ABSTRACT
Ground water is the source of drinking water for many people around the world, especially in rural areas. A lot of parameters such as the concentrations of Arsenic, Copper, Zinc and other heavy metals in conjunction with other physico-chemical properties contribute to the suitability of water for drinking and for other purposes. This study was carried out in the Bibiani-Anhwiaso-Bekwai District of the Western region of Ghana to assess the presence and the levels of selected heavy metals (As, Cu, Zn) pollution of boreholes from different villages in the study areas using Inductively Coupled Plasma Mass Spectrometry (ICP-MS). The result of the study revealed that, the mean concentrations of As, Cu and Zn in the analyzed samples were 0.00143, 0.0186 and 0.0329 mg/L, respectively. The analysis of variance showed that there were significant differences in the Arsenic concentrations among the eight boreholes (F=4.078, P=0.033). However, the differences in the concentrations of Copper (F=1.592, P=0.264) and Zinc (F=0.741, P=0.647) from the eight boreholes were not significant. Concentrations of the selected heavy metals in analysed water samples were below the acceptable limits of World Health Organization (WHO) and Ghana Environmental Protection Agency (GEPA). The concentration of the selected heavy metals may be attributed to the activities of panners, improper disposal of sewage and solid materials containing toxic chemicals and the indiscriminate use of farming inputs such as fertilizers and pesticides which have impacted on the water quality of the selected boreholes in the study areas. Although the levels of the selected heavy metals in these water samples did not exceed WHO and GEPA permissible limits, it is necessary for residents in the study areas to be provided with potable water.
Key words: Ground water, heavy metals, Arsenic, Copper, Zinc.
Heavy metals are unique among pollutants that cause adverse health effects in that they occur naturally and, in many instances, are ubiquitous in the environment. Regardless of how metals are used in consumer products or industrial processes, some level of human exposure is, in most instances, inevitable. Furthermore, many are biologically essential but become toxic with increasing dosage. Metals are an important emerging class of human carcinogens. Several more metals and their compounds are suspected to have carcinogenic potential in humans (IARC, 1980). The toxic heavy metals entering the ecosystem may lead to geo-accumulation, bioaccumulation and biomagnifications. Heavy metals like Fe, Cu, Zn, Ni and other trace elements are important for proper functioning of biological systems and their deficiency or excess could lead to a number of disorders. Food chain contamination by heavy metals has become a burning issue in recent years because of their potential accumulation in bio-systems through contaminated water, soil and air. Therefore, a better understanding of heavy metal sources, their accumulation in the soil and the effect of their presence in water and soil on plant systems seem to be particularly important issues of present-day research on risk assessments (Lokeshwari and Chandrappa, 2006). Gold mining in recent times has become unpopular as it is regarded as a significant source of Hg, Pb and heavy metal contamination of the environment owing to activities such as mineral exploitation, ore transportation, smelting and refinery, disposal of the tailing and waste waters around mines (Essumang et al., 2007; Hanson et al., 2007; Obiri, 2007; Singh et al., 2007). In the Bibiani-Anhwiaso-Bekwai District, the major economic activities have developed around important resources like gold, bauxite, food and cash crops which in effect has necessitated the emergence of service rendering industries. The district is one of the leading producers of cocoa in the region and has the highest concentration of bauxite, which is still being actively mined. These resources and industries have attracted migrants in search of jobs in the district, including gold and bauxite mining. The gold and bauxite mining work is conducted all over the district and during raining season the metals and other contaminates present in mines enter into the surface and ground water by leaching or by the overflow of water from mines. In artisanal and small-scale mining, it is a common practice for waste material to be removed and piled in large mounds at the mining site. These piles of tailings often contain heavy metals found in the ore and in many instances also contain mercury waste that was used during the amalgamation of gold (Cobbina et al., 2015). In such instances, these tailings are exposed to the elements and can be easily weathered, releasing toxic metals into the soil, adjacent water bodies and ultimately groundwater. Although the small-scale mining is a lucrative business, the practice is a dangerous activity as heavy metals are released to the environment (Aryee et al., 2003). However, once mining has taken place, the minerals are broken down due to exposure to oxygen and water. The toxicity level of a few heavy metals can be just above the background concentrations that are being present naturally in the environment [Fatah, 2008]. Hence thorough knowledge of heavy metals is rather important for allowing providing proper defensive measures against their excessive contact. Therefore, it is necessary to monitor these selected heavy metals of the selected boreholes from different villages of the district for safety assessments of the environment and human health. The aim of this study is to assess the selected heavy metals (As, Cu, Zn) pollution of boreholes from different villages in the Bibiani-Anhwiaso-Bekwai District of the Western Region of Ghana.
The Bibiani-Anhwiaso-Bekwai District is located in the North-eastern part of the Western region. It covers an area of 873 km2 representing 8.6% of the total land area of the region. Bibiani, the district capital is 88 km from Kumasi in the Ashanti region and 356 km from the regional capital, Sekondi. Bibiani-Anhwiaso-Bekwai District is located between latitude 60 3” N and longitude 20 3” W. The district is bounded on the north by the Atwima Mponua District in the Ashanti region, south by the Wassa Amenfi in the Western region, west by the Sefwi-Wiawso district in the Western region and east by the Denkyira North and Amansie East in the Central region and Ashanti region respectively (Figure 1).
The lowest and highest points in the district are 350 and 660 m above sea level. The district is also located in the equatorial climate zone with annual rainfall averages between 1200 and 1500 mm. The pattern of rainfall is bimodal, falling between March-August and September to October. Humidity is relatively high averaging between 75% in the afternoon and 95% in the nights and early mornings (Fatah, 2008).
Experimental procedure
750 ml of water sample was collected from each borehole two times for a period of two months from villages in the Bibiani-Anhwiaso-Bekwai District of the Western Region. Samples were collected from one borehole each located at Akaaso, Kwawkrom, Subri and Surano. Samples were also taken from two boreholes each from Chirano and Anyinase because these villages are more populated and therefore have more boreholes than the other villages. A total of eight water samples were collected during each visit. Samples were collected from May to June 2017. During the sample collection at the sites, each pre-cleaned plastic bottle was thoroughly rinsed with water from that particular borehole and a reasonable amount of the water was pumped out before they were collected. All collected samples were placed in an ice chest before they were transported to the Environmental, Health and Safety Laboratory of SGS Laboratory Services Ghana Limited Tema, for analysis. Each sample was thoroughly mixed by shaking and 50 ml transferred into a Griffin beaker. 1 ml of 1:1 HNO3 and 1 ml of 1:1 HCl were added to the Griffin beaker containing 50 ml of de-ionised water. The Griffin beaker with its content was then placed in a heat block and the temperature adjusted to 950C to provide evaporation for two and a half hours. The digested sample was cooled and topped up to the 50 ml mark with de-ionised water ready for analysis using ICP-MS (Agilent 7900 model). A blank prepared from de-ionised water as well as the standard reference solution for each parameter was used to calibrate the instrument. The instrument was adjusted until the acceptable calibration was achieved. Once the required calibration was achieved, the samples were run to determine the concentration of the metals (As, Cu, Zn) in the sample.
Heavy metal concentrations in borehole water
The total levels of As, Cu and Zn in borehole water sampled from the Bibiani-Anhwiaso-Bekwai District are shown in Table 1. The minimum and maximum levels of As in the borehole water was <0.0005 and 0.0046 mg/L respectively with mean total of 0.00143 mg/L. The mean As concentrations in the borehole water were below the WHO and GEPA permissible limits of 0.01 and 1.0 mg/L respectively. The mean total of Cu concentration in the borehole water was 0.0186 mg/L with minimum and maximum levels of 0.002 and 0.125 mg/L respectively. The mean Cu concentrations in the borehole water were below the WHO permissible limits of 2.0 mg/L. From Table 1 the minimum level of Zn recorded was 0.01 mg/L and maximum of 0.10 mg/L with total mean of 0.0329 mg/L. This was however lower than the WHO and GEPA permissible limits of 3.0 and 10.0 mg/L respectively. In artisanal and small-scale mining, it is common practice for waste material to be removed and piled in large mounds at the mining site. These piles of tailings often contain heavy metals found in the ore and in many instances, also contain mercury waste that was used during the amalgamation of gold (Cobbina et al., 2015). In such instances, these tailings are exposed to the elements and can be easily weathered, releasing toxic metals into the soil, adjacent water bodies and ultimately groundwater. Although the small-scale mining is a lucrative business, the practice is a dangerous activity as heavy metals are released to the environment (Aryee et al., 2003). This study considered As, Cu and Zn contamination of borehole water sampled from the Bibiani-Anhwiaso-Bekwai District in the Western Region of Ghana.
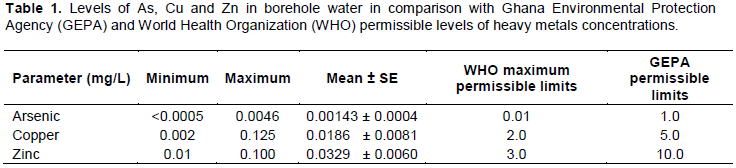
As concentrations in borehole water
Figure 2 shows the level of As in some sampled borehole water. The mean As concentrations recorded for all the boreholes were below the WHO and GEPA permissible limits. However, in comparing the individual borehole water, the level of As was higher in Akaaso (BH5) with mean of 0.0045 mg/L. The second borehole water from Chirano (BH2) recorded higher levels of As compared to the first borehole (BH1). On other hand, the As levels were higher in the water for the first borehole (BH3) compared to the second (BH4) sampled in Anyinase. The borehole water sampled from Surano (BH7) and Kwawkrom (BH8) recorded the least As levels of <0.0005 mg/L. However, analysis of variance showed that there were significant differences in the As concentrations among the eight boreholes (F = 4.078, P=0.033). GEPA guideline for dissolved As concentration is 0.01 mg/L. The borehole water sampled from Surano and Kwawkrom recorded As levels below the detection limit of the laboratory. However, water sampled from the boreholes in Akaaso and Anyinase recorded mean values that were higher compared to the other borehole although the levels were within GEPA permissible guideline value. As is found in the deep bedrock materials as well as the shallow glacial materials in the study area. They are also found alongside gold ores such as arsenopyrites (FeAsS) (Smedley and Kinniburgh, 2002). As is usually present in the environment in inorganic form. The inorganic As easily dissolves and enters underground and surface waters. The presence of As in the environment may be attributed to residual As from former pesticidal use and smelter emission from ores of gold such as arsenopyrites from the sulphur treatment plant. Thus, during ore crushing and panning by the small scale gold miners, arsenopyrite like As is released into the environment and it finally finds its way into sediments of underground and surface water (Kaye, 2005). Also in most aquifers, bio-geological interactions dominate as the source of As, interaction of As with organic and mineral colloids can elevate its concentration (He and Charlet, 2013). Franblau and Lillis (1989) reported cases of sub-chronic As intoxication resulting from ingestion of contaminated well water. Acute gastrointestinal symptoms, central and peripheral neuropathy, bone marrow depression, hepatic toxicity, skin pigmentation occurred when the mean level of As from the contaminated well from which the inhabitants depend were between 0.03 and 0.08 mg/L. Comparing the mean As values in the Franblau and Lillis (1989) study to the calculated As concentration obtained in this study, which residents in Bibiani-Anhwiaso-Bekwai district ingest daily from drinking the borehole water, it was found out that the mean As concentration in water is lower than the estimated As value used in Franblau and Lillis (1989) study. Hence, symptoms associated with As intoxication would be less for residents in the study area. Drinking water contaminated with As is one of the major causes for As toxicity in more than 30 countries in the world (Chowdhurry et al., 2000). If the As level in ground water is 10-100 times the value given in the WHO guideline for drinking water (10 μg/L), it can be a threat to human health (Hoque et al., 2011). Most of the reports of chronic As toxicity in man focus on skin manifestations because of its specificity in diagnosis. Pigmentation and keratosis are the specific skin lesions that indicate chronic As toxicity (Martin and Griswold, 2009).
Cu concentrations in borehole water
Figure 3 shows the level of copper in some sampled borehole water. The mean Cu concentrations recorded for all the boreholes were below the WHO and GEPA permissible limits. However, comparing the individual borehole water, the level of Cu was higher in Kwawkrom (BH8) with mean of 0.0745 mg/L. The second borehole water from Chirano (BH2) recorded higher levels of Cu compared to the first borehole (BH1). On other hand, the Cu levels were higher in the water for the first borehole (BH3) compared to the second (BH4) sampled in Anyinase. The borehole water sampled from Subri and Surano recorded the least Cu levels of 0.0060 and 0.0065 mg/L respectively. However, analysis of variance showed that the differences in the concentration of Cu from the eight boreholes were not significant (F = 1.592, P=0.264). Generally, very low levels of Cu were observed for all the water samples at the sampling sites when compared with the GEPA and WHO guidelines limit of 5 mg/l for drinking water. The mean values of Cu in the boreholes recorded at Kwawkrom were higher than the other borehole water samples.
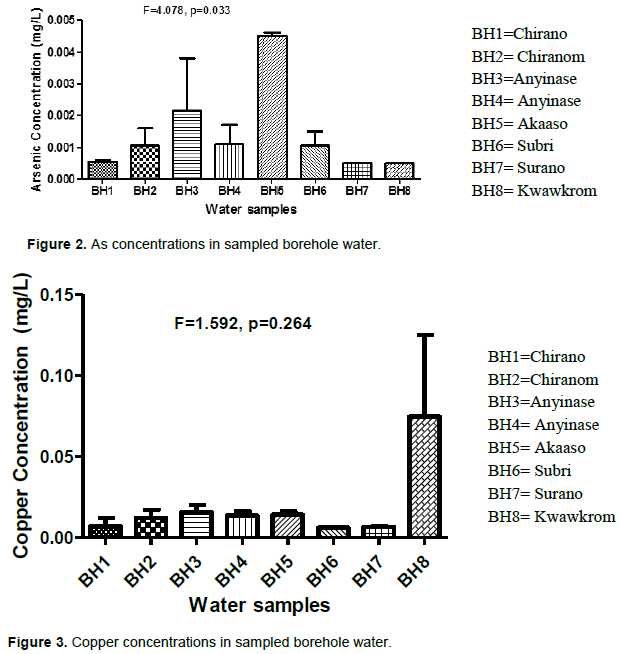
The high concentration of Cu in the BH8 (Kwawkrom) may be due to the improper disposal of Cu wire by the inhabitants which may leach down into the boreholes. The borehole water sampled from Subri and Surano recorded the very low Cu levels. The presence of Cu in the study area may be due to the excavations made by panners in the course of prospecting for gold in the Bibiani traditional area. Such activities lead to the weathering and leaching of this metal from waste rock dumps (Obiri, 2007). Other sources of Cu are the weathering of the rocks, which contain high levels of Cu. Similarly, improper disposal of Cu wire may also account for the presence of Cu in the study area. Most Cu compounds will settle and be bound to either water sediment or soil particles. Soluble Cu compounds form the largest threat to human health. Long-term exposure to Cu can cause irritation of the nose, mouth and eyes and it causes headaches, stomach aches, dizziness, vomiting and diarrhoea. Intentionally high uptakes of Cu may cause liver and kidney damage and even death (Obiri et al., 2010). Osredkar and Sustar (2011) reported that long-term exposure to high concentrations of Cu has a link with a decline in intelligence with young adolescents.
Zn concentrations in borehole water
The level of Zn in some sampled borehole water is presented in Figure 4. The analysis of variance shows that the Zn concentrations do not vary significantly among the eight boreholes (F = 0.741, P=0.647). However, comparing the individual borehole water, the level of Zn was higher in Kwawkrom (BH8) with mean of 0.0625 mg/L. This may be as a result of the leaching of Zn from piping and fittings in the area. The second borehole water from Chirano (BH2) and Anyinase (BH4) recorded higher levels of Zn compared to the boreholes BH2 and BH3. The borehole water sampled from Subri (BH6) and Surano (BH7) recorded the least Zn levels of 0.0160 and 0.0170 mg/L respectively. The mean Zn concentrations recorded for all the boreholes were below the WHO and GEPA permissible limits. No significant difference was observed in the concentrations of Zn in the water samples from the different sources. In natural surface waters, the concentration of Zn is usually below 10 µg/L, and in ground waters, 10-40 µg/L (Van Leeuwen, 2000). In tap water, the Zn concentration can be much higher as a result of the leaching of Zn from piping and fittings. Taking too much Zn into the body through food, water, or dietary supplements can also affect health. The levels of Zn that produce adverse health effects are much higher than the Recommended Daily Allowances (RDAs) for Zn of 15 mg/day for men and 12 mg/day for women.
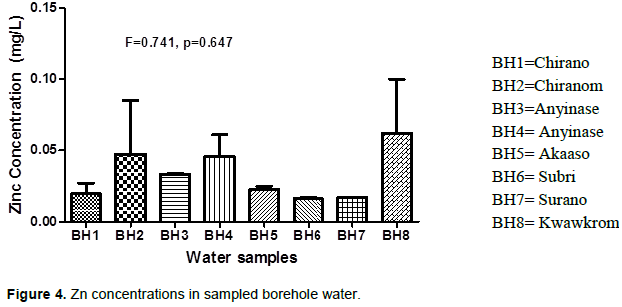
If large doses of Zn (10-15 times higher than the RDA) are taken by mouth even for a short time, stomach cramps, nausea, and vomiting may occur (Hotz and Brown, 2004; Roohani et al., 2013). Ingesting high levels of Zn for several months may cause anemia, damage of pancreas and decrease levels of high-density lipoprotein (HDL) cholesterol (Plum et al., 2010). Zn content in the borehole water samples ranged from 0.01 to 0.1 mg/L with a mean value of 0.0329 mg/L is within WHO maximum allowable of 3.0 mg/L for drinking water (Table 1). This indicates that water from the sampled boreholes contain the right proportion of Zn which is an essential plant and human nutrient element. The low concentration further implies the boreholes do not have caustic taste, hence ideal for consumption and other domestic uses.
The results of the study showed that the boreholes from the various sampling sites are contaminated with heavy metals; however, the mean concentrations do not exceed WHO/GEPA permissible level for drinking water. This raises less concern about the quality of drinking water being used by residents in the study area. The study has established that the concentrations of heavy metals, mainly As, Cu and Zn recorded in the borehole water from Chirano, Anyinase, Akaaso, Subri, Surano and Kwawkrom were below GEPA and WHO standards, rendering the water somewhat safe for domestic use. However, it is important for residents in the study area to be provided with more potable water.
The authors have not declared any conflict of interests.
REFERENCES
|
Aryee BN, Ntibery BK, Atorkui E (2003). Trends in the small-scale mining of precious minerals in Ghana: a perspective on its environmental impact. Journal of Cleaner Production 11(2):131-140.
Crossref
|
|
|
|
Chowdhurry UK, Biswas BK, Chowdhurry HR, Samanta G, Mandal BK, Basu GC, Borti D (2000). Groundwater arsenic contamination in Bangladesh and west Bengal, India, Environmental Health Perspective 108(5):393-397.
Crossref
|
|
|
|
|
Cobbina SJ, Duwiejuah AB, Quansah R, Obiri S, Bakobie N (2015). Comparative assessment of heavy metals in drinking water sources in two small-scale mining communities in northern Ghana. International Journal of Environmental Research and Public Health.
Crossref
|
|
|
|
|
12(9):10620-10634.
|
|
|
|
|
Essumang DK, Dodoo DK, Obiri S, Yaney JY (2007). Arsenic, Cadmium, and Mercury in Cocoyam (Xanthosoma sagititolium) and water cocoyam (colocasia esculenta) in Tarkwa a mining community. Bulletin of Environmental Contamination and Toxicology 79:377-379.
Crossref
|
|
|
|
|
Fatah L (2008). The impacts of coal mining on the economy and environment of South Kalimantan Province, Indonesia. ASEAN Economic Bulletin 25(1):85-98.
Crossref
|
|
|
|
|
Franblau A, Lillis R (1989). Acute arsenic intoxication from environmental arsenic exposure. Archives of Environmental Health 44:385-390.
Crossref
|
|
|
|
|
Hanson R, Dodoo DK, Essumang DK, Blay Jr., Yankson JK (2007). The effect of some selected pesticides on the growth and reproduction of fresh water oreochromis niloticus; chrysicthys nigrodigitatus and clarias gariepinus. Bulletin of Environmental Contamination and Toxicology 79:544-547.
Crossref
|
|
|
|
|
He J, Charlet L (2013). A review of Arsenic presence in China drinking water. Journal of hydrology 492:79-88.
Crossref
|
|
|
|
|
Hoque MA, Burgess WG, Shamsudduha M, Ahmed KM (2011). Delineating low - arsenic groundwater environments in the Bengal Aquifer system Bangladesh. Applied Geochemistry 26(4):614-623.
Crossref
|
|
|
|
|
Hotz C, Brown KH (2004). Assessment of the risk of zinc deficiency in populations and options for its control. The Food and Nutrition Bulletin 25(1):194-195.
|
|
|
|
|
IARC (1980). Monographs on the carcinogenic risk to humans and their supplements: some metals metallic compounds. International Agency for Research on Cancer, IARC monographs, volume 23.
|
|
|
|
|
Kaye A (2005). The effects of mine drainage water from Carrock Mine on the water quality and benthic macro-invertebrate communities of Grainsgill Beck: a preliminary study. Earth and Environment 1:120-154.
|
|
|
|
|
Lokeshwari H, Chandrappa GT (2006). Impact of heavy metals contamination of Bellandur lake on soil and cultivated vegetation. Current Science 91(5):622-627.
|
|
|
|
|
Martin S, Griswold W (2009). Human health effects of heavy metals. Environmental Science and Technology Briefs for Citizens 15:1-6.
|
|
|
|
|
Obiri S (2007). Determination of Heavy Metals in Boreholes in Dumasi in the Wassa West district of Western region of the Republic of Ghana. Environmental Monitoring and Assessment 130:455-463.
Crossref
|
|
|
|
|
Obiri S, Dodoo DK, Essumang DK, Armah FA (2010). Cancer and non-cancer risk assessment from exposure to arsenic, copper, and cadmium in borehole, tap, and surface water in the Obuasi municipality, Ghana. Human and Ecological Risk Assessment 16(3):651-665.
Crossref
|
|
|
|
|
Osredkar J, Sustar N (2011). Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. Journal of Clinical Toxicology S3:001.
Crossref
|
|
|
|
|
Plum LM, Rink L, Haase H (2010). The essential toxin: impact of zinc on human health. International Journal of Environmental Research and Public Health 7(4):1342-1365.
Crossref
|
|
|
|
|
Roohani N, Hurrell R, Kelishadi R, Schulin R (2013). Zinc and its importance for human health: An integrative review. Journal of Research in Medical Sciences 18(2):144-157.
|
|
|
|
|
Singh N, Koku JE, Balfor B (2007). Resolving water conflicts in mining areas of Ghana through public participation; A Community Perspective. Journal of Creative Communications 2:361-382.
Crossref
|
|
|
|
|
Smedley PL, Kinniburgh DG (2002). A review of the source behavior and distribution of arsenic in natural waters. Applied Geochemistry 17:517-568.
Crossref
|
|
|
|
|
Van Leeuwen FXR (2000). Safe drinking water: the toxicologist's approach. Food and Chemical Toxicology 38:S51-S58.
Crossref
|
|