ABSTRACT
During the present investigation of Wular lake in terms of species composition and biomass of annelids, 10 taxa were recorded which belonged to two major classes namely Oligochaeta (7) and Hirudineae (3). The class Oligochaeta included Limnodrilus hoffmeisteri, Tubifex tubifex, Branchiura sowerbyii, Nais sp., Aelosoma sp., Pristina sp. and an unidentified taxion. Similarly, the class Hirudinaea was comprised by Erpobdella sp., Placobdella sp. and Glossiphonia sp. The seasonal mean value for biomass of annelids fluctuated between 0.31 g/m2 at site I in winter to 14.92 g/m2 at site III in summer. The annual mean biomass was highest at site IV (10.82±2.02 g/m2), followed by site III (10.47±2.07 g/m2), site II (8.94±1.90 g/m2), site I (1.85±0.94 g/m2) and site V (1.71±0.50 g/m2).
Key words: Biomass, species composition, annelid, Lake and Ramsar site.
In an aquatic ecosystem the life of aquatic biota is closely dependent on the physical, chemical and biological charac-teristics of water that directly acts as a controlling factor (Yaqoob and Pandit, 2009). Macrozoobenthos is an impor-tant constituent of aquatic ecosystem and has functional importance in assessing the trophic status. Thus as the abundance of benthic fauna mainly depends on physical and chemical properties of the substratum, the benthic communities are known to respond to changes in the quality of water or habitat. The benthic macroinvertebrates are associated with bottom or any solid liquid interface, which includes a heterogeneous assemblage of organisms belonging to various phyla like Arthropoda, Annelida, Mollusca and others. The benthos occupies an important position in the lake ecosystem, serving as a link between primary producers, decomposers and higher trophic levels (Pandit, 1980). They also play an important role in the detrital food web which in turn affects the cycling of minerals (Gardner et al., 1981). Macroinvertebrates are used as indicators of pollution as their communities change in response to changes in physicochemical factors and available habitats (Sharma and Chowdhary, 2011). According to Jumppanen (1976) the first signs of eutrophication and pollution in a lake are reflected in the benthic flora and fauna as the suspended waste imme-diately sink to the bottom to decompose and thus cause a change in the benthic composition and abundance. The lakes and wetlands having soft bottom sediments are characterized by annelids either as the dominant or one of the most abundant group. Of the fresh water annelids, the oligochaetes display the greatest diversity and have the greatest indicator value. Oligochaetes worms are diverse and occur in a wide spectrum of freshwaters from unproductive to extremely eutrophic lakes and rivers. Leeches are found in warm water of shallow standing sites (Peckarsky et al., 1990) and are generally pollution tolerant. Oligochaeta, especially the Tubificidae family, have been universally applied on bioassessment assays, as bioindicators to reflect the organic pollution (Lin and Yo, 2008). This is because their capacity to increase in number with increasing organic matter, replacing other benthic macroinvertebrates, less tolerant for this condition (Schenkova and Helesic, 2006). It is in the backdrop of paucity of researches on Wular Lake, the largest freshwater body of Indian subcontinent, being recognised as a Ramsar Site, that the present study on community structure and biomass of annelids has been undertaken during 2012.
Study area and study sites
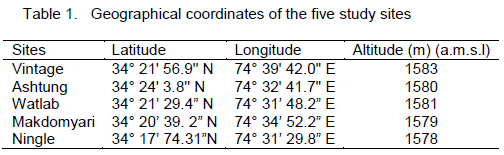
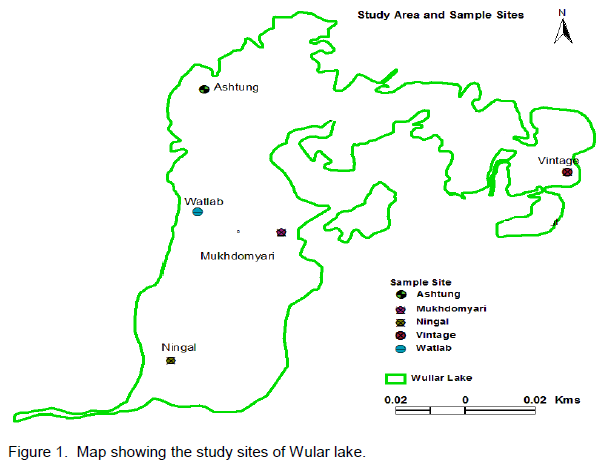
Wular lake is the largest freshwater lake of Indian subcontinent, located in the flood plains of Jhelum river with an open water area of 24 km2 (Pandit, 2002). The rural valley lake in the north-west of Kashmir extends from Bandipore to Sopore and is at a distance of about 54 km from Srinagar city and is situated at an altitude of 1,580 m (amsl), lying between 34°16´-34°20´N latitudes and 74°33´-74°44´E longitudes. The lake is mono-basined, elliptical in shape and is of fluviatile origin, formed by the meandering of Jhelum River. Its depth on an average is 3.6 m throughout length, reaching 5.8 m at its deepest point. The major inflows to Wular Lake are Jhelum, Madumati and Erin. The lake plays a significant role in the hydrographic system of Kashmir valley by acting as a large reservoir and by absorbing high annual flood of the Jhelum River. In 1990, this shallow lake was designated as a Ramsar Site, a Wetland of International Importance. For the present investigation, five sampling sites were selected which are described in Table 1 and Figure 1.
The benthic fauna encompassing annelids was collected from all five sites of the lake, with an Ekman Dredge (15 x 15 cm). The sites differered in water depth, vegetation and bottom sediments. The samples were taken in triplicate. The sediment samples collected were sieved carefully in order to remove fine sediments and other extraneous material without damaging the fragile organisms, using sieves of 1mm and 0.5 mm mesh size for checking annelids. The macroscopic organisms were collected with the help of forceps and brushes. The organisms collected were preserved in 70% alcohol for detailed examination.
Identification
Preserved annelids were identified by observing them under a microscope and identification was done with the help of standard taxanomical works of Edmondson (1992), APHA (1998), Pennak (1978), Adoni (1985), Brinkhurst (1971) and Kiemm (1995).
Biomass
Biomass of annelids was determined as fresh weight. The organisms were kept on filter paper for some time until the samples dry and weight became constant. Then organisms were weighed by using electronic balance with the accuracy of 1 mg. The biomass was calculated by using the formula:
Where, n = weight of organisms counted; a = extraction area of dredge; s = number of samples.
Species composition
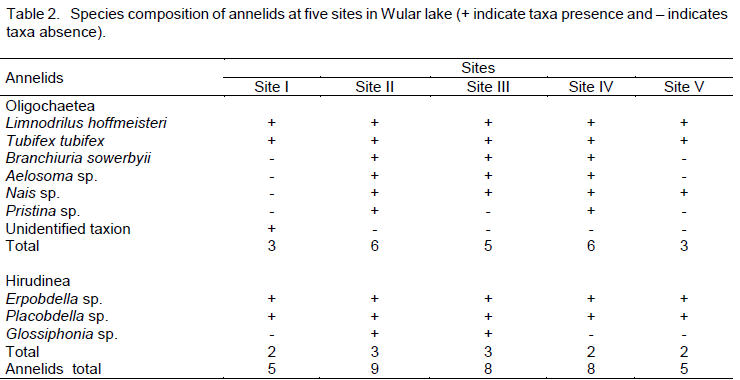
During the present investigation of Wular lake, 10 taxa of annelids were recorded which belonged to two major classes namely Oligochaeta (7) and Hirudinaea (3). The class Oligochaeta included Limnodrilus hoffmeisteri, Tubifex tubifex, Branchiura sowerbyii, Nais sp., Aelosoma sp., Pristina sp. and an unidentified taxion. Similarly, the class Hirudinea was comprised by Erpobdella sp., Placobdella sp. and Glossiphonia sp. Amongst the 10 species listed, the highest taxa richness was obtained at site II (9), followed by site III (8), site IV (8), and decreasing to the minimum of 3 species each at sites I and V (Table 2). The most common taxa encountered across all the sites were L. hoffmeisteri, T. tubifex, Erpobdella sp. and Placobdella sp. The unidentified annelid taxion was found only at site I. Among the two classes, Oligochaeta dominated both qualitatively and quantitatively at each site and was represented by a maximum number of 6 species each at sites II and IV, followed by site III (5), till it reached a minimum number of 3 species each at sites I and V. The class Hirudinea was represented by 3 taxa each at sites II and III and 2 taxa each at sites I, IV and V.
Collection and preservation
Biomass
The annelid biomass showed a considerable variation between the study sites. Depending upon the species composition biomass of annelids varied seasonally. The seasonal mean value for biomass of annelids fluctuated between a lowest of 0.31 g/m2 at site I in winter to a highest of 14.92 g/m2 at site III in summer. However, the annual mean biomass was revealed to be maximum at site IV (10.82±2.02 g/m2), followed by site III (10.47±2.07 g/m2), site II (8.94±1.90 g/m2), site I (1.85±0.94 g/m2) and decreasing to the lowest ebb (1.71±0.50 g/m2) at site V.
At site I, the annual mean biomass of Hirudineae was highest (1.18±0.61 g/m2) as compared to class Oligochaeta (0.67±0.33 g/m2). Among Oligochaetes, the L. hoffmeisteri acquired maximum mean biomass (0.41±0.15 g/m2) while the unidentified taxion made a minimum mean biomass (0.09±0.06 g/m2). Among Hirudineae, only Placobdella sp. maintained the greatest biomass (0.71±0.48 g/m2). On a seasonal basis, the oligochaetes registered the maximum biomass during summer (1.26 g/m2) and the minimum in winter (0.07 g/m2) whereas the Hirudineae registered the highest biomass in spring (2.90 g/m2) and the lowest in winter (0.24 g/m2) (Table 3).
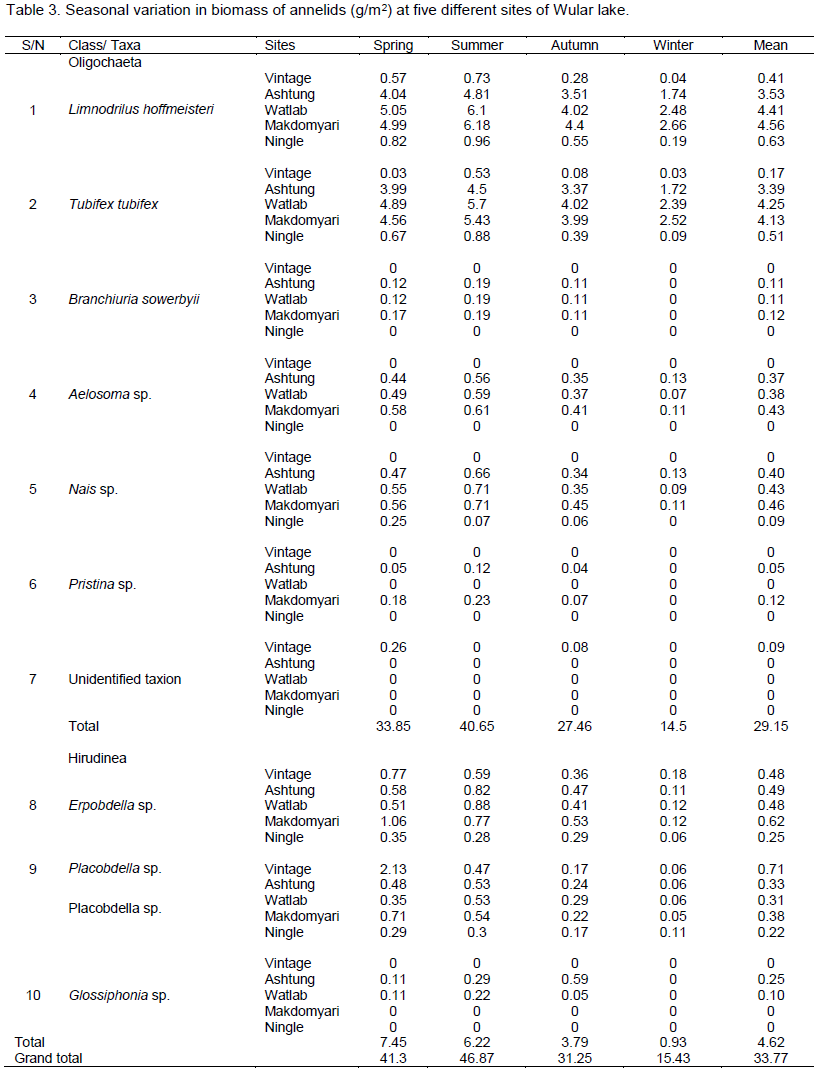
At site II, the annual mean biomass of Oligochaeta was highest (7.86±1.51 g/m2) as compared to class Hirudinea (1.08±0.39 g/m2). Further, among Oligochaeta, L. hoffmeisteri attained the maximum mean biomass (3.53± 0.65 g/m2) whereas Pristina sp. had the minimum mean biomass (0.05±0.02 g/m2). On a seasonal basis, the annelids showed maximum biomass in summer (12.48 g/m2) and lowest in winter (3.89 g/m2). Erpobdella sp. had the highest mean biomass (0.50±0.15 g/m2) whereas Glossophonia sp. had the lowest mean biomass (0.25± 0.13g/m2) in class Hirudinae (Table 3).
At site III, the annual mean biomass of annelids varied between 9.58±1.76 g/m2 (Oligochaeta) and 0.89±0.31 g/m2 (Hirudinea). L. hoffmeisteri obtained maximum mean biomass (4.41± 0.77g/m2) and B. soewerbyii registered the minimum mean biomass (0.11±0.04 g/m2). Erpobdella sp. obtained the highest mean biomass (0.48±0.16 g/m2) and Glossophonia sp. the lowest mean biomass (0.10± 0.05g/m2). On a seasonal basis, the annelids showed a maximum biomass during summer (14.92 g/m2) and the lowest in winter (5.21 g/m2) as depicted in Table 3.
At site IV, the mean biomass of L. hoffmeisteri was maximum (4.56±0.73 g/m2), followed by T. tubifex (4.13±0.61 g/m2) while B. soewerbyii and Pristina sp. registered lower mean biomass (0.12±0.05 g/m2 for each). Erpobdella sp. recorded its greatest biomass (0.62±0.20 g/m2) while Placobdella sp. had its lowest biomass (0.38± 0.15g/m2). The annual mean biomass of annelids varied between 9.82±1.67 g/m2 for Oligochaeta and 1.0±0.35 g/m2 for Hirudinea. In general, on a seasonal basis, the annelids showed maximum biomass (14.66 g/m2) in summer and minimum (5.57 g/m2) in winter (Table 3).
At site V, the annelids registered their maximum biomass (2.49 g/m2) in summer and their minimum (0.45 g/m2) in winter. The biomass of Oligochaeta was highest (1.24±0.39 g/m2) than the biomass of Hirudinea (0.47±0.11 g/m2). Among Oligochaetes, the L. hoffmeisteri attained greatest mean biomass (0.63±0.17 g/m2) while the lowest mean biomass (0.10±0.05 g/m2) was found for Nais sp. Erpobdella sp. was the dominant maintained leeches (0.25±0.06 g/m2) and Placobdella sp. was the less abundant one (0.22± 0.05 g/m2) (Table 3).
Assessment of species composition, distribution and biomass of macroinvertebrate community often gives an important clue to the functional status of a water body. Benthic macroinvertebrates are tools that enable rapid bioassessment which is cost effective and quick assess-ment strategy to determine the health of an ecosystem (Loeb and Spacie, 1994). According to Jumppanen (1996) the first sign of eutrophication and pollution is reflected by the benthic biota, because the pollutants are rapidly deposited into the sediments where they evolve an impact on the benthic organisms. Therefore, it is essential to study the benthic composition to help in the evaluation of the trophic status of the water bodies.
During the present study, 10 taxa of annelids belonging to two major classes namely Oligochaeta (7) and Hirudinea (3) were recorded. Amongst the 10 species listed, the maximum number of species was obtained at site II (9), followed by site III (8), site IV (8), and decreasing to the minimum of 3 species each at sites I and site V. The lowest species richness at site V may be due to the physical heterogeneity of substrate, being reflected in the number of taxa (Marshall and Winterbourn,1979) as site V is chracterized by sandy sediment with small boulders and diminished macrophytic growth. Conversely the highest richness at sites II, III and IV may be due to the dense macrophytic cover and soft bottom sediments as macrophytes provide suitable habitat for macroinvertebrates. The above finding corroborates the fact that oligochaete community thrives well in soft depositing substrates rather than stony beds (Bhat and Pandit, 2010).
Biomass is a potential renewable source of energy, which is related to the ecological conditions of the habitat. Estimation of biomass is necessary for understanding the trophic dynamics and productivity of an ecosystem (Mir and Yousuf, 2003). During the course of the present study, biomass of annelids fluctuated between 0.31 g/m2 at site I and 14.92 g/m2 at site III. This reflected the higher productivity and trophic status of site III and inversely, the relatively lower productivity at site I. Biomass showed a seasonal trend with maximum in spring and summer and minimum in winter. Thus, biomass fluctuated in close rela-tion with density of annelids, which also showed higher value during summer and lower in winter, indicating a positive correlation between the two parameters. Sunder and Subla (1986) and Mortensen and Simonson (1983) also noticed a positive relationship between density and biomass of zoobenthos.
During the present investigation 10 taxa of annelids belonging to two major classes namely Oligochaeta (7) and Hirudinea (3) were recorded. The most common taxa encountered across all the sites included L. hoffmeisteri, T. tubifex, Erpobdella sp. and Placobdella sp. However, the unidentified annelid taxion was found only at site I. Among the two classes, Oligochaeta dominated both qualitatively and quantitatively at each site and was represented by a maximum number of taxa at each site. The presence of L. hoffmeisteri and T. tubifex on an average in great numbers is indicative of organically rich sediments. The class Hirudinea was represented by three taxa each at sites II and III and two taxa each at sites I, IV and V. In general, Oligochaeta comprised 95% of the total annelid community and remaining 5% was made by Hirudinea. During the course of the present study, bio-mass of annelids fluctuated from the lowest of 0.31g/m2 at site I to the highest of 14.92 g/m2 at site III. However, the biomass of oligochaetes fluctuated from 0.67 to 9.82 g/m2 and the biomass of Hirudinea varied from 0.47 to 1.18 g/m2, indicating that overall biomass was dominated by oligochaetes and hence reflecting the moderate eutrophication of lake.
The authors have not declared any conflict of interests.
REFERENCES
|
APHA (1998). Standard Methods for Examination of Water and Waste Water. 20th ed. American Public Health Association, New York. |
|
|
|
Adoni AD (1985). Work Book of Limnology, Pratiba Publication Sagar, (M.P), India. |
|
|
|
Bhat S, Pandit AK (2010). Ecological study of benthic communities in three limnocrene fresh water springs in Kashmir Himalaya. J. Natural Sciences and Mathematics, 3(2):89-96. |
|
|
|
Brinkhurst RO (1971). A Guide for the Identification of British Aquatic Oligochaeta. Freshwater Biological Association, Scientific Publication No.22. |
|
|
|
Edmondson WT (1992). Ward and Whiple Freshwater Biology. 2nd ed. Intern. Books and Periodicals Supply Service, New Delhi. |
|
|
|
Jumppanen K (1976). Effects of waste waters on a lake ecosystem. Ann. Zool. Fennici. 13:85-138. |
|
|
|
Kiemm DJ (1995). Identification Guide to the Freshwater Leeches (Annelida: Hirudinea) of Florida and Other Southern States. Bioassesment and Ecotoxicology branch, Ecological Monitoring Research Division, Environmental Monitoring System Laboratory, U.S. Environmental Protection Agency, Cincinnati, Ohio- 45268. Leob SL, Spacie A (1994). Biological Monitoring of Aquatic Systems. Lewis Publishers, Florida. 381p. |
|
|
Lin KJ, Yo SP (2008). The effect of organic pollution on the abundance and distribution of aquatic oligochaetes in an urban water basin, Taiwan. Hydrobiologia, 596(1):213-223.
Crossref |
|
|
Marshall JW, Winterbourn MJ (1979). An ecological study of a small New Zealand stream with particular reference to the Oligochaeta. Hydrobiologia, 65:199-208.
Crossref |
|
|
|
Mir MF, Yousuf AR (2003). Oligochaete community of Dal lake, Kashmir. Oriental Sci. 8:83-87. |
|
|
Mortensen E, Simonsen IJ (1983). Production estimates of the benthic invertebrate community in a small Danish stream. Hydrobiol. 102: 155-162.
Crossref |
|
|
|
Pandit AK (2002). Plankton as indicators of trophic status of wetlands. p. 341-360. In: Ecology and Ethology of Aquatic Biota. (Arvind Kumar, ed.). Daya Publishing House, New Delhi-110002. |
|
|
|
Pandit AK (1980). Biotic factor and food chain structure in some typical wetlands of Kashmir. Ph.D. thesis, University of Kashmir, Srinagar-190006, J&K, India. |
|
|
|
Peckarsky BL, Fraissinet PR, Penton MA, Conklin DJ (1990). Freshwater macroinvertebrates of northeastern North America. Cornell Univ. Press, U.S.A. pp.442. |
|
|
|
Pennak RW (1978). Freshwater Invertebrates of United States of America. Wiley Interscience Pub., New York. |
|
|
Schenkova J, Helesic J (2006). Habitat preferences of aquatic Oligochaeta (Annelida) in the Rokttna river, Czech Republic- a small highland stream. Hydrobiologia, 564(1):117-126.
Crossref |
|
|
|
Sharma KK, Chowdhary S (2011). Macroinvertebrate assemblages as biological indicators of pollution in a Central Himalayan River, Tawi (J&K). Int. J. Biodivers. Conserv. 3(5):167-174. |
|
|
|
Sunder S, Subla BA (1986). Macrobenthic fauna of a Himalayan river. Indian J. Ecol., 13(1):127-132. |
|
|
|
Yaqoob KU, Pandit AK (2009). Distribution and abundance of macrozoobenthos in Dal lake of Kashmir Valley. J. Res. Dev. 9:20-29. |