Review
ABSTRACT
Most coastal waters are exposed to high influx of pollutants due to the obvious elevated human activities. In order to adequately evaluate the extent of toxicity of contaminants in the ecosystem, and their synergistic effects, marine ecologists prefer biomonitoring to chemical approach. The benthic macroinvertebrates, due to their sedentary mode of life and residence at the sediment-water microcosm, are regarded as the most veritable tool for biomonitoring. This is because these organisms are impacted by the interstitial forces of the sediment-water interface in the marine ecosystem and also serve as the main channel for the interchange of biomass. This review focuses on the biomonitoring status and prospects of a coastal lagoon with reference to the benthic macroinvertebrates. The levels of biomonitoring activities which are suborganismal, organismal, population, community and ecosystem levels are critically analyzed. However, most of the molecular and biochemical assays, and biomarkers used in biomonitoring studies at the suborganismal and organismal levels of biological development are being outsourced. This poses great challenges to holistic biomonitoring programs in the vast Nigerian coastal ecosystem.
Key words: Biomonitoring, coastal waters, Lagos lagoon, bioindicators, benthic macroinvertebrates.
INTRODUCTION
The anthropogenic stress exerted the coastal waters has resulted in pollution hazards at an alarming rate in such aquatic ecosystems. For some obvious reasons, coastal environments are choices for localization of industries, high human population concentration, developmental projects and agricultural and fishing activities. The combination of all these generate variant pollutants that are directly or indirectly released into the nearby coastal waters as the last sink (Nkwoji et al., 2020). Monitoring according to Cullen (1990) involves the process of repetitively observing one or more elements of the environment, for defined purposes and according to prearranged schedules in space and time using comparable methods for environmental sensing and data collection. Environmental monitoring is both systematic and repetitive and this differentiates it from environmental survey.
Biomonitoring is defined as the systematic use of living organisms or their responses to determine the condition or changes in the environment (Rosenberg, 1998; Gerhardt, 1999; Oertel and Salanki, 2003). It involves the direct measurements of pollution impacts on organisms of interest as opposed to the use of abiotic chemical surrogates. One advantage of this is its capability to integrate and also to measure subtle variables caused by minor and intermittent contaminants. Biological assessment of the outcomes of the fluctuating factors that drive any ecosystem would seem more sensible than attempting to measure these varying driving factors and then to estimate how they might affect biological production’ (Cullen, 1990).
According to Schöne and Krause (2016) and Prabhakaran et al. (2017), when chemicals are directly employed to assess the level of contamination in water and sediment, the extent of toxicity of contaminants and their synergistic effects in the ecosystem would not be evaluated. This is because some toxicants which are introduced into the aquatic environments in levels that are undetectable through chemical means could still be bioavailable to the biota for uptake and subsequently bioaccumulated in their tissues despite the low concentration (Schöne and Krause, 2016). According to Li et al. (2010), there is an urgent need for more holistic and methodological approaches to evaluate the actual state of these ecosystems and to monitor their rate of alterations.
In a complex natural ecosystem such as lagoons, the use of chemical means to evaluate the characteristics of pollutants is often difficult. It is therefore, important that the effects of such pollution should be studied in relation to biological systems (Parmar et al., 2016; Cerveny et al., 2016). According to Masese et al. (2013), the aquatic communities are better indicators of anthropogenic stressors in their environment at different levels because they integrate the different variations. This underscores the need for biomonitoring in aquatic ecosystem management and conservation.
According to Zhou et al. (2008), biomonitoring exhibits obvious advantages over the routine chemical monitoring in the following ways: It reveals both the subtle biological changes of organisms affected by exogenous chemicals, which is usually missed by the conventional chemical analysis, and the integrated effects of the complex pollutants on the organisms in the environment. When the organisms are exposed to pollutants, they respond rapidly and this sensitivity is often harnessed. Some pollutants below the detection limits by the instrumental analytical techniques could still be biomonitored due to the occurrence of the chronic toxicities of the pollutants in the organisms under long-term exposure.
Biomonitoring gives a complete evaluation of the exact impacts of the pollutants by considering both the potential impacts and the actual joint toxicities of the toxicants on the environment. This is possible due to the fact that biological responses could get stimulated at chemical concentration levels below the conventional analytical detection limits, and may persist even at the end of the chemical exposure (Zuykov et al., 2013). According to Prabhakaran et al. (2017), the success of any biomontoring project would depend on the right choices of the biomonitors. While any of such terms as bioindicators, biological monitors and sentinel organisms may be used to mean the biota employed as biomonitors (Hellawell, 2012), it is important to state here that, while bioindicators show an ecological effect either by their presence or absence, ecological monitors are species that indicate the actual degree of ecological alterations by the behavioural, as well as their physiological and biochemical responses (Tsygankov et al., 2017; Müller and Müller, 2018).
The definition of biomonitoring by Zhou et al. (2008) as “a scientific technique for assessing environment including human exposures to natural and synthetic chemicals, based on sampling and analysis of an individual organism’s tissues and fluids”, could be disputed. This is because the definition which emphasizes the chemical analysis of tissues and fluids of organisms is consistent with biomarker which is more of a biomonitor than a bioindicator, and therefore does not represent the general definition of biomonitoring. The technique takes advantage of the fact that when organisms are exposed to certain chemicals, they leave markers that reflect the extent and duration of the exposure. These markers could be the chemical itself, components of the chemical or other biological variations in the biota resulting from the effects of the chemicals on the organism (Zhou et al., 2008).
BIOMONITORING TECHNIQUES
The multi dynamic interactions in the aquatic ecosystem by its biotic and abiotic components make it a complex one. This complexity includes the bioassessment of some stress factors which often have synergistic, additive or antagonistic effects in the aquatic environment (Solimini et al., 2009). In order for a more holistic assessment, biomonitoring should be addressed at the different levels of biological organization; suborganismal, organismal, population, community and ecosystem. This however, is not always the case as many biomonitoring programs tend to be restricted to a few levels of biological organization, thereby limiting the potential spectrum of measurable of cause-effect responses to different anthropogenic impacts (Cortes et al., 2016). In this review, different biomonitoring techniques, based on the specific aim, have been adopted.
Bioaccumulation
When an organism absorbs a toxic substance at a rate greater than that at which the substance is eliminated, biomonitoring occur. According to Waykar et al. (2011), this results from a dynamic equilibrium between exposure from the outside environment and uptake, excretion, storage, and degradation within an organism. The processes of uptake, storage and elimination of toxicants are involved during bioaccumulation. Understanding of the dynamic process of bioaccumulation is a critical consideration in the regulation of chemicals such as aquatic metals (Zhou et al., 2008).
The mechanism and quantity of toxicant’s accumulation in the tissues and organs of biota in the aquatic ecosystem is key to assessing the adverse effects of those toxicants on the ecosystem. For example, Liu et al. (2016) stated that the organochloride pesticide contamination in Lake Chaohu in China could be determined by the correlation between the OCP contamination in the aquatic biota with that in the water/or suspended matter. By interacting with the environment, aquatic biota accumulates pollutants by directly picking them up from their surroundings as well as through the food chain at the various trophic levels. The extent of accumulation of pollutants by the aquatic biota will determine the bioavailability in the water and sediment as well as along the trophic levels.
The accumulation of these toxicants constitutes threat to the ecosystem, its biogeochemical processes, and risks to human health (Prabhakaran et al., 2017). In the case of Polycyclic Aromatic Hydrocarbons (PAHs), their level of accumulation in marine biota is dependent on the length of exposure and concentration of the toxicants in that environment as well as extent of the mobilization (Meador et al., 1995). Aquatic biota that accumulates PAHs during short-term acute incidences (Prabhakaran et al., 2017) may have the opportunity of system clean up when returned to pristine condition. If however the exposure is chronic and continuous, the ability to eliminate the toxicant may not be possible. According to Prabhakaran et al. (2017), fishes, oligochaetes and crustaceans easily metabolise PAHs while molluscs and bivalves are the least in the ability to metabolise PAH. The implication is that consumers of molluscs have high risk of the shellfish toxicity. In most bivalves, the gills are the main sites for metal accumulation. This is because the bivalves are mostly filter feeders and in the process of feeding, the first contact point is the gills. Also, the gills have large surface area and are lined with mucus, thus making them better accumulator of such metals as cadmium, lead, and zinc in the feeding ambient water than the other soft body tissues (Zhou et al., 2008).
Biochemical alteration
Biomontoring adopted using biochemical alteration involves the bioassessment at the suborganismal level of the biological organization. The tools employed at this level are the biomarkers. Biomarkers are measured at the suborganismal level to identify the biological effects of some toxicants in the environment at an early stage for effective quality assessment of that environment (McCarthy and Shugart, 1990).
According to Kumar et al. (2017), biomarkers are linker between environmental contamination (cause) and its effects in respect to changes in biological systems. Prabhakaran et al. (2017) stated that alteration of the biochemical defense mechanism is the first typical response to any toxic assault by xenobiotics. The protective nature of antioxidative enzymes makes them effective biomarker for identifying pollutants in the environment at an early stage. The measurement of this excitation, according to Schlenik et al. (2008), can serve as sensitive indicators of an altered cell function. This is because according to Capela et al. (2016), biochemical responses are triggered in aquatic organisms even at a low concentration of the toxicants. Moore et al. (2004) has proposed that biomarkers should be related to the different functions of the organisms and that different levels of the biological scale of the organisms studied in order “for better understanding of the mechanisms underlying the effect of the stressors” (Lavarías et al., 2016) on the aquatic environment.
Biomarkers such as metallothionein and cytotoxicological responses such as genotoxicity, lysosomal alterations, immunocompetence and gencholinesterase activities (Zhou et al., 2008) have currently been developed. There is need however, to take some precautions at the sensitivity of these biomarkers at each developmental stage of the organisms. Some special protein could be purified and harnessed to serve as biomarker for some metal exposure (Zhou et al., 2008). Biomarkers should be well selected for specificity on particular pollutants as well as organisms and areas of interest.
MORPHOLOGICAL AND BEHAVIOURAL OBSERVATIONS
Observing the effects of toxicants on the morphology and behaviour of organisms is the most direct form of bioassessment. In bioassay analysis, the sublethal effects of toxicants on aquatic biota can easily be visually observed through the changes in their physical appearance and behavioural pattern. Behavioural ecotoxicology deals with morphological and behavioural observation of organisms in relation to environmental quality (Prabhakaran et al., 2017). Behavioural changes in aquatic biota in response to chemical stress in their environment are among the early and most sensitive observable parameters, and they incorporate both the biochemical alterations at the sub-organismal level and changes at the whole organism level (Hartmann et al., 2016).
The study by Zhou et al. (2008) on the masculinization phenomena exhibited in prosobranch gastropod, Rapana venosa is a typical example of morphological observation in biomonitoring. The imposex was as a result of organotin pollution that the gastropod was continuously exposed to. In imposex-affected species, the entire female genital system is conserved but superimposed by male organs such as penis and/or vas deferens (Zhou et al., 2008). This could result to infertility in the female species thereby affecting the population. According to the study, imposex occurred in some snails in southeast China indicating the feasibility of the biomonitoring technique based on imposex investigation in the assessment of organotin contamination caused by frequent marine traffic (Zhou et al., 2008).
In biomonitoring, the sublethal effect of chemical exposures in the aquatic ecosystem could be bioassessed through some behavioural patterns of the organisms. The organism exhibits such behaviours like avoidance, feeding depression and valve closure behaviour (Zhou et al., 2008).
A study carried by Lopes et al. (2004) showed that Daphnia longispina avoid certain concentration of copper and further revealed that avoidance to copper was much more sensitive than lethality by copper in the organisms. Lopes et al. (2004) therefore, recommended that the avoidance assays be used as a complementary tool, for ecological risk assessments and effluent biomonitoring since such assays can provide cost-effective and ecologically relevant information.
A research conducted by Zhou et al. (2008) indicated that certain concentration of cadmium and zinc at a sublethal level would result in the significant reductions of the feeding rate of the Decapod, Atyaephyra desmarestii and Amphipoda, Echinogammarus meridionalis. They therefore, posited that chronic feeding assays be used in biomonitoring studies because they are rapid, cheap and effective. According to Liao et al. (2007) and Zhou et al. (2008), the valve closure behaviour in the freshwater clam, Corbicula fluminea in response to copper concentration, could be used in the bioassessment of the aquatic heavy metals.
POPULATION AND COMMUNITY LEVEL APPROACH
Biomonitoring approach at the level of population and community is also very critical. At this level of biomonitoring, the bioindicators are mostly employed for the assessment. The population and community level approaches in aquatic biomonitoring are based on the understanding of the influence of environmental factors on patterns of distribution, abundance and species diversity of aquatic communities (Prabhakaran et al., 2017). The community structure can be described by computing the richness and diversity indices and used to determine the impact of any toxicants and the general health of the ecosystem (Hering et al., 2006). The relevant indices are discussed later in this paper.
Researches by De Castro-Catala et al. (2015), Chiu et al. (2016), and Hasenbein et al. (2016) indicate that biomonitoring approach at the population and community level of an ecosystem is very necessary in the bioassessment of its aquatic biota and general health. However, according to Prabhakaran et al. (2017), emphasis on this biomonitoring approach cannot be considered as an all-purpose concept to determine all aspects of biodiversity loss. This is because in a situation where an abrupt change in environmental conditions has caused the loss or total elimination of certain species, the community could be replaced by ecologically similar ones capable of occupying the new niche. It is important to note that population and community approach of biomonitoring can only capture the loss of species, and this might not be reflected in the overall species diversity assessment (Feld et al., 2014).
Due to its masculinisation properties on some molluscs, organotin pollution, because of the imposex response to tributyltin compound, are commonly used in biomonitoring for populations of some gastropods (Axiak et al., 2003). The organotin may cause infertility in the female gastropod thereby obstructing reproduction and drastically affecting their population. Researches have shown the water quality of any natural aquatic ecosystem could be evaluated by the population dynamics of a particular species (Crespo et al., 2015). According to Ajao and Fagade (1990a), loss in biodiversity is the most obvious effect of pollution. Alterations in population and community level indicate disturbances in the normal balance in the studied ecosystem but may also be an indication that the population of a peticular ecosystem is advanced (Zhou et al., 2008).
BIOMONITORING OF COASTAL WATERS: BENTHIC MACROINVERTEBRATES AS SUITABLE BIOINDICATORS
Zhou et al. (2008), in the review, “Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem”, posited that “suitable bioindicators usually give great help to the biomonitoring” and that a perfect bioindicator is expected to have the following characters: (1) it can accumulate high levels of pollutants without death; (2) it lives in a sessile style, thus definitely representing the local pollution; (3) it has enough abundance and wide distribution for the repetitious sampling and comparison; (4) its life is long enough for the comparison between various ages; (5) it can afford suitable target tissue or cell for the further research at microcosmic level; (6) easy sampling and easy raising in the laboratory; (7) it keeps alive in water; (8) it occupy the important position in food chain; (9) well dose-effect relationship can be observed in it.”
According to Markert et al. (2003), bioindicators are organisms or communities of organisms whose content of certain elements or chemical (organic) compounds and/or whose morphological, histological or cellular structure, metabolic-biochemical processes, behaviour or population structure(s), including changes in these parameters, supply information on the quality of the environment or the nature of environment changes. It then implies that a bioindicator should be able to provide enough information to ascertain the quality of part or all of an environment. Additionally, “an ‘ideal’ indicator should have the characteristics as follows: (a) taxonomic soundness (easy to be recognized by nonspecialist); (b) wide or cosmopolitan distribution; (c) low mobility (local indication); (d) well-known ecological characteristics; (e) Numerical abundance; (f) suitability for laboratory experiments; (g) high sensitivity to environmental stressors; (h) high ability for quantification and standardization” (Li et al., 2010). All these qualities are inherent in the benthic macroinvertebrates, and thus make them most suitable bioindicators for pollution assessment in the coastal waters.
Macroinvertebrates are organisms without backbones that can be seen without the aid of a microscope. As a result of their habitat choice, macroinvertebrates are often referred to as benthos, a term that refers collectively to organisms that lives on, in or near the bottom. These organisms reside in aquatic systems for long enough to reflect chronic effects of pollutants (Reboredo-Fernández et al., 2014), and thus, data on these organisms are used individually or in combination with other environmental characteristics, to assess the extent of environmental impairment (Huh, 2019) and the general health of an aquatic ecosystem.
The benthic macroinvertebrates constitute the key components of aquatic food webs, linking organic matter and nutrient resources with higher trophic levels (Kiljunen et al., 2020). Their sedentary lifestyle makes them “representative of site-specific ecological conditions” (Nkwoji et al., 2016). With the sensitive life stage and relatively long lifespan, the benthic macroinvertebrates have the ability to integrate the effects of short-term environmental variations. Besides, these assemblages are made up of many species among which there is a wide range of trophic levels and pollution tolerances (Muzón et al., 2019), thereby providing vital information for interpreting cumulative effects. According to Kiljunen et al. (2020), the community structure of the assemblages frequently changes in response to environmental disturbances in predictable ways and this has formed “the basis for development of biocriteria to evaluate anthropogenic influences” (Karrouch et al., 2017).
DIVERSITY INDICES
Diversity indices are mathematical expressions which use three components of community structure; namely, richness (number of species present), evenness (uniformity in the distribution of individuals among the species) and abundance (total number of organisms present), to describe the response of a community to the quality of its environment (Metcalfe?Smith, 1994). They are efficient in describing responses of a community of organisms to variation (Ghosh and Biswas, 2015) in the environment.
The underlying assumption in the diversity approach is that undisturbed environments will be characterized by a high diversity or richness, an even distribution of individuals among the species, and moderate to high counts of individuals (e.g., Shannon-Wiener Index, Simpson Index and Margalef Index) (Metcalfe, 1989). However, it is not in all cases that this assumption has proved to be true. High diversity does not necessarily imply unpolluted water, and low diversity on the other hand, does not necessarily indicate pollution.
Shannon index
This index is more popular among other diversity indices. It is referred to as Shannon’s diversity index, the Shannon-Wiener index, the Shannon-Weaver index and the Shannon entropy (Niklaus et al., 2001; Sax, 2002). The index was first introduced by Claude E. Shannon to quantify the entropy (uncertainly or information content) in strings of text. The concept is that the more diverse letters there are, and the more equal their proportional abundances in string of interest, and the more difficult it would be to correctly predict which letter will be the next one in the string. The Shannon entropy was therefore, introduced to quantify the uncertainly (entropy or degree of surprise) (Shannon, 1948) associated with this prediction (Shannon, 1948; Tandon et al., 2007). Applied in ecology, the Shannon entropy quantifies the uncertainty in predicting the species identity of an individual that is taken at random from the dataset (Sarma and Das, 2015). The Shannon diversity index (H) is applied in community structure studies to characterize their species diversity. Shannon diversity index (H) is not a diversity in itself, but is an index used as a determinant of diversity. It is expressed in the following equation:
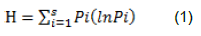
where H = Shannon index of diversity, Pi = fraction of the entire population made up of species i, that is, pi is the proportion (n/N) of individuals of one particular species found (n) divided by the total number of individuals found (N), S = Numbers of species encountered, ln = natural logarithm, and ∑= sum from species 1 to species S (Shannon, 1948).
Generally, the Shannon index ranges between 1.5 and 3.5 in most ecological studies, and could hardly exceed 4. The index increases as both the richness and the evenness of the community increase and vice versa. Shannon index is the preferred of all other indices by ecologists because it incorporates both components of biodiversity. However, this could be seen as both strength and weakness. It is a strength because it provides a simple, synthetic summary, but it is also a weakness because it makes it difficult to compare two communities that have much difference in richness (Chao and Shen, 2003).
Simpson’s index
Simpson’s index of diversity is used to calculate the diversity that incorporates both the number of species that are present and the relative abundance of each species in the community (Gorelick, 2006). It is based on probability of any two individuals drawn at random from an infinitely large community belonging to the same species (Ardura and Planes, 2017). The index is useful especially when researchers are dealing with very large quantities of data such that the level of diversity within that data becomes difficult to ascertain by merely reading from the table of results.
The Simpson’s index gives relatively little weight to the rare taxa and more weight to the common taxa, as it weighs towards the abundance of the most common taxon (Ghosh and Biswas, 2015). The value, D is a measure of dominance, so as D increases, diversity (in the sense of evenness) would decreases. This index is therefore, usually reported as its complement 1-D (Hurlbert, 1971). Since D takes on values between zero and one and approaches one in the limit of a monoculture, (1-D) provides an intuitive proportional measure of diversity that is much less sensitive to species richness (Magurran and McGill, 2011).
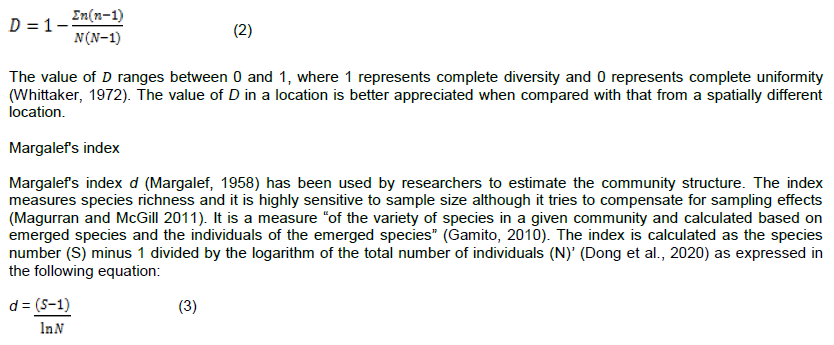
Unlike Shannon and Simpson indices which may use proportional values or densities, absolute values or the total numbers must be used while calculating Margalef index. The sole use of the index by researchers to determine the health of an aquatic ecosystem is highly discouraged because species richness alone does not reflect the true health status of the environment as it depends largely on the size of the sample.
BIOMONITORING OF THE LAGOS LAGOON: STATUS AND PROSPECTS
The strategic location of the Lagos lagoon (Figure 1), coupled with its economic and ecological relevance, made it one of the most widely researched coastal lagoons in the world. Its link with the Atlantic ocean, the concentration of industries, and high human population around the lagoon have caused the lagoon to be much impacted with pollutants from maritime, industrial and domestic sources respectively. There is need for regular biomonitoring of the lagoon so as to maintain its ecosystem functions and reduce health hazards on the human population.
The first published report on the ecology of Lagos lagoon was by Webb (1958). In this report, no mention was made about the living resources of the lagoon as the study was basically on the geomorphology and depositional features of the lagoon system. Many subsequent studies on Lagos lagoon have focused on heavy metals and other associated toxicants in water and sediment (Okoye, 1991; Okoye et al., 1991; Olatunji and Abimbola, 2010; Lawson, 2011; Alani et al., 2013; Benson et al., 2014; Elijah and Isa, 2015; Olayinka et al., 2016; Olafisoye et al., 2016; Bawa-Allah et al., 2018) without due recourse to the biota of the lagoon.
Don-Pedro et al. (2004) however, monitored the trends of heavy metal concentration of the lagoon with reference to the bioaccumulation of the metals in the body tissues of benthic fauna, Typanotonus fuscatus and Clibanarius africanus. More recent studies on the biomonitoring of Lagos lagoon include “the use of aquatic macrophytes to monitor the distribution of heavy metals” (Adesuyi et al., 2018) and Palaemonetes africanus to monitor the toxicity of dumpsite leachate (Amaeze and Abel-Obi, 2015).
The earliest and robust biomonitoring survey of the Lagos lagoon that used the community structure of the benthic macroinvertebrates as bioindicators (Nkwoji et al., 2020) was conducted by Ajao and Fagade (1990a). In this study however, more emphasis was placed on the sediment, the habitat of the benthos rather than the benthos themselves.
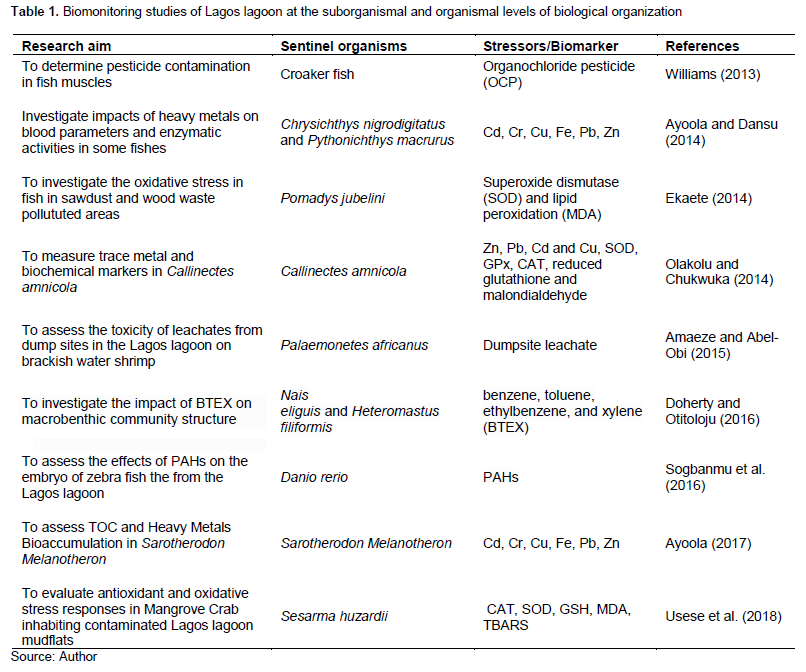
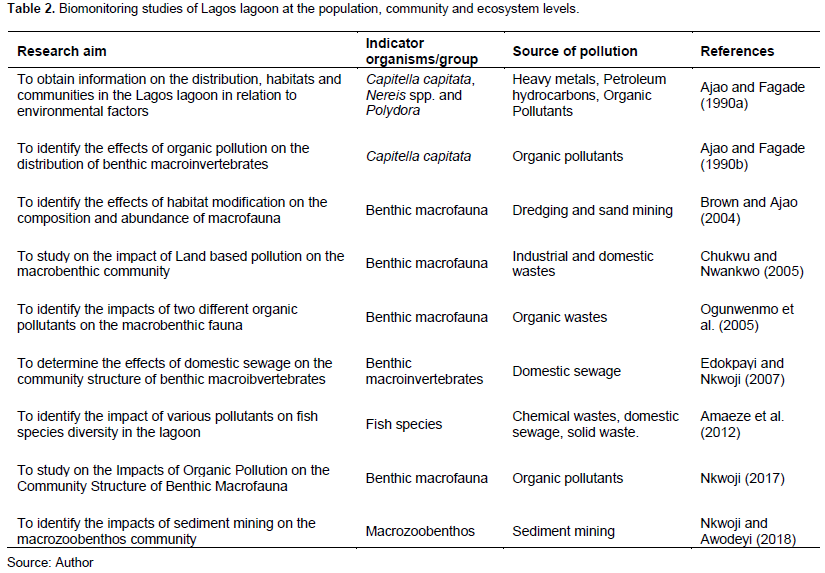
The characteristics of the sediments were analysed in details and the benthic macroinvertebrates studies were conducted only to the extent it related to the sediment composition and type. Moreover, no biomonitoring was conducted at the suborganismal and organismal levels and the identification of the sentinel organisms was purely morphological. Some of the biomonitoring studies of the Lagos lagoon at both the suborganismal and organismal levels are shown in Table 1 while the studies at population, community and ecosystem levels are shown in Table 2.
CONCLUSION AND FUTURE DIRECTION
Biological monitoring of the Lagos lagoon would serve as the reference for other Nigerian coastal waters. However, most studies on biomonitoring of the coastal water have centered on the use of bioindicators, especially the benthic macroinvertebrates as sentinel organisms. Unfortunately, modern biomonitoring techniques that incorporate the different levels in biological organization are yet to be developed in Nigeria. The molecular and biochemical assays, as well as the biomarkers used for assessment at the suborganismal level of biological development are being sourced outside the country. Their cost, availability and accessibility pose great challenges and constitute serious impediments to biomonitoring programs. Incidentally, biomonitoring at this level, because of their early warning signal features, is key to identifying the xenobiotics in our coastal ecosystem and help to forestall any serious future damage to the ecosystem. Biomonitoring at the organismal level also has its challenges in Nigeria. There is erratic public power supply and this is the bane of experimental procedures. For instance, the bioassay setups need constant power for aeration and general conditioning of the experimental environment to assume a near natural ecosystem and for acclimatization of the test organisms. Private power generators pose the problem of emitting carbon fumes and gases that add pollutants to the bioassay setup thereby distorting the experiment. There is the need for concerted effort by the government and non-governmental bodies to fund researches on biomonitoring of Nigerian coastal waters and Lagos lagoon in particular. Infractural deficit should be tackled and green and clean energy provided. These are necessary for laboratory experimental setups required for biomonitoring studies.
CONFLICT OF INTERESTS
The author has not declared any conflict interests.
REFERENCES
|
Adesuyi AA, Njoku KL, Akinola MO, Jolaoso AO (2018). Biomonitoring of Heavy Metals Level in Wetland Plants of Lagos Lagoon, Nigeria. Journal of Applied Sciences and Environmental Management 22(9):1489-1498. |
|
|
Ajao EA, Fagade SO (1990a). A study of the sediments and communities in Lagos Lagoon, Nigeria. Oil and Chemical Pollution 7(2):85-117. |
|
|
Ajao EA, Fagade SO (1990b). The ecology of Capitella capitata in Lagos Lagoon, Nigeria. Archiv fur Hydrobiologie. Stuttgart 120(2):229-239. |
|
|
Alani R, Olayinka K, Alo B (2013). Studies on persistent organic pollutants (POPs) in the Lagos Lagoon 1: occurrence and levels of polycyclic aromatic hydrocarbons (PAHs) in surface waters of the lagoon. Journal of Emerging Trends in Engineering and Applied Sciences (JETEAS) 4(6):811-818. |
|
|
Amaeze NH, Abel-Obi CJ (2015). Coastal Dump Sites in the Lagos lagoon and toxicity of their leachate on brackish water shrimp (Palaemonetes africanus). Journal of Applied Sciences and Environmental Management 19(3):503-510. |
|
|
Amaeze NH, Egonmwan RI, Jolaoso AF, Otitoloju AA (2012). Coastal environmental pollution and fish species diversity in Lagos Lagoon, Nigeria. International Journal of Environmental Protection 2(11):8-16. |
|
|
Ardura A, Planes S (2017). Rapid assessment of non-indigenous species in the era of the eDNA barcoding: A Mediterranean case study. Estuarine, Coastal and Shelf Science 188:81-87. |
|
|
Axiak V, Micallef D, Muscat J, Vella A, Mintoff B (2003). Imposex as a biomonitoring tool for marine pollution by tributyltin: some further observations. Environment International 28(8):743-749. |
|
|
Ayoola SO (2017). Comparative Assessment of Total Hydrocarbon Content and Bioaccumulation of Heavy Metals in Sarotherodon Melanotheron at Atlas Cove Area and Okobaba of Lagos Lagoon. Iranian Journal of Energy and Environment 8(2). |
|
|
Ayoola SO, Dansu FM (2014). The Impact of Heavy Metals on Haematological Parameters and Enzymatic Activities in Chrysichthys Nigrodigitatus and Pythonichthys Macrurus. World Applied Sciences Journal 31(5):794-800. |
|
|
Bawa-Allah KA, Saliu JK, Otitoloju AA (2018). Heavy Metal Pollution Monitoring in Vulnerable Ecosystems: A Case Study of the Lagos Lagoon, Nigeria. Bulletin of Environmental Contamination and Toxicology 100(5):609-613. |
|
|
Benson NU, Essien JP, Asuquo FE, Eritobor AL (2014). Occurrence and distribution of polycyclic aromatic hydrocarbons in surface microlayer and subsurface seawater of Lagos Lagoon, Nigeria. Environmental Monitoring and Assessment 186(9):5519-5529. |
|
|
Brown CA, Ajao EA (2004). Effects of topographical modification on the composition and abundance of macrofauna in Southern Lagos lagoon (Ikoyi). West African Journal of Applied Ecology 5(1):41-50. |
|
|
Cerveny D, Turek J, Grabic R, Golovko O, Koba O, Fedorova G, Randak T (2016). Young-of-the-year fish as a prospective bioindicator for aquatic environmental contamination monitoring. Water Research 103:334-342. |
|
|
Chao A, Shen TJ (2003). Nonparametric estimation of Shannon's index of diversity when there are unseen species in sample. Environmental and Ecological Statistics 10(4):429-443. |
|
|
Chiu MC, Hunt L, Resh VH (2016). Response of macroinvertebrate communities to temporal dynamics of pesticide mixtures: A case study from the Sacramento River watershed, California. Environmental Pollution 219:89-98. |
|
|
Chukwu LO, Nwankwo DI (2005). The impact of Land based pollution on the hydrochemistry and macrobenthic community of a tropical West African creek. Fundamentals of Limnology, SB Nangia for APH Publishing Corporation 5:7-15. |
|
|
Cortes R, Hughes S, Coimbra A, Monteiro S, Pereira V, Lopes M, Carrola J (2016). A multiple index integrating different levels of organization. Ecotoxicology and Environmental Safety 132:270-278. |
|
|
Crespo D, Dolbeth M, Leston S, Sousa R, Pardal MÂ (2015). Distribution of Corbicula fluminea (Müller, 1774) in the invaded range: a geographic approach with notes on species traits variability. Biological Invasions 17(7):2087-2101. |
|
|
Cullen P (1990). Biomonitoring and environmental management. Environmental Monitoring and Assessment 14(2-3):107-114. |
|
|
De Castro-Català N, Muñoz I, Armendáriz L, Campos B, Barceló D, López-Doval J, Riera JL (2015). Invertebrate community responses to emerging water pollutants in Iberian river basins. Science of the Total Environment 503:142-150. |
|
|
Doherty VF, Otitoloju AA (2016). Occurrence and distribution of monocyclic aromatic hydrocarbons (BTEX) and the impact on macrobenthic community structure in Lagos lagoon, Nigeria. Environmental Monitoring and Assessment 188(10):571-587. |
|
|
Dong S, Lei Y, Li T, Jian Z (2020). Response of benthic foraminifera to pH changes: Community structure and morphological transformation studies from a microcosm experiment. Marine Micropaleontology 156:101819. |
|
|
Don-Pedro KN, Oyewo EO, Otitoloju AA (2004). Trend of heavy metal concentrations in Lagos lagoon ecosystem, Nigeria. West African Journal of Applied Ecology 5(1):103-114. |
|
|
Edokpay CA, Nkwoji JA (2007). Annual changes in the physico-chemical and macroinvertebrates characteristics of the Lagos lagoon sewage dump site at Iddo, Southern Nigeria Ecology, Environment and Conservation 13(1):13-18. |
|
|
Ekaete AG (2014). Oxidative stress in fish living in coastal water polluted with sawdust and wood waste along Lagos lagoon, Nigeria. Researcher 6(3):1-5. |
|
|
Elijah FB, Isa E (2015). Environmental Sustainability Impact of the Okobaba Sawmill Industry on some biogeochemistry characteristics of the Lagos Lagoon. Poultry, Fisheries and Wildlife Sciences pp. 1-19. |
|
|
Feld CK, De Bello F, Dolédec S (2014). Biodiversity of traits and species both show weak responses to hydromorphological alteration in lowland river macroinvertebrates. Freshwater Biology 59(2):233-248. |
|
|
Gamito S (2010). Caution is needed when applying Margalef diversity index. Ecological Indicators 10(2):550-551. |
|
|
Gerhardt A (1999). Biomonitoring for the 21st century. In Biomonitoring of polluted water. Reviews on actual topics. Environmental Research Forum 9:1-12. |
|
|
Ghosh D, Biswas JK (2015). Macroinvertebrate diversity indices: A quantitative bioassessment of ecological health status of an oxbow lake in Eastern India. Journal of Advances in Environmental Health Research 3(2):78-90. |
|
|
Gorelick R (2006). Combining richness and abundance into a single diversity index using matrix analogues of Shannon's and Simpson's indices. Ecography 29(4):525-530. |
|
|
Hartmann JT, Beggel S, Auerswald K, Stoeckle BC, Geist J (2016). Establishing mussel behavior as a biomarker in ecotoxicology. Aquatic Toxicology 170:279-288. |
|
|
Hasenbein S, Lawler SP, Geist J, Connon RE (2016). A long?term assessment of pesticide mixture effects on aquatic invertebrate communities. Environmental Toxicology and Chemistry 35(1):218-232. |
|
|
Hellawell JM (Ed.) (2012). Biological indicators of freshwater pollution and environmental management. Springer, Netherlands, Science and Business Media 546 p. |
|
|
Hering D, Feld CK, Moog O, Ofenbo T (2006). Cook book for the development of a multimetric index for biological condition of aquatic ecosystems: experiences from the European AQEM and STAR projects and related initiatives. Hydrobiologia 566:311-324. |
|
|
Huh MK (2019). Community Analysis and Water Quality of Benthic Macroinvertebrates at Gwangseok stream in Korea. European Journal of Engineering Research and Science 4(1)97-100. |
|
|
Hurlbert SH (1971). The nonconcept of species diversity: a critique and alternative parameters. Ecology 52(4):577-586. |
|
|
Karrouch L, Chahlaoui A, Essahale A (2017). Anthropogenic impacts on the distribution and biodiversity of benthic macroinvertebrates and water quality of the Boufekrane River, Meknes, Morocco. Journal of Geoscience and Environment Protection 5(07):173. |
|
|
Kiljunen M, Peltonen H, Lehtiniemi M, Uusitalo L, Sinisalo T, Norkko J, Karjalainen J (2020). Benthic?pelagic coupling and trophic relationships in northern Baltic Sea food webs. Limnology and Oceanography 65(8):1706-1722. |
|
|
Kumar N, Krishnani KK, Meena KK, Gupta SK, Singh NP (2017). Oxidative and cellular metabolic stress of Oreochromis mossambicus as biomarkers indicators of trace element contaminants. Chemosphere 171:265-274. |
|
|
Lawson EO (2011). Physico-chemical parameters and heavy metal contents of water from the Mangrove Swamps of Lagos Lagoon, Lagos, Nigeria. Advances in Biological Research 5(1):8-21. |
|
|
Li L, Zheng B, Liu L (2010). Biomonitoring and bioindicators used for river ecosystems: definitions, approaches and trends. Procedia Environmental Sciences 2:1510-1524. |
|
|
Liao CM, Lin CM, Jou LJ, Chiang KC (2007). Linking valve closure behavior and sodium transport mechanism in freshwater clam Corbicula fluminea in response to copper. Environmental Pollution 147(3):656-667. |
|
|
Lopes I, Baird DJ, Ribeiro R (2004). Avoidance of copper contamination by field populations of Daphnia longispina. Environmental Toxicology and Chemistry: An International Journal 23(7):1702-1708. |
|
|
Magurran AE, McGill BJ (Eds.). (2011). Biological diversity: frontiers in measurement and assessment. Oxford University Press 344 p. |
|
|
Margalef R (1958). Information theory in ecology. General Systems 3:36-71. |
|
|
Markert B, Breure T, Zechmeister H (Eds.) (2003). Bioindicators and biomonitors - principles, concepts and applications. Amsterdam: Elsevier 1017 p. |
|
|
Masese FO, Omukoto JO, Nyakeya K (2013). Biomonitoring as a prerequisite for sustainable water resources: a review of current status, opportunities and challenges to scaling up in East Africa. Ecohydrology and Hydrobiology 13(3):173-191. |
|
|
McCarthy JF, Shugart LR (1990). Biological markers of environmental contamination. In: McCarthy, JF., Shugart, L.R. (Eds.), Biomarkers of Environmental Contamination. Lewis Publishers, Boca Raton 457 p. |
|
|
Metcalfe JL (1989). Biological Water Quality Assessment of Running Waters Based on Macroinvertebrate Communities: History and Present Status in Europe. Environmental Pollution 60:101-139. |
|
|
Metcalfe?Smith JL (1994). Biological water?quality assessment of rivers: use of macroinvertebrate communities. The rivers handbook: Hydrological and Ecological Principles, pp. 144-170. |
|
|
Müller WE, Müller IM (2018). Sponge cells and tissue as biological monitors of aquatic pollution. In Microscale Testing in Aquatic Toxicology (pp. 97-112). CRC Press. |
|
|
Muzón J, Ramos LS, del Palacio A (2019). Urban Aquatic Insects. in Aquatic Insects (pp. 349-364). Springer, Cham. |
|
|
Niklaus PA, Kandeler E, Leadley PW, Schmid B, Tscherko D, Korner C (2001). A link between plant diversity, elevated CO2 and soil nitrate. Oecologia 127:540-548. |
|
|
Nkwoji JA, Yakub AS, Abiodun AO, Bello BO (2016). Hydrochemistry and community structure of benthic macroinvertebrates in Ilaje coastal waters, Ondo State, Nigeria Regional Studies in Marine Science 8:7-13. |
|
|
Nkwoji JA (2017). The Impacts of Organic Pollution on the Hydrochemistry and Community Structure of Benthic Macrofauna of Lagos Lagoon, Southwest Nigeria. Journal of Applied Sciences and Environmental Management 21(2):225-233. |
|
|
Nkwoji JA, Awodeyi SI (2018). Impacts of sediment mining on the hydrochemistry and macrozoobenthos community in a coastal lagoon, Lagos, Nigeria. Archives of Agriculture and Environmental Science 3(3):209-215. |
|
|
Nkwoji JA, Ugbana SI, Ina-Salwany MY (2020). Impacts of land-based pollutants on water chemistry and benthic macroinvertebrates community in a coastal lagoon, Lagos, Nigeria, Scientific African, https://doi.org/10.1016/j.sciaf.2019.e00220. |
|
|
Oertel N, Salánki J (2003). Biomonitoring and bioindicators in aquatic ecosystems. In Modern trends in applied aquatic ecology (pp. 219-246). Springer, Boston, MA. |
|
|
Ogunwenmo CA, Okuonghae O, Olofin I, Chilaka G (2005). The macrobenthic fauna at two point sources of pollution in estuarine Lagos lagoon of southwestern Nigeria. Journal of environmental biology 26(2 Suppl):369-374. |
|
|
Okoye BC (1991). Nutrients and selected chemical analysis in the Lagos Lagoon surface waters. International Journal of Environmental Studies 38(2-3):131-135. |
|
|
Okoye BC, Afolabi OA, Ajao EA (1991). Heavy metals in the Lagos Lagoon sediments. International Journal of Environmental Studies 37(1-2):35-41. |
|
|
Olafisoye OB, Senkale TM, Osibote AO (2016). Determination of the Level of Pesticides in Sediment and Water from the Lagos Lagoon. Journal of Advanced Agricultural Technologies 3(3). |
|
|
Olakolu FC, Chukwuka AV (2014). Trace metal concentrations and antioxidant activity in ovarian tissue of blue crab Callinectes amnicola from Lagos lagoon and implications for reproductive success. Zoology and Ecology 24(3):278-284. |
|
|
Olatunji AS, Abimbola AF (2010). Geochemical evaluation of the Lagos Lagoon sediments and water. World Applied Sciences Journal 9(2):178-193. |
|
|
Olayinka KO, Oladosu NO, Abayomi AA, Alo BI (2016). Assessment of nitrogen and phosphorus loading by atmospheric dry deposition to the Lagos Lagoon, Nigeria. Environmental Monitoring and Assessment 188(7):423. |
|
|
Parmar TK, Rawtani D, Agrawal YK (2016). Bioindicators: the natural indicator of environmental pollution. Frontiers in Life Science 9(2):110-118. |
|
|
Prabhakaran K, Nagarajan R, Franco FM, Kumar AA (2017). Biomonitoring of Malaysian aquatic environments: A review of status and prospects. Ecohydrology and Hydrobiology 17(2):134-147. |
|
|
Reboredo-Fernández A, Prado-Merini Ó, García-Bernadal T, Gómez-Couso H, Ares-Mazás E (2014). Benthic macroinvertebrate communities as aquatic bioindicators of contamination by Giardia and Cryptosporidium. Parasitology Research 113(5):1625-1628. |
|
|
Rosenberg DM (1998). A national aquatic ecosystem health program for Canada: We should go against the flow. Bulletin - Entomological Society of Canada 30(4):144-152. |
|
|
Sarma P, Das D (2015). Application of Shannon's Index to Study Diversity with Reference to Census Data of Assam. Asian Journal of Management Research 5(4):620-628. |
|
|
Sax DF (2002), Equal diversity in disparate species assemblages: a comparison of native and exotic woodlands in California. Global Ecology and Biogeography 11(1):49-57. |
|
|
Schlenk D, Handy R, Steinert S, Depledge M, Benson W (2008). Biomarkers. In: Di giulio, R.T., Hinton, D.E. (Eds.), The Toxicology of Fishes. CRC Press, Boca Raton, FL. pp. 683-715. |
|
|
Schöne BR, Krause Jr. RA (2016). Retrospective environmental biomonitoring-Mussel Watch expanded. Global and Planetary Change 144:228-251. |
|
|
Shannon CE (1948). A mathematical theory of communication. The Bell System Technical Journal 27(3):379-423. |
|
|
Sogbanmu TO, Nagy E, Phillips DH, Arlt VM, Otitoloju AA, Bury NR (2016). Lagos lagoon sediment organic extracts and polycyclic aromatic hydrocarbons induce embryotoxic, teratogenic and genotoxic effects in Danio rerio (zebrafish) embryos. Environmental Science and Pollution Research 23(14):14489-14501. |
|
|
Solimini AG, Ptacnik R, Cardoso AC (2009). Towards holistic assessment of the functioning of ecosystems under the Water Framework Directive. TrAC Trends in Analytical Chemistry 28(2):143-149. |
|
|
Tandon P, Abrol YP, Kumaria S (Eds.) (2007). Biodiversity and its significance. IK International Pvt Limited. |
|
|
Tsygankov VY, Boyarova MD, Lukyanova ON, Khristoforova NK (2017). Bioindicators of organochlorine pesticides in the Sea of Okhotsk and the Western Bering Sea.Archives of Environmental Contamination and Toxicology 73(2):176-184. |
|
|
Usese AI, Chukwu OL, Moruf OR, Lawal-Are AO (2018). Biomarker Responses to Environmental Stressors in the Hairy Mangrove Crab, Sesarma huzardii (Graspidae) from a Tropical Lagoon Mudflat in Nigeria. Alexandria Journal for Veterinary Sciences 57(1):4-10. |
|
|
Waykar B, Satish S, Gajanan D (2011). Bioaccumulation of metal in freshwater Pelecypod Molluscs under experimental condition. The Bioscan 6(4):537-542. |
|
|
Webb JE (1958). The ecology of Lagos Lagoon I. The lagoons of the Guinea coast. Philosophical Transactions of the Royal Society of London B. 241(683):307-318. |
|
|
Whittaker RH (1972). Evolution and measurement of species diversity. Taxon 21(2-3):213-251. |
|
|
Williams AB (2013). Pesticide contamination in muscle tissues of croaker fishes from Lagos lagoon, Nigeria. Transnational Journal of Science and Technology 3(1):71-83. |
|
|
Zhou Q, Zhang J, Fu J, Shi J, Jiang G (2008). Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Analytica Chimica Acta 606(2):135-150. |
|
|
Zuykov M, Pelletier E, Harper DA (2013). Bivalve mollusks in metal pollution studies: from bioaccumulation to biomonitoring. Chemosphere 93(2):201-208. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0