patentamt , DE 3518005 A1, 1.
|
|
|
|
|
Kordsachia O, Patt R, Rose B (2002). New Available Technologies. In Seventh International Conference on SPCI, Stockholm, Sweden, 26.
|
|
|
|
|
Ligero P, Villaverd JJ, Vega A (2010). Delignification of Miscanthus x Giganteus by the Milox process. Bioresour. Technol. 101:3183-3193.
Crossref
|
|
|
|
|
Lonnberg B, Laxen T, Sjoholm R (1987). Chemical pulping of softwood chips by alcohols, I. Cooking Paperi ja Puu 69:757-762.
|
|
|
|
|
Lora JH, Aziz J (2000). Organosolv pulping: A versatile approach to wood refining. Tappi J. 68(8):94-97.
|
|
|
|
|
Monte M, Fuente E, Blanco A, Negro C (2009). Waste management from pulp and paper production in the European Union. Waste Manage. 29:293-308.
Crossref
|
|
|
|
|
Pooja T, Virendra K, Gyanesh J, Sat P, Suresh P, Sanjay N, Raman N (2013). A Comparative study on physico-chemical properties of pulp and paper mill effluent. Int. J. Eng. Res. Appl. 3(6):811-818.
|
|
|
|
|
Preeti N (2008). Studies on the effluent generated during the pulping process in paper industry. Curr. World Environ. 3(1):189-193.
|
|
|
|
|
Rousu P, Rousu P, Anttila J (2002). Sustainable pulp production from agricultural waste. Resources. Conserv. Recycl. 35:85-103.
Crossref
|
|
|
|
|
Schneider T (2011). Is environmental performance a determinant of bond pricing? Evidence from the U.S. Pulp and paper and chemical industries. Contemp. Account. Res. 28:1537-1561.
Crossref
|
|
|
|
|
Zhu X, Wang J, Jiang Y, Cheng Y, Chen F, Ding S (2012). Feasibility study on satisfing standard of water pollutants for pulp and paper industry. Appl. Mech. Mater. 178-181:637-640.
Crossref
|
|
|
|
|
Zuby A, Ajay S (2014). Impact of pulp and paper mill effluent on water quality of River Aami and its effect on aquatic life (Fish). Glob. J. Pharmacol. 8 (2):140-149.
|
|