ABSTRACT
This study was carried out to investigate the potential uptake of heavy metals (Pb, Ni, and Cd) by Pteridium aquilinum plant from aqueous solutions. The whole plant strands were cultured in 36 experimental pots containing 5, 10 and 15 (mg/L) of Pb2+, Ni2+ and Cd2+ aqueous solutions. The plants were harvested after 24, 48, 72 and 96 hours of exposure and segmented into leaves, stem and root, respectively. The heavy metals content in the plant parts were analyzed using Atomic Absorption Spectrophotometer version VGP 210. The highest actual accumulations for Pb, Ni and Cd were recorded in the root at 15 mg/L of the aqueous solution after 96 hours of exposure with mean values of 4.29±0.04, 4.06±0.01 and 0.65±0.00 mg/kg, respectively. The highest total actual accumulations recorded in the plant were 9.19±0.14, 10.80±0.03, and 1.23±0.04 mg/kg for Pb, Ni and Cd, respectively. The accumulation of the heavy metals in the plant parts was in this order; root > stem > leaves. The results reveal that, the accumulation of heavy metals in the plant increases with increase in the concentration of the aqueous solutions and the duration of exposure to the aqueous solutions. The uptake of heavy metals by the plant is in this order; Ni > Pb > Cd. The translocation factor (TF) recorded for Pb, Ni and Cd in the plant were all greater than 1 (>1) except for Cd at exposure duration of 96 h in the 15 mg/L Cd2+ aqueous solutions, with the highest TF values of 2.500, 1.886 and 1.601, respectively. This indicates that more of the heavy metals were stored in the shoot. P. aquilinum is therefore described as a good accumulator plant, which could be used for phytoextraction of Pb, Ni and Cd in aqueous solutions of the metals.
Key words: Phytoremediation, Pteridium aquilinum, heavy metals, contamination, translocation factor.
Due to increase in anthropogenic activities, environmental contamination by heavy metals has increased considerably and has become a serious environmental concern (Saha et al., 2017; Yan et al., 2020). It has been known that heavy metals pollution of the biosphere is caused by the increased growth of the chemical industries. Nearly 1000 new chemicals are being synthesized every year and once these chemicals get into the environment, they pose potential health risk to humans and also have great impact on soil, ground water and plants (Shukla et al., 2010). Other factors such as excessive use of pesticides in agriculture, waste from de-acidifying soils and crude oil processing are known to contribute to environmental pollution (Szczygłowska et al., 2011; Uwazie et al., 2020).
Contamination by heavy metals is considered as the most critical threat to soil and water resources as well as humans, because of their non-bio-degradable nature, long biological half-life and their potential to accumulate in different body parts. Also heavy metals are capable of causing serious clinical problems to humans even at relatively lower concentration (Oti and Nwabue, 2013; Ibrahim and El Afandi, 2020). Heavy metals such as Cd can result in muscular weakness and cancers in humans; they can also cause a decrease in the lipid content and plant growth. For instance Ni can reduce proteins and enzymes production while Pb inhibits root and shoot growth (Fernandes and Henriques, 1991; Sarma et al., 2009).
Soil and water contaminated with toxic metals such as Cd, Pb, As, Zn, Ni, and Cu, as a result of worldwide industrialization has increased noticeably within the past few years (Ahmadpour et al., 2012; Li et al., 2019). Environmental pollution is therefore a global problem, and the development of inventive remediation technologies for the de-contamination of impacted sites is therefore of paramount importance.
Physical, chemical and biological methods are available for the remediation of contaminated sites (Kummling et al., 2001). These remediation technologies may be used in conjunction with one another to reduce the contaminants to a safe and acceptable level (Hardiman et al., 2005; Jadia and Fulekar, 2009). Some of these methods (conventional) used for the remediation of contaminated sites include: excavation or dredging, pump and treatment, thermal desorption, solidification and stabilization, precipitation, nitrification, soil washing and air striping (Jadia and Fulekar, 2009). In spite of being efficient, these methods are quite expensive, time consuming and environmentally destructive and some of these methods can also strip the soil of its natural nutrient, even if it has been decontaminated, and also generate large amount of waste to be disposed of (Danh et al., 2009).
Phytoremediation, which is a fast growing technology is considered among the best available technologies and recognized as an effective, appealing method for the remediation of contaminated sites because of its cost effectiveness, aesthetic advantage and long term applicability (Cunningham and Ow, 1996; Cluis, 2004). The term phytoremediation actually refers to diverse collection of plant based technologies that use either naturally occurring or genetically engineered plants for cleaning up contaminated sites (Flathman and Lanzan, 1996; Yoon et al., 2006). It is a green technology.
Studies have been conducted in areas of phytoremediation, using various types of plants in the phytoremediation of contaminated soil and water. Ma et al. (2001) applied Pteris vittatta to accumulate 14,500 mg/kg of arsenic in contaminated soil while Schnoor (1997) applied sunflower to remediate soil contaminated with uranium from 350 ppb to 5 ppb, achieving a 95% reduction in 24 h. Saha et al. (2017) achieved a 99.5% removal of Cr (IV) using water hyacinth. Pteridium aquilinun has also been used in the phytoremediation of zinc (Olaifa and Ajagbe, 2017) and copper (Olaifa and Omekam, 2014), using Clarias gariepinus for bioassay. Positive results were recorded. Some other studies investigated the use of phytoremediation in the remediation of soils contaminated with petroleum hydrocarbons and organics (Uwazie et al., 2020). This study therefore aims at assessing the phytoremediation capability of P. aquilinum uptake of heavy metals (Pb, Ni and Cd) from contaminated aqueous solution over a given period of time in varying concentrations, following a perceived phytoremediating capability of P. aquilinum in its natural habitat.
Appropriate quality control measures and precautions were put in place in order to obtain reliable data. Samples were carefully handled to avoid contamination, glassware apparatuses were properly cleaned and reagents and salts used throughout the analysis were of analytical grade. Stock solution and working standard solutions were prepared using standard procedures. Distilled water was used throughout the sample preparation and analysis. To prevent heavy metal contamination, all glass and non-glass ware apparatus used in this research work were washed with distilled water and immersed in 2% nitric acid for 24 h. Glass ware used throughout the analysis had no metal linings that could contaminate the samples.
Description of sampling area
This study was carried out in Niger Delta University New Site Campus within Amassoma, Bayelsa State, Nigeria. Here, P. aquilinum fern grows wildly around swampy environments with roots spreading all over the ground; it serves as a filtering medium for moving water bodies and also for adorning the environment with aesthetic beauty.
Amassoma is situated around Latitude 5.89° North of the Equator and Longitude 5.68o East of the Greenwich Meridian. The town is characterized by high temperature and heavy rainfall all year round. The town experiences tropical wet and dry climate with a lengthy wet and short dry season. The rainy season runs from March through October with a short spell of dryness in August, while the dry season is between the months of November and February.
Bracken fern (P. aquilinum) is a non-flowering vascular plant that possesses true root, stem and complex leaves and it reproduces by spores. It belongs to lower division Pteridophyta, having leaves usually unrolled from a tight fiddlehead (https://en.wikipedia.org/wiki/Fern). Pteridium aquilinum is a wild naturally growing plant in Niger Delta that has relatively low vegetative value. As fern grows in swampy areas with its roots spreading all over the covered ground, and serving as a filtering medium for moving water bodies, it is thought that the plant could also have some phytoremediation capabilities for the uptake of heavy metals from aqueous solutions. Hence, this research work was designed to investigate the potential of P. aquilinum fern for the phytoremediation uptake of heavy metals (such as Pb, Ni, and Cd) in aqueous solutions.
Sample collection and treatment
Plant samples collection and preparation was conducted on the 21st of April, 2017. A total of 144 P. aquilinum strands were collected. The plant strands were then packed and kept in a well labeled clean polyethylene bags and transported to the laboratory. The plants samples were placed under running tap water to remove any soil particles and finally rinsed twice with distilled water. The plant strands were then conditioned in distilled water for 24 h in order to eliminate contamination from the environment.
Standard solutions (5, 10 and 15) mg/L of Pb, Ni and Cd were prepared from Pb (NO3)2, NiCl2.6H2O, and CdCl2.H2O salts respectively and put into 36 different experimental pots. After 24 hour(h) of conditioning of the plant strands in distilled water, the plant strands were removed from the distilled water and then cultured into 36 different experimental pots containing the various concentrations (5 , 10 and 15) mg/L of Pb2+, Ni2+ and Cd2+ and control pots containing only distilled water (0.00 mg/L) respectively. The 0.00 mg/L solution (distilled water) in different pots was treated as the controls. Each set up contained three plant strands for triplicate determinations. The plant strands were harvested after a period of 24, 48, 72 and 96 h of exposure to the various metal solutions from each of the experimental and control pots respectively. The plant samples were then segmented using stainless steel knife into the leaves, stems and roots after each harvest and air dried for 7 days and then further oven dried at 70 oC until stable weights were obtained. The dried plant samples were then ground with ceramic mortar and pistil and then stored in well labeled plastic containers awaiting further laboratory analysis.
Plant digestion and heavy metals analysis
One gram (1.0 g) of the sieved plants samples (leave, stem and root) was weighed in triplicates and transferred into different 100 ml glass beakers. It was digested with 25 ml of 3:1 mixture of aqua regia (HNO3:HCl) at 100°C using corning PC- 351 model hot plate in a fume cupboard until a clear solution was obtained. The digested samples were left to cool for 5 min and then filtered using Whatman no.42 paper into a 100ml volumetric flask. The solution was made up to the mark with distilled water. The filtrate was transferred into a well labeled dry plastic containers awaiting heavy metal content analysis. The extractions were done in triplicates. The concentrations of the heavy metals in the digested plant parts were determined using Buck Scientific VGP 210 Atomic Absorption Spectrophotometer. The metals analyzed in the plant parts were Pb, Ni and Cd.
Calculation of heavy metals accumulation in plant
The actual accumulations of heavy metals (Pb, Ni and Cd) in plant parts were calculated from the various mean concentrations of these metals in P. aquilinum plant parts. They were estimated and determined using Equation 1 below:
AA = Cplant part from aqueous solution – Cplant parts from control (1)
Where: AA = Actual accumulation of heavy metal in the plant part Cplant part from aqueous solution = Concentration of heavy metals in plant parts obtained from various aqueous solutions of heavy metals.
Cplant parts from control = Concentration of heavy metals in plant parts obtained from the controls.
The actual total accumulation was calculated with Equation 2:
Total AA = AA in leaves + AA in stem + AA in root (2)
Where: Total AA = Total Actual accumulation of heavy metal in the plant
AA in leaves = Actual accumulation in the leaves
AA in stem = Actual accumulation in the stem
AA in root = Actual accumulation in the root
Translocation factor (TF)
In order to evaluate the potential of plants for phytoremediation, the Translocation Factor (TF) was determined. This is the ability of the plant to translocate metals from the roots to the aerial parts of the plant (Marchiol et al., 2004). Metals that are accumulated by plants and largely stored in the roots of plants are indicated by TF values <1. While the TF values >1 indicates that more of the heavy metals are being stored in the stems and the leaves (shoot) above the ground level than in the root.
In this study, Translocation Factors were calculated to estimate the transfer of heavy metals (Pb, Ni and Cd) from the roots to shoot (leaf + stem) and the Translocation Factor values of these metals were determined using Equation 3:
Where: CSHOOT = concentration of heavy metal in shoot
CROOT = concentration of heavy metal in Root
Data analysis
Microsoft Excel was used to determine the mean concentrations and standard deviations. Student t-test was also carried out to compare the mean concentrations of heavy metal in the P. aquilinum plant (leaves, stems, and roots) harvested from the various concentrations of the aqueous solution.
The results obtained are as stated and discussed in the subsections that follow.
Lead content in Pteridium aquilinum plant parts
The actual accumulations of Pb uptake in plant parts recorded during the experimental period from the different concentrations of the aqueous solutions are presented in Table 1. These values were calculated from the mean concentrations of Pb accumulation in P. aquilinum plant parts. Comparison of means was also carried out using the student’s t-test to check for statistical differences amongst the mean concentrations of Pb determined in the plant parts. The comparison was set at 95% confidence limits.
Pb accumulation in plant parts in the 5mg/L solution after 24 h of exposure showed no significant difference between the leaf and stem; leaf and root; and the stem and root at 95% confidence limits. In the 10 mg/L after 24 h of exposure, leaf and stem had no significant difference, while leaf and root; and the stem and root had significant differences. At 15 mg/L after 24 h of exposure, there was a significant difference between the various plant parts. This shows that as the concentration of the Pb solution increased, more accumulation took place in the plant parts following the trend root>stem>leaves. A similar trend was observed by Mkumbo et al. (2012). The difference in the levels of accumulation can be described as being proportional to the density of the tissues of the various plant parts. The roots were denser followed by the stem and then the leaves.
The findings indicate that P. aquilinum has the potential for phytoremediation of Pb, since the concentration of Pb in the shoot is higher than that in the roots, TF > 1 (Cheraghi et al., 2011). The trends of Pb uptake by P. aquilinum from Figure 1 show that the uptake kept increasing even at 96 h. It is a strong indication that the uptake would have still increased beyond 96 h. The trend is similar to the report made by Maha (2012) in its study of two aquatic macrophytes, Ceratophyllum demersum and Lemna gibba, in removing two toxic metals (Pb and Cr). The plants were grown at four different concentrations in single metal and separately harvested after day 2, 4, 6, 9 and 12 under laboratory experiment, achieving 95% reduction of Pb and 85% reduction of Cr during 12 days’ incubation period. However, the removal continued throughout the 12 days and up to the highest value on the twelfth day of the experiment.

Being limited by the period of exposure, which was 96 h in this study, it was difficult to ascertain if P. aquilinum is an hyperaccumulator of Pb as stated by Tangahu et al. (2011).
The results recorded for Pb in the P. aquilinum plant parts after 24, 48, 72, and 96 h of exposure to the various aqueous solutions (5, 10 and 15 mg/L) show that there was a steady increase in the level of Pb concentration in the plant parts, hence as the duration of exposure of the plant to the aqueous solutions increases, so also was the concentration of Pb in the plant parts. The results further revealed that as the concentration of the aqueous solutions increases, the more the uptake of Pb in the plant parts and the concentration of Pb accumulation in the plant from the various aqueous solutions recorded after 24, 48, 72 and 96 h of exposure followed the order of root > stem > leaf. This result agreed with the report by Subhashini and Swamy (2013), in their study of phytoremediation of Pb and Ni contaminated soils using Catharanthus reseus (L). They achieved a total accumulation of 0.92, 8.80 and 67.34mg/kg of Pb in the leaf, stem and root respectively throughout the experimental period.
Addition of amendments as nutrients also would have enhanced the plants to live longer due to available nutrients and the period of study would have been longer as studied by Placek et al. (2016), that worked on improving the phytoremediation of heavy metals contaminated soil by use of sewage sludge as a source of nutrients; thus the remediation of the P. aquilinum would have been better. Shrestha et al. (2019) also studied the enhancement of the phytoremediation capability of switchgrass by applying different compost and coir fibre amendments. They recorded significant improvements.
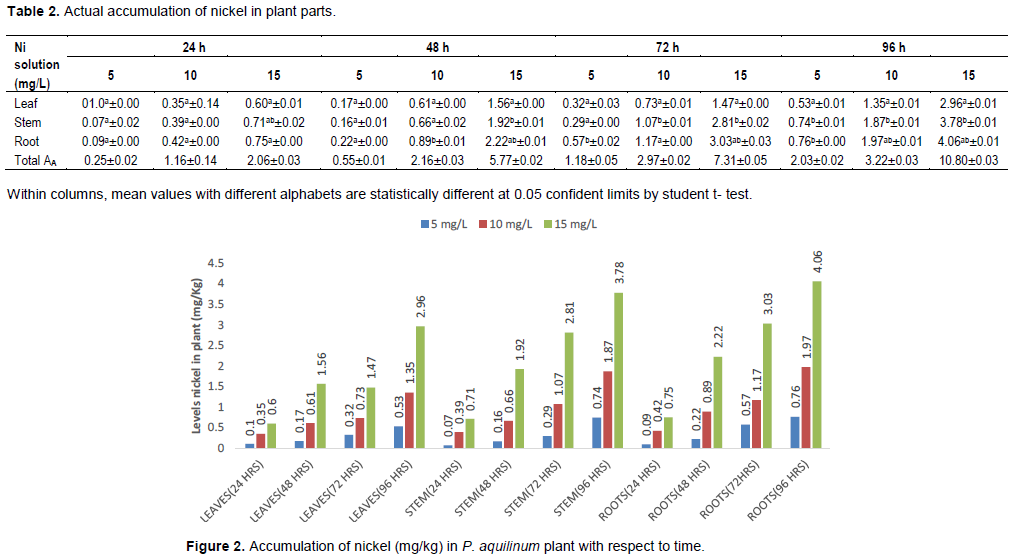
Nickel content in P. aquilinum plant
The accumulations of Ni in plant parts recorded during the experimental period from the different concentrations of the aqueous solutions were calculated from the mean concentrations of Ni accumulation in P. aquilinum plant parts. The mean concentrations of Ni in the plant parts are as presented in Table 2. The levels of Ni in the plant parts increased with increase in the concentration of Ni solution in which the plant is cultured as in the case of Pb. That is the amount accumulated in the plant followed the trend 15 mg/L > 10 mg/L >5 mg/L for the three solutions of different concentrations that the plant was cultured.
Comparison of the means using the student’s t-test at 95% confidence limit showed that there was no significant difference for the lower concentration (5 mg/L) of the Ni solution at 24 and 48 h’ exposures but as the period of exposure increased (72 and 96 h) there was a glaring difference in the amounts of Ni in the various plant parts which were significantly different with roots having higher concentrations. At 10 and 15 mg/L after 96 h of exposure, there were significant differences between the various plants parts at 95% confidence limit respectively. Similar trend as shown in Figure 2 was observed in the work reported by Mojiri et al. (2013), where they worked on the ability of Southern carttai (Typha domigenesis) plant for uptake of Pb, Ni and Cd in contaminated urban leachate within the period of 24, 48 and 92 h and achieved accumulation of 0.9725 mg/kg Pb, 0.468 mg/kg Ni and 0.392 gm/kg Cd, respectively, after the experimental period.
The results recorded for Ni in the P. aquilinum plant parts after 24, 48, 72, and 96 h of exposure to the various aqueous solutions (5, 10, and 15 mg/L) revealed that there was a steady increase in the level of Ni concentration in the plant parts, hence as the duration of exposure of the plant to the aqueous solutions increases, so also was the concentration of Ni in the plant parts. The results also show that as the concentration of the aqueous solution increases, the higher the uptake of Ni in the plant parts.
The level of Ni accumulation in the plant followed the order of root > stem > leaf, which shows similar trends with the work reported by Mojiri et al. (2013).
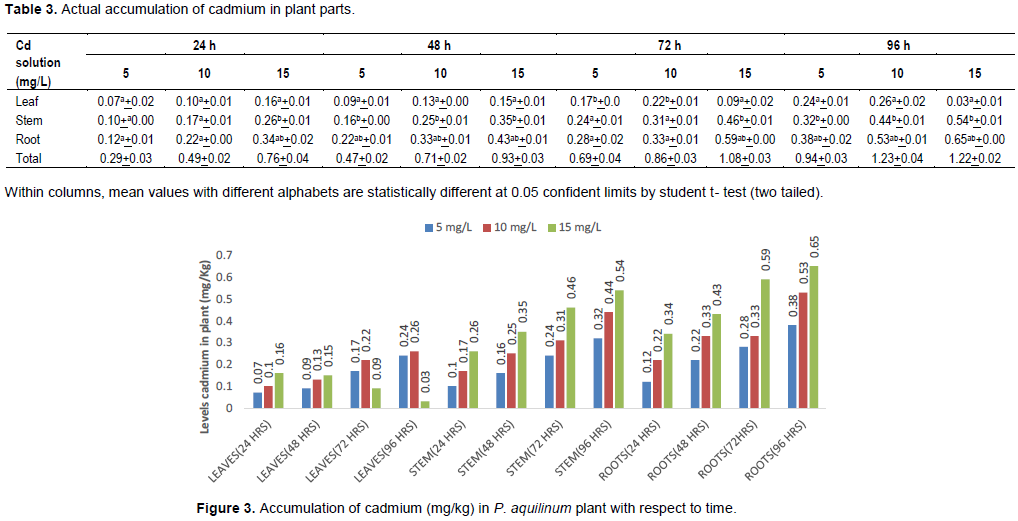
Cadmium content in P. aquilinum plant
The results recorded in Table 3 show the accumulation of Cd in the leaf, stem and root of the plant during the experimental period. There was significant difference between leaves and stem; leaves and roots; and the roots and the stem except at 5 and 10 mg/L and 24 h exposure where there was no significant difference between the roots and the stem. This indicates that there is good migration of Cd from the roots to the stems and to the leaves at low concentration and short period of time.
At the lower concentration (5 mg/L) and shorter period of exposure (24 h), there does not seem to be difference in the levels of Cd in the various plant parts (Table 3) but as the concentrations of the culture medium increase and the duration of exposure increases, there tends to be a difference between the plant parts with the trend roots>stem>leaves. This indicates that there is poor migration from the roots to the stem and also from the stem to the leaves. The trend is similar to the result reported by Sabeen et al. (2013), in their study of the potential uptake of Arundo donax L for the phytoextraction of Cd from contaminated soil and water after 21 days of exposure. However, the results obtained from Cd accumulation in plant parts also established that the higher the concentration of the aqueous solution, the higher the uptake of Cd in the stem and root of P. aquilinum plant. The level of Cd accumulation in plant parts followed the order of root>stem>leaf (Figure 3). The result also agreed with the report made by Sabeen et al. (2013) in their study of Cd phytoremediation by Arundo donax L. from contaminated soil and water.
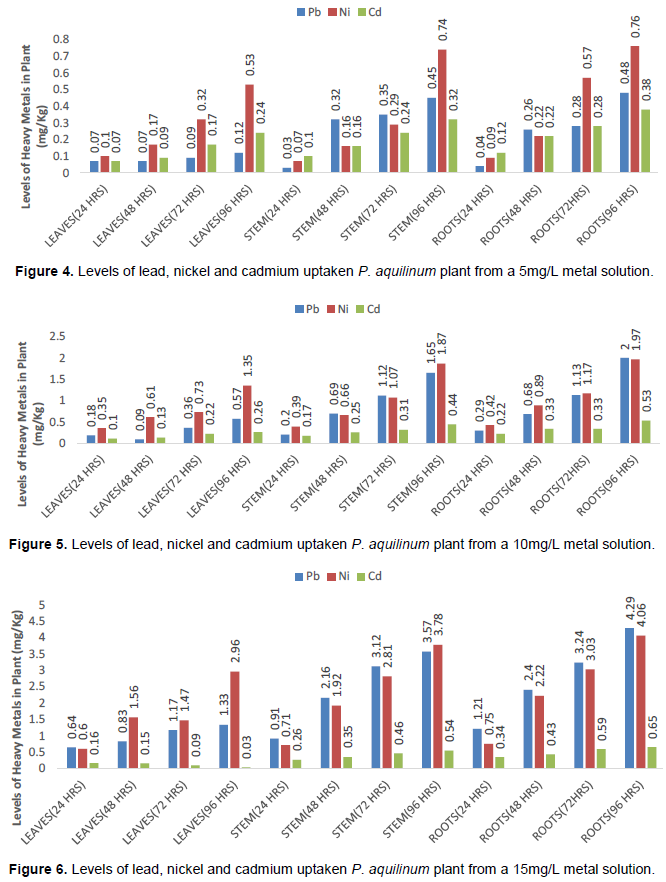
Levels of accumulation of lead, nickel and cadmium by P. Aquilinum
The overall results show that P. aquilinum plant accumulates more of the Ni than Pb and then Cd, that is Ni > Pb > Cd. The highest amounts of Ni, Pb and Cd taken-up by the P. aquilinum plant were (10.80 ± 0.03, 9.19 ± 0.14 and 1.23 ± 0.04) mg/kg respectively. Aside other factors, the uptake of heavy metals by plants is influenced by the relevance of the heavy metal in the plant’s metabolism; thus plants do not accumulate metals beyond their metabolic needs, which usually is in the range of 10 to 15 ppm (Tangahu et al., 2011), except the plants that are hyperaccumulators. Ni is an essential element in plant’s metabolism while Pb and Cd are toxic to plants (Fernandes and Henriques, 1991; Sarma et al., 2009). This could possibly explain the trend, Ni > Pb > Cd in the heavy metal uptake by P. aquilinum. Figures 4 to 6 are pictorial presentations of the trends of the levels of uptake of Pb, Ni and Cd by P. aquilinum. It is observed that as the duration of exposure increases, Pb competed favourably with Ni and even exceeded Ni especially at higher concentrations of the culture medium (10 and 15 mg/L). This was observed mostly in the roots and stems. It is therefore an indication that there is effective translocation of Pb and Ni as the duration of exposure and concentration of the culture medium increases.
Translocation factors of Lead, Nickel and Cadmium in P. aquilinum plant
The translocation factors (TFs) for Pb, Ni and Cd in the plant parts are presented in Table 4. The translocation factors recorded during the experimental period were all greater than unity (>1) except for Cd at exposure duration of 96 h in the 15 mg/L Cd solution. Figures 7 to 9 are Surface Contour Maps illustrating the TFs for Pb, Ni and Cd showing the concentrations and durations that favour the translocation of the heavy metals from the roots to the shoots. The results show that there is relatively higher TFs at a solution concentration of 5 mg/L.
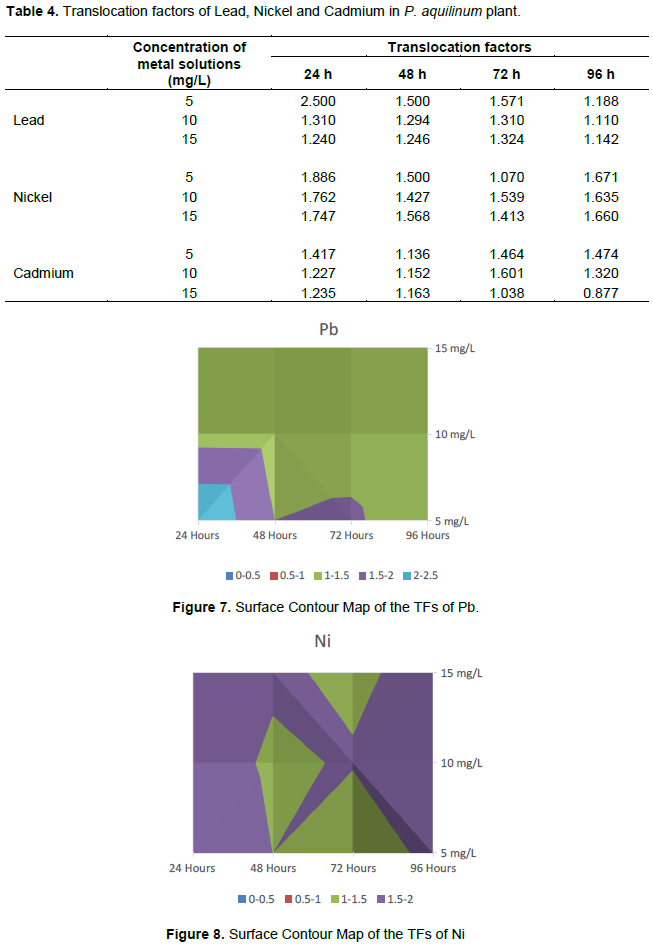

The highest TF value recorded for Pb in the plant was observed after 24 h of exposure at 5 mg/L of the aqueous solution with a TF value of 2.500, while 0.877 recorded for Cd as the least TF value was observed at 15 mg/L of the aqueous solution after 96 h of exposure. The results were similar to those reported by Mojiri et al. (2013) in their study of phytoremediation of heavy metals from urban waste leachate by southern cattail (Typha domingenesis) after 24, 48 and 72 h of exposure to the waste leachate. From the contour maps of Pb TFs (Figure 7) it is obvious that at lower concentrations and lower exposure times the migration rate of Pb from the roots to the shoot was high. As the exposure time increases the rates starts dropping thereby resulting to lower TFs. The same applies to concentration of the culture medium.
The trend in the translocation factors of Ni in P. aquilinum plant parts are as presented in Figure 8. The results show that the translocation factor recorded for Ni in the plant parts during the experimental period were all greater than 1 (>1) and fell within the range 1.143 to 1.886. Even at 24 h’ duration, the migration from the roots to the shoot was almost complete, indicating a higher rate of Ni translocation as compared to Pd. That is why no much difference is observed between the lower and higher duration of exposure. The same applies for the concentrations of the culture media. There is high correlation of observations from this study with finding from studies carried out by Mojiri et al. (2013) and Subhashini and Swamy (2013).
The TF values for Cd recorded in the plant parts during the experimental period from the various concentrations of the culture medium were as presented in Figure 9. The results show that the translocation factors of Cd in the plant parts were all greater than 1 (>1) except after 96 h at 15 mg/L, where the TF value was less than 1 (0.877). The highest TF values were recorded after 72 h at 10 mg/L with a TF value of 1.601, while the lowest TF value was recorded at 15 mg/L after 96 h of exposure with a TF value of 0.877.
The high TF values were recorded within longer periods of duration and lower concentrations of the culture medium. Similar observations were recorded by Mojiri et al. (2013) in their study of phytoremediation of heavy metals from urban waste leachate by southern cattail (Typha domingenesis) after 24, 48 and 72 h of exposure to the waste leachate. Furthermore, the TF expresses the capacity of the plant to store the heavy metals in its upper part, hence the translocation factor (TF) results recorded for Pb, Ni and Cd from the various concentration of the aqueous solutions after 24, 48, 72 and 96 h of exposure were all greater than 1 (>1); TF value greater than 1 indicates the translocation of the metals from root to above ground parts (Jamil et al., 2009) showing that more of the heavy metals are stored in the shoot. The translocation factor (TF) patterns were in the order Ni > Pb > Cd.
This study shows that the level of heavy metals uptake by the P. aquilinum plant increases with increase in the concentration of the aqueous solutions. It was further established that the longer the duration of exposure by the plant in the aqueous solutions, the higher the level of heavy metals uptake by the plant. The levels of heavy metals accumulation in the plant parts were in the order; root > stem > leaves. The findings reveal that the plant accumulates more of Ni, followed by Pb and lastly Cd. The translocation factors (TF) in the shoot of the plant were > 1 indicating that more of the heavy metals were stored in the shoot. In conclusion, P. aquilinum can be described as an effective accumulator plant which could be used for phytoextraction of Pb, Ni and Cd from aqueous solutions.
The authors have not declared any conflict of interests.
REFERENCES
|
Ahmadpour P, Ahmadpour F, Mahmud TMM, Abdu A, Soleiman M, Tayefeh FH (2012). Phytoremediation of Heavy metals: A Green Technology. African Journal of Biotechnology 11:14036-14043.
|
|
|
|
Cheraghi M, Lorestani B, Khorasani N, Yousefi N, Karami M (2011). Findings on the Phytoextraction and Phytostabilization of Soils Contaminated with Heavy Metals. Biological Trace Element Research 144:1133-1141.
Crossref
|
|
|
|
|
Cluis C (2004). Junk - Greedy Greens; phytoremediation as a new option for soil decontamination. Biotech Journal 2: 61-62.
|
|
|
|
|
Cunningham SO, Ow DW (1996). Promise and prospects of root zone of crops, phytoremediation. Plant Physiology 110:715-719.
Crossref
|
|
|
|
|
Danh LT, Truong P, Mammucari R, Tran T, Foster N (2009). Vetiver Grass, Vetiveria zizanoides. A choice plant for phytoremediation of heavy metals and organic wastes. International Journal of Phytoremediation 1:664-691.
Crossref
|
|
|
|
|
Fernandes JC, Henriques FS (1991). Biochemical, physiological and structural effects of excess copper in plants. Botanical Review 57:246-273.
Crossref
|
|
|
|
|
Flathman PE, Lanza GR (1996). Phytoremediation current views on an emerging green technology. Journal of Soil Contamination 7(4):415-432.
Crossref
|
|
|
|
|
Hardiman RF, Jacoby B, Banin A (2005). Factors affecting the distribution of copper, cadmium and Pb and their influence upon yield and Zn content in bush beans (Phaseolus vulgaris). Plant and Soil 81:17-27.
Crossref
|
|
|
|
|
Ibrahim N, El Afandi G (2020). Phytoremediation uptake model of heavy metals (Pb, Cd and Zn) in soil using Nerium oleander. Heliyon 6(7):e04445.
Crossref
|
|
|
|
|
Jadia CD, Fulekar MH (2009). Phytoremediation of heavy metal: recent techniques. Africa Journal of Biotechnology 8(6):921-928.
|
|
|
|
|
Jamil S, Abhilash PC, Singh N, Sharma PN (2009). Jatrophacurcas: A potential crop for phytoremediation of local fly ash. Journal of Hazardous Materials 172:269-275.
Crossref
|
|
|
|
|
Kummling KE, Gray DJ, Power JP, Woodland SE (2001). Gas phase chemical reduction of hexachlorocyclohexane and other chlorinated compounds, waste treatment experience and applications. 6th International HCH and Pesticides Forum.
|
|
|
|
|
Li C, Ji X, Luo X (2019). Phytoremediation of Heavy Metal Pollution: A Bibliometric and Scientometric Analysis from 1989 to 2018. International Journal of Environmental Research and Public Health 16(23):4755.
Crossref
|
|
|
|
|
Ma LQ, Komar KM, Tu C, Zhang W, Cai X, Kennelley ED (2001). A fern that hyperaccumulate arsenic. Nature 409:579.
Crossref
|
|
|
|
|
Maha AMA (2012). Phytoremediation of heavy metals from aqueous solutions by two aquatic Macrophytes, Ceratophyllum demersum and Lemna gibba L. Environmental Technology 33(14):1609-1614.
Crossref
|
|
|
|
|
Marchiol L, Assolari S, Sacco P, Zerbi G (2004). Phytoextraction of heavy metals by Canola (Brassica napus) and radish (Raphanus sativus) grown in multi-contaminated soil. Environmental Pollution 132:21-27.
Crossref
|
|
|
|
|
Mkumbo S, Mwegoha W, Renman G (2012). Assessment of the phytoremediation potential for Pb, Zn and Cu of indigenous plants growing in a gold mining area in Tanzania. International Journal of Environmental Sciences 2(4):2425-2434.
|
|
|
|
|
Mojiri A, Aziz HA, Zahed MA, Aziz SO, Razip M, Selamat B (2013). Phytoremediation of Heavy Metals from Urban Waste Leachate by Southern Cattail (Typha domingensis). International Journal of Scientific Research in Environmental Sciences 1(4):63-70.
Crossref
|
|
|
|
|
Olaifa FE, Ajagbe AO (2017). Accumulation of Zinc by Pteridium aquilinum (bracken fern) with different plant stimulants and bioassay with Clarias gariepinus. Journal of Environmental Research and Management 8(2): 011-017.
Crossref
|
|
|
|
|
Olaifa FE, Omekam AJ (2014). Studies on Phytoremediation of Copper Using Pteridium aquilinum (Bracken Fern) In the Presence of Biostimulants and Bioassay Using Clarias gariepinus Juveniles. International Journal of Phytoremediation 16:219-234.
Crossref
|
|
|
|
|
Oti WJO, Nwabue FI (2013). Heavy metals effects due to contamination of vegetable from Enyigba lead mine in Ebonyi state, Nigeria. Environment and Pollution 1(2):19-26.
Crossref
|
|
|
|
|
Placek A, Grobelak A, Kacprzak M (2016). Improving the phytoremediation of heavy metals contaminated soil by use of sewage sludge. International Journal of Phytoremediation 18(6):605-618.
Crossref
|
|
|
|
|
Sabeen M, Mahmood Q, Irshad M, Fareed I, Khan A, Ullah F, Hussain J, Hayat Y, Tabassum S (2013). Cadmuim Phytoremediation by Arundo donax L. from contaminated soil and water. Hindawi Biomed Research International 2013:9.
Crossref
|
|
|
|
|
Saha P, Shinde O, Sarkar S (2017). Phytoremediation of industrial mines wastewater using water hyacinth, International Journal of Phytoremediation 19(1):87-96.
Crossref
|
|
|
|
|
Sarma H, Sarma A, Sarma CM (2009). Physiological studies of some weeds grown under heavy metal and industrial effluent discharge zone of fertilizer factory. Journal of Ecology and the Natural Environment 1(7):173-177.
|
|
|
|
|
Schnoor JL (1997)."Phytoremediation'' University of Lowa, Department of Civil and Engineering 1:62.
|
|
|
|
|
Shrestha P, Bellitürk K, Görres JH (2019). Phytoremediation of Heavy Metal-Contaminated Soil by Switchgrass: A Comparative Study Utilizing Different Composts and Coir Fiber on Pollution Remediation, Plant Productivity, and Nutrient Leaching. International Journal of Environmental Research and Public Health 16(7):1261.
Crossref
|
|
|
|
|
Shukla KP, Singh NK, Sharma (2010). Bioremediation: Developments, Current Practices and Perspectives. Genetic Engineering and Biotechnology Journal 3:1-20.
|
|
|
|
|
Subhashini V, Swamy AVV (2013). Phytoremediation of Pb and Ni contaminated soils using Catharanthus roseus (L). Universal Journal of Environmental Research and Technology 3:465-472.
|
|
|
|
|
Szczygłowska M, Pukarsha A, Konieczka P, Namiesnik J (2011). Use of brassica plants in the phytoremediation and biofumigation processes. International Journal of Molecular Sciences 12:7760-7771.
Crossref
|
|
|
|
|
Tangahu BV, Abdullah SRS, Basri H, Idris M, Anuar N, Mukhlisin M (2011). A Review on HeavyMetals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Hindawi International Journal of Chemical Engineering pp. 1-31.
Crossref
|
|
|
|
|
Uwazie MC, Obijiaku JC, Onukwuli OD, Babayemi AK, Umeuzuegbu JC (2020). Remediation ability of melon grass in a crude oil polluted soil in a tropical region. International Journal of Engineering Technologies and Management Research 7(6):89-101.
Crossref
|
|
|
|
|
Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S, Chen Z (2020). Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Frontiers in Plant Science 11:359.
Crossref
|
|
|
|
|
Yoon J, Cao X, Zhou Q, Ma LQ (2006). Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Science of the Total Environment 368(2-3):456-464.
Crossref
|
|