Full Length Research Paper
ABSTRACT
Produced water (PW) during oil and gas production operations contains various hazardous substances including heavy metals (HM) with adverse impact on the environment. Disposal of PW interferes with environmental sustainability, making PW treatment obligatory prior to discharge into the environment. Among previously available treatments of PW, environmentally sustainable methods using low-pore space bio-adsorbents require further development. This study investigated the efficacy of chemically-modified activated carbon (cMAC) of Luffa cylindrica (LC) and Banana Peel (BP) for the treatment of PW obtained from Niger-Delta oilfield, treated (2, 4, and 6 h) with finely ground (425 μm) carbonized LC and BP impregnated separately with phosphoric-acid and sodium hydroxide. The derived cMAC was characterized by proximate analysis and FTIR spectroscopy. Treated PW was analysed for HM using AAS, Langmuir, and Freundlich isotherms. Obtained values of the surface area for the cMAC from LC were 880 (NaOH), 830 (H3PO4) m2/g and BP was 810 (NaOH), and 920 (H3PO4) m2/g. The existence of active functional groups is revealed on the FTIR spectra. Results revealed a substantial drop in HM concentrations (Zn: 30-86%, Cu: 78-88%, Ni: 33-55%, Fe: 17-52%) in PW after treatment with cMAC at varying contact times. All metals (Zn, Cu, Ni) in the treated PW except Fe were below WHO and USEPA guideline limits. Treatment of Niger Delta oilfield PW was effectively improved with acid-modified carbonized L. cylindrica.
Key words: Activated carbon, adsorption, bio-adsorbent, heavy metals, oil-produced water, treatment
Abbreviation: AAS, Atomic absorption spectrophotometer; BP, banana peel; CBP, carbonized banana peel; CBPN, sodium hydroxide impregnated carbonized banana peel; CBPP, phosphoric acid impregnated carbonized banana peel; CLC, carbonized Luffa cylindrical; CLCN, sodium hydroxide impregnated carbonized Luffa cylindrical; CLCP, phosphoric acid impregnated carbonized Luffa cylindrical; Cu, copper; cMAC, chemically modified activated carbon; Fe, iron; FTIR, Fourier transform infrared; HM, heavy metals; LC, Luffa cylindrical; MBP, modified banana peel; MLC, modified Luffa cylindrical; Ni, nickel; PW, produced water; TDS, total dissolved solids; TSS, total suspended solids; UN, United Nations; Zn, zinc.INTRODUCTION
Petroleum is produced with large volumes of water (Igunnu and Chen, 2014; Nonato et al., 2018; Gbadegesin et al., 2022) typically called Produced Water (PW). Thus, the petroleum industry is characterized by prolific wastewater generation during extraction and development activities (Beech, 2006; Udeagbara et al.,2021). Produce water constitutes the largest volume wastewater stream from oil field operations and contains large amounts of salt, scale products, and hazardous substances including polycyclic aromatic hydrocarbons (PAH), benzene, toluene, ethylbenzene, and xylene (BTEX), radioactive materials, heavy metals (HM) and others resulting in the contamination of the environment (Neff et al., 1992; Igunnu and Chen, 2014; Olajire, 2020; Udeagbara et al., 2021). Among these contaminants, heavy metals are of particular interest because of their non-biodegradability, long-term persistence in the environment, and adverse impacts on human and environmental health (Kostecka et al., 2014; Nowicki et al., 2016; Ani et al., 2019; Briffa et al., 2020). Hence, PW during oil and gas extraction and development operations contain various hazardous substances including HM with adverse impact on the environment.
In Nigeria, PW is a major environmental pollutant in areas where oil exploration and production activities are ongoing, the disposal of the PW interferes with environmental sustainability, making PW treatment mandatory prior to discharge into the environment. This has led to increase in research works on plausible treatment management options (Joel et al., 2010; Isehunwa and Onovae, 2011; Onyema et al., 2015; Ofili et al., 2015; Udeagbara et al., 2021). Whilst, many treatment technologies for the management of PW exists, efficient treatment methods that are cost-effective and environmentally sustainable remain a challenge (Nwosi-Anele et al., 2016; Nonato, 2018) and as such requires further development. Among previously available treatments of PW, environmentally sustainable methods using low-pore space bio-adsorbents require further development. Current research efforts on cost-effective methods for the removal of toxic HM and other contaminants have received increased attention (Rasheed et al., 2017; Aclione et al., 2018; Oumam et al., 2020; Udeagbara et al., 2021; Gbadegesin et al., 2022).
Currently, appropriate management strategies are not only necessary for the treatment of PW to meet specified limits of water quality required by regulatory agencies for safe discharge and/or reuse (Nwosi-Anele et al., 2016; Olajire, 2020) but also to achieve relevant Sustainable Development Goals (Clean water and sanitation, Climate Action, Life below water, and Life on land) strategic to environmental sustainability and water use (UN, 2015). In particular, a major current and future priority of PW management should be directed to developing an eco-friendly and cost-effective technology with zero pollutant discharge (Olajire, 2020).
One way to achieve the aforementioned is by treating PW using bio-adsorbents made from natural organic materials, such as agricultural wastes or other raw materials which are readily available and low-cost. Some previous studies have evaluated the effectiveness of bio-adsorbents for the treatment of PW. Recently, Udeagbara et al. (2021) reported 18 to 100% removal of HMs from PW using non-carbonized bio-adsorbents produced from organic materials. Furthermore, other studies have demonstrated improved removal efficiency of various contaminants in PW using bio-adsorbents processed into activated carbon. Studies investigating possible treatments of PW from Niger Delta oilfields have recently reported improved removal efficiency for crude oil (50 = 74%) (Akinsete and Araoye, 2021) and HM (59 - 80%) (Popoola et al., 2022) using activated carbon of bio-adsorbents. The removal efficiency of contaminants, especially HM can be further enhanced through the modification of activated carbon using activating agents such as phosphoric acid, nitric acid, hydrochloric acid, zinc chloride, sulphuric acid, potassium hydroxide, and sodium hydroxide (Jankowska et al., 1991; Kumar and Namasivayam, 2009; Reffas et al., 2010; Li et al., 2015; Rahman et al., 2015; Ani et al., 2020; Zi?zio et al., 2020). The increased removal efficiency of 61 to 83% of crude oil from PW using phosphoric acid-modified activated carbon has been demonstrated (Akinsete and Araoye, 2021).
Organic materials such as banana peel, a readily available agro-waste, and sponge gourd (Luffa cylindrica), a fast-growing vine that produces an inedible sponge-like structure when mature are promising candidates for oilfield PW treatment. Many organic materials do not only serve as raw materials but have the advantages of availability, low-cost, and can be readily processed into activated carbon (Zi?zio et al., 2020).
Efficient treatment technology that is cost-effective for PW treatment to meet regulatory standards prior to discharge and/or reuse has remained a big challenge for the oil and gas industry (Olajire, 2020). Therefore, this comparative study was conducted to assist in the identification of an efficient oilfield PW treatment technology. The study adopted the adsorption technology using non-carbonized bio-adsorbent and activated carbons (AC) prepared from L. cylindrica and banana peel and modified with two activating agents (H3PO4 and NaOH) for the removal of HMs (Cu, Fe, Ni, and Zn) and other contaminants from oilfield PW at varying time intervals.
MATERIALS AND METHODS
The PW sample (Figure 1a) was collected from the Gbetiokun oilfield of Niger Delta, Nigeria. Banana peels (Figure 1b) and dried mature fruit of L. cylindrica (Figure 1c) were sourced from a local market in Ibadan, Oyo State, Nigeria. All reagents used were of high analytical grade. The pH was attuned (from red colour) to desired values (blue colour) using NaOH pellets.
Preparation of adsorbents
This study investigated the efficiency of chemically-modified activated carbon (CMAC) of Banana Peel (BP) and L. cylindrical (LC) for the treatment of PW.
Pre-treatment of banana peels and L. cylindrica
Banana peels (BP) and L. cylindrica (LC) were thoroughly washed with distilled water to remove dirt, cut into pieces, and air-dried for 7 days. Excess water was removed by oven-drying (100°C) for 5 h (BP) and 3 h (LC) owing to the difference in their texture. Completely dried samples of BP (ground and sieved) and LC (cut into small pieces only due to very light texture) were stored in airtight containers.
Modified bio-adsorbent
The non-carbonized bio-adsorbent of the BP and LC was pre-treated with sodium hydroxide (1.25 M NaOH) by immersing 20 g of non-carbonized bio-adsorbent in 50 mL NaOH solution at room temperature for 24 h. The alkali solution was drained out and the adsorbents were thoroughly washed with distilled water (to remove NaOH) until pH 7 was attained. The obtained adsorbent samples (MBP and MLC) were sun-dried (6 h), then oven-dried (2 h), and stored in air-tight containers.
Production of activated carbon
The ground BP and LC samples were carbonized (Figure 2) in a muffle furnace (Heraeus RO/ROF Tube Furnace) in a steady flow of nitrogen gas (N2), at a temperature of 450°C for 1 and 0.5 h, respectively. The carbonized samples were left to cool down to room temperature. Chemical activation of the carbonized samples (CBP and CLC) was achieved by impregnation using phosphoric acid (H3PO4; CBPP and CLCP) and sodium hydroxide (NaOH; CBPN and CLCN) at a ratio of 1:3 for 24 h. Briefly, 40 g of activated carbon was placed in a beaker and 120 mL of the activating agent was added. After the 24 h impregnation process, the CBP and CLC were filtered and washed repeatedly with water purified by distillation (for acid and alkali removal from the pore spaces) to obtain a neutral pH. The washed chemically activated carbons of BP (CBPP and CBPN) and LC (CLCP and CLCN) were dried at 200°C for 5 and 2 h, respectively.
Characterization of activated carbons and produced water
Activated carbons were characterized for moisture content, ash content, and volatile materials using methods described by Oladimeji et al. (2021). Total and fixed carbon was determined by the method described by Isehunwa and Onovae (2011), while the bulk density and surface area were determined according to the methods described by Sugumaran et al. (2012). Additionally, the functional groups of activated carbons were determined by Fourier transform infrared spectroscopy (FTIR; PerkinElmer Spectrum BXII) which is an essential technique for determining the chemical properties of activated carbon (Rahman et al., 2015).
Moreover, the PW (untreated and treated) was characterized for total dissolved solids (TDS) and total suspended solids (TSS) according to Oladimeji et al. (2021). The concentrations of heavy metals (HM) in PW (untreated and treated) were determined using atomic absorption spectrophotometer (AAS; PerkinElmer Analyst 200).
The effect of contact time was determined by adding 1 g of activated carbon sample to 50 mL of distilled water in a bottle and placed on a mechanical shaker for different contact times (2, 4, and 6 h). After shaking, the samples were filtered and HM, TDS, and TSS were determined in the filtrate.

Adsorption batch experiments
The batch experiments for adsorption equilibrium were carried out to estimate the adsorption isotherms of metal ions onto the surface of the adsorbents used in this study. Equation 1 gives the quantity of adsorbed metal ion at time t:
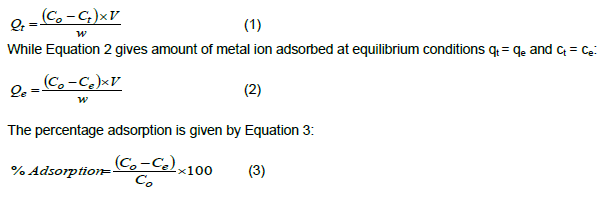
Adsorption isotherms
In this study, equilibrium isotherms were used to describe the experimental bio-sorption data. The equations of Langmuir and Freundlich are the most frequently used to describe experimental data of adsorption isotherms. The parameters and assumptions of these equilibrium isotherms continually provide certain acumen into the sorption mechanism, the surface properties, and the affinity of the sorbent (Langmuir, 1917).
The Langmuir isotherm
This Langmuir isotherm correlates the amount of extracted material to its equilibrium concentration in the majority of solutions. Langmuir (1971) showed that the model is only acceptable for monolayer adsorption. Langmuir isotherm model (Equation 4) assumes uniform adsorption on the surface and transmigration in the plane of the surface.
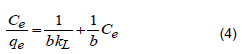
where qe signifies adsorption capacity at equilibrium in mg/g, Ce is the equilibrium concentration (mg/L), kL is the Langmuir constant in mL/mg and b is the adsorption capacity of the substrate (gram solute/gram adsorbent). Plotting Ce/qe against Ce gives a straight line of slope 1/b and an intercept 1/bkL that is equal to homogenous and the adsorption energies to be equivalent for each adsorption site. The key features of the Langmuir isotherm given in a dimensionless equilibrium parameter RL are defined by Equation 5:

where Co is the original solute concentration, b is the Langmuir’s adsorption constant (L/mg) and RL value gives the grouping of the isotherm to be either unfavorable (RL>1), linear (RL =1), favorable (0< RL <1) or irreversible (RL = 0) (El-Nafaty et al., 2013).
The Freundlich Isotherm
This is an empirical model describing the adsorption process occurring on multilayer (that is, heterogeneous) surfaces (Freundlich, 1906); the model (Equation 6) suggests that adsorption capacity is correlated to the adsorbent concentration (Udegbara et al., 2021).
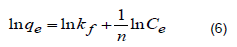
where qe represents the equilibrium concentration (mg/g), Ce denotes equilibrium concentration in solution (mg/L), kf signifies the Freundlich adsorption isotherm constant and 1/n indicates the heterogeneity of the data distribution which is correlated to the degree of the adsorption driving force (Konggidinata et al., 2017).
RESULTS AND DISCUSSION
Adsorbent characterization
Chapter One
Characteristics of the adsorbent after the activation with H3PO4 and NaOH are shown in Table 1. The total ash content ranging from 2.8 to 3.9% indicates that the produced adsorbents contained high amounts of organic materials. Additionally, the high carbon content indicated that the biomass used in this study could serve as a good bio-adsorbent in adsorption studies. The bulk density of the chemically activated carbons (0.22 – 0.32 g/cm3) are similar to the values obtained for phosphoric acid impregnated carbonized plantain stem (0.32 g/cm3) (Ekpete et al., 2017) but slightly lower than (0.39-0.56 g/cm3) phosphoric acid impregnated carbonized spent coffee grounds (Zi?zio et al., 2020). The obtained values of the surface area for the chemically activated carbons from BP were 810.0 (NaOH) and 920.0 (H3PO4) m2/g and from LC were 880.0 (NaOH) and 830.0 (H3PO4) m2/g.
The large surface area exhibited by the chemically activated carbons in this study is higher than (Ramutshatsha-Makhwedzha et al., 2022) but comparable (Zi?zo et al., 2020) to those reported in the literature for activated carbons using similar activation agents. Generally, a larger surface area indicates better-developed porosity for the increased adsorption process (Zi?zio et al., 2020; Udeagbara et al., 2021).

FTIR spectroscopy analysis of adsorbents
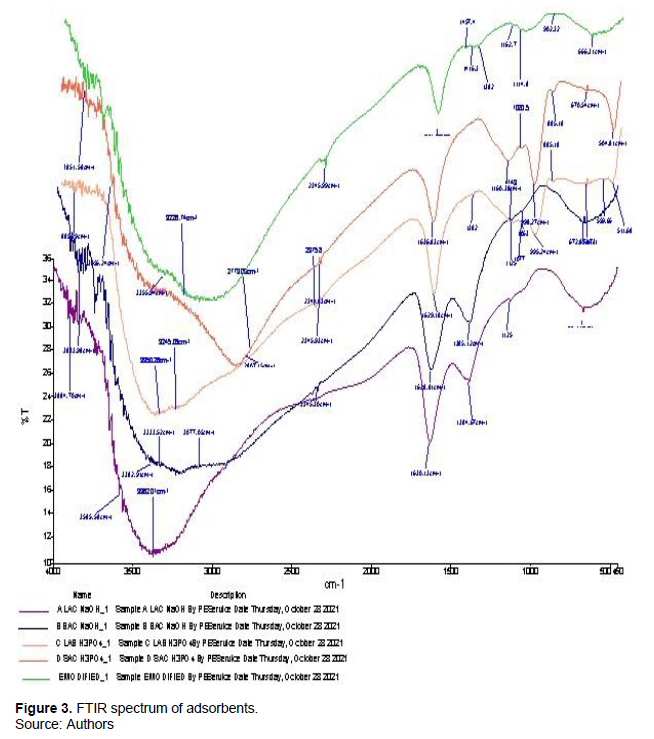
The Fourier Transform Infrared (FTIR) spectra of the chemically activated carbons, recorded between 4000 and 400 cm-1 are as shown in Figure 3. Varying absorption peaks were displayed on the spectra of the activated carbons, suggesting the presence of active functional groups that will increase the removal of HM from PW (Popoola et al., 2022). In this study, all activated carbons revealed common board peaks from 1628.21 to 1637.94 cm-1 corresponding to the C=O stretch of amine (Ekpete et al., 2017). The defined peaks ranging from 3833.98 to 3885.29 cm-3 were assigned to the O–H stretch of alcohol. This range is similar to values previously reported for activated carbons from banana waste (Sugumaran et al., 2012). The presence of the hydroxyl (OH) group observed in all the activated carbons has been reported to be important for the adsorption of metals (Ramutshatsha-Makhwedzha et al., 2022). The bands observed around 1053 and 1080 cm-1 can be associated with C–O stretching vibrations of carboxylic groups. Similar FTIR spectra were observed for the different activating agents regardless of the carbonized material. Sodium hydroxide impregnated activated carbons had similar bands between the regions of 550 and 3500 cm-1, while the bands between 500 and 2800 cm-1 were similar for phosphoric acid impregnated activated carbons. Additionally, the phosphoric acid activated carbons displayed pronounced bands within the region of 902.32 to 995.24 cm-1 corresponding to the P–H band of phosphine, which is absent when the activating agent was sodium hydroxide.
Produced water treatment
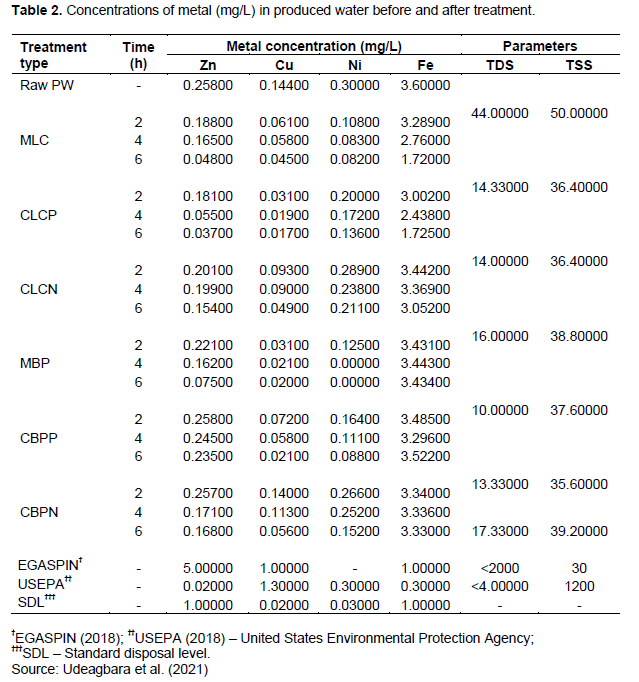
Table 2 shows the adsorption of heavy metals; Zinc (Zn), Nickel (Ni), Iron (Fe), and Copper (Cu) from produced water using the prepared activated carbons. The PW used in this study contained higher concentrations of HMs than reported in recent studies from the Niger Delta area of Nigeria (Udeagbara et al., 2021; Popoola et al., 2022). The results obtained demonstrated the efficiency of the bio-adsorbent in removing heavy metals. The results revealed a substantial decrease of heavy metal concentrations present in produced water after treatment with the prepared activated carbons at varying contact times. The pattern of removal efficiency of HM was 2 < 4 < 6 h, indicating the highest removal efficiency was achieved at 6 h (Figure 4). This confirms that increasing contact time improves the effectiveness of HM removal by bio-adsorbents (Udeagbara et al., 2021). At 6 h, higher Zn (86%) and Cu (88%) were removed via the phosphoric acid impregnated LC activated carbon compared to corresponding 9 and 85% for NaOH impregnated BP activated carbon. Overall, CLCP was most effective in the removal of all HMs compared with the other activated carbons. Only Ni was completely removed (100%) at 4 and 6 h using banana peel modified activated carbon (MBP), thus demonstrating the high adsorption capacity of Ni. Again, at 6 h, the most adsorbed HMs by the bio-adsorbents were Cu, corresponding to 61 to 88% removal, while the least was Fe corresponding to 2 to 52% removal. While all the prepared bio-adsorbents demonstrated effective removal of Cu, only those prepared from LC adsorbed Fe better. This confirms that locally prepared activated carbon has a strong affinity to removing heavy metals from PW at varying contact times (Popoola et al., 2022). At 6 h, the modified and chemically activated carbons are better adsorbents for Zn, Cu, and Ni when compared with non-carbonized adsorbents prepared from similar materials used in a related study (Udeagbara et al. 2021). All metals in the treated PW except Fe were below the guideline limits by the regulatory bodies (Table 2).

Analysis based on adsorption isotherms
To study the connection between adsorption capacity (qe) and aqueous concentration (Ce) at equilibrium, Langmuir’s adsorption isotherm reported by Naseem et al. (2019), Ramutshatsha-Makhwedzha et al. (2022) and Freundlich’s adsorption isotherm stated by Rasmey et al. (2018), Ramutshatsha-Makhwedzha et al. (2022) models were deployed to fit the equilibrium data in this study. Table 3 presents the correlation coefficient for the adsorbents using the Langmuir and Freundlich isotherm models. Results of the dimensionless equilibrium parameter (RL) revealed that the sorption of Zn, Cu, Ni, and Fe on the adsorbents is satisfactorily good as values fall between the favorable range of 0<RL<1. The adsorption of Zinc by L. cylindrica (LC) favored multilayer as agreed by Freundlich isotherm, while adsorption of Zinc by Banana Peel (BP) favored monolayer as agreed by Langmuir. Results also showed that the Langmuir isotherm is good for the equilibrium study of the adsorption of Copper by L. cylindrica and banana peel, which suggests homogeneous treatment of the adsorbate on the adsorbent surface for the Copper ion. Nickel and Iron give the Freundlich-type model a better result than the Langmuir model, which gives an indication of multilayer coverage of the adsorbate on the adsorbent surface for the ions.
CONCLUSION
Modified and chemically activated carbons were prepared from banana peel and L. cylindrica for the treatment of produced water at different contact times. All prepared activated carbons were characterized by large surface area and the presence of active functional groups was revealed on the FTIR spectra. The bio-adsorbents showed improved surface area.
The efficiency of prepared activated carbons at varying contact times, indicating the highest HMs removal was achieved at 6 h (2 < 4 < 6 h). Overall, phosphoric acid impregnated activated carbon of L. cylindrica was most effective in the removal of all heavy metals compared with the other activated carbons. Copper was the most adsorbed heavy metal by the bio-adsorbents while the least adsorbed was iron. While all the prepared bio-adsorbents demonstrated effective removal of copper, only those prepared from L. cylindrica adsorbed iron better.
Results further showed that Langmuir isotherm gave the best fit for Copper and Zinc, while Freundlich isotherm gave the best fit for Iron and Nickel for L. cylindrica while Freundlich isotherm gave the best fit for Iron and Copper and Langmuir isotherm gave the best fit for Nickel and Zinc for Banana Peel. Treatment of Niger Delta oilfields PW was effectively improved with acid-modified L. cylindrica activated carbon.
The prepared activated carbons have the ability to effectively treat produced water contaminated with Cu, Zn, and Ni. The efficiency of heavy metal removal demonstrated in this study confirms that locally prepared activated carbon enhanced with activating agents is an environmentally sustainable method for the treatment of produced water from the oil industry.
ABBREVIATIONS
AAS, Atomic absorption spectrophotometer; BP, banana peel; CBP, carbonized banana peel; CBPN, sodium hydroxide impregnated carbonized banana peel; CBPP, phosphoric acid impregnated carbonized banana peel; CLC, carbonized Luffa cylindrical; CLCN, sodium hydroxide impregnated carbonized Luffa cylindrical; CLCP, phosphoric acid impregnated carbonized Luffa cylindrical; Cu, copper; cMAC, chemically modified activated carbon; Fe, iron; FTIR, Fourier transform infrared; HM, heavy metals; LC, Luffa cylindrical; MBP, modified banana peel; MLC, modified Luffa cylindrical; Ni, nickel; PW, produced water; TDS, total dissolved solids; TSS, total suspended solids; UN, United Nations; Zn, zinc.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Aclione A, Thyara CN, Mauricio I, Ramon L (2018). Produced Water from oil - A Review of the Main Treatment Technologies. Journal of Environmental Chemistry 2(1):23-27. |
|
|
Akinsete OO, Araoye AO (2021). Adsorption of Crude Oil Spill from Aqueous Solution using Agro-Wastes as Adsorbents. Journal of Scientific Research and Reports 27(4):27-52. |
|
|
Ani JU, Akpomie KG, Okoro UC, Aneke LE, Onukwuli OD, Ujam OT (2020). Potentials of activated carbon produced from biomass materials for sequestration of dyes, heavy metals, and crude oil components from aqueous environment. Applied Water Science 69(10):1-11. |
|
|
Beech SJ (2006) Oil removal for produced water treatment and micellar cleaning of ultrafiltration membranes. Ph.D. dissertation, Texas A&M University. |
|
|
Briffa J, Sinagra E, Blundell R (2020). Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6(9):e04691. |
|
|
EGASPIN (2018). In: Environmental Guidelines and Standards for the Petroleum Industry in Nigeria, third ed. Department of Petroleum Resources. |
|
|
Ekpete OA, Marcus AC, Osi V (2017). Preparation and characterization of activated carbon obtained from plantain (Musa paradisiaca) fruit stem. Hindawi Journal of Chemistry 6 p. |
|
|
El-Nafaty UA, Muhammad IM, Abdulsalam S (2013). Biosorption and kinetic studies on oil removal from produced water using banana peel. Journal of Civil Environmental Research 3(7):125-136. |
|
|
Freundlich HMF (1906). Over the adsorption in solution. Journal of Physical Chemistry 57(385471):1100-1107. |
|
|
Gbadegesin A, Fadairo A, Ling K, Rasouli V, Ogunkunle T, Ayoo J, Olowu A (2022). Evaluating the Performance of Natural Organic Sorbent for Produced Water Treatment. In: 46th SPE Nigeria Annual International Conference and Exhibition. |
|
|
Igunnu ET, Chen GZ (2014). Produced water treatment technologies. International Journal of Low-Carbon Technologies 9:157-177. |
|
|
Isehunwa SO, Onovae S (2011). Evaluation of produced water discharge in the Niger Delta. ARPN Journal of Engineering and Applied Sciences 6(8):66-72. |
|
|
Jankowska H, Swiatkowski A, Choma J (1991). Active Carbon. Ellis Horwood, New York. |
|
|
Joel OF, Amajuoyi CA, Nwokoye CU (2010). Characterization of formation water constituents and the effect of freshwater dilution from land rig location of the Niger Delta, Nigeria. Journal of Applied Sciences and Environmental Management 14(2):37-41. |
|
|
Konggidinata MI, Chao B, Lian Q, Subramaniam RZM, Gang DD (2017). Equilibrium, kinetic and thermodynamic studies for adsorption of BTEX onto Ordered Mesoporous Carbon (OMC). Journal of Hazardous Materials 336:249-259. |
|
|
Kostecka J, Koc-Jurczyk J, Brudzisz K (2014). Waste management in Poland and European Union. Archiwum Gospodarki Odpadami I ochrony Srodowiska 16(1):1-10. |
|
|
Kumar R, Namasivayam C (2009). Development and characteristics of activated carbons from jatropha husk, an agro-industrial solid waste by chemical activation methods. Environmental Engineering and Management Journal 19(3):173-178. |
|
|
Langmuir I (1971). The constitution and fundamental properties of solids and liquids. II. Liquids. Journal of the American chemical Society 39(9):1848-1906. |
|
|
Li Y, Zhang X, Yang R, Li G, Hu C (2015). The role of H3PO4 in the preparation of activated carbon for NaOH-treated rice husk residue. RSC Advances 5(41):32626-32636. |
|
|
Naseem K, Begum R, Wu W, Usman M, Irfan A, Al-Sehemi AG, Farooqi ZH (2019). Adsorptive removal of heavy metal ions using polystyrene-poly (N-isopropylmethacrylami de-acrylic acid) core/shell gel particles: Adsorption isotherms and kinetic study. Journal of Molecular Liquid 277:522-531. |
|
|
Neff JM, Sauer TC, Maciolek N (1992). Composition, Fate, and Effects of Produced Water Discharge to Nearshore Marine Waters. In: Ray JP, Engelhardt FR (eds) Produced Water. Environmental Science Research, 46 Springer, Boston, MA. |
|
|
Nonato TCM, De A Alves AA, Sens ML, Delsasso RL (2018). Produced water from oil - A review of the main treatment technologies. Journal of Environmental Toxicology 2(1):23-27. |
|
|
Nowicki P, Kazmierczak-Razna J, Skibiszewska P, Wisniewska M, Nosal-Wiercinska A, Pietrzak R (2016). Production of activated carbons from biodegradable waste materials as an alternative way of their utilization. Adsorption 22(4):489-502. |
|
|
Nwosi-Anele AS, Iledare OO (2016). Produced Water Treatment Methods and Regulations: Lessons from the Gulf of Mexico and North Sea for Nigeria. American Journal of Engineering Research 5(12):46-57. |
|
|
Ofili O, Temisanren T, Olafuyi O (2015). A review of produced water management processes: A case study of a brownfield in Niger Delta. In: 39th SPE Nigeria Annual International Conference and Exhibition; 4-6 August 2015, Nigeria. |
|
|
Oladimeji TE, Odunoye BO, Elehinafe FB, Obanla OR, Odunlami OA (2021). Production of activated carbon from sawdust and its efficiency in the treatment of sewage water. Heliyon 7:e05960. |
|
|
Olajire AA (2020). Recent advances on the treatment technology of oil and gas produced water for sustainable energy industry-mechanistic aspects and process chemistry perspectives. Chemical Engineering Journal Advances 4:100049. |
|
|
Onyema HK, Iwuanyanwu JO, Emeghara GC (2015). Evaluation of Some Physicochemical Properties and Heavy Metals in Post-Treated Produced Water from Offshore Locations in the Niger Delta Area, Nigeria. Journal of Applied Sciences and Environmental Management 19(4):767-770. |
|
|
Oumam M, Abourriche A, Mansouri S, Mouiya M, Benhammou A, Abouliatim Y, Hafiane YE, Hannache H, Birot M, Pailler R, Naslain R (2020). Comparison of chemical and physical activation processes at obtaining adsorbents from Moroccan oil shale. Oil Shale 37(2):139-157. |
|
|
Popoola LT, Yusuff AS, Adeyi AA, Omotara OO (2022). Adsorptive removal of heavy metals from oil well produced water using citrullus lanatus peel: Characterization and optimization. South African Journal of Chemical Engineering 39(1):19-27. |
|
|
Rahman MM, Samsuddin SH, Miskon MF, Yunus K, Yusof AM (2015). Phosphoric acid activated carbon as borderline and soft metal ions scavenger. Green Chemistry Letters and Reviews 8(2):9-20. |
|
|
Ramutshatsha-Makhwedzha D, Mbaya R, Mavhungu ML (2022). Application of activated carbon banana peel coated with Al2O3-Chitosan for the adsorptive removal of lead and cadmium from wastewater. Materials 15:860. |
|
|
Rasheed A, Sana S, Kashif S, Umer Z, Khatoon M (2017). To evaluate the efficiency of char and biochar for wastewater treatment. Resources, Recycling, and Waste Management 2(2):7. |
|
|
Rasmey AH, Aboseidah AA, Youssef AK (2018). Application of Langmuir and Freundlich isotherm models on Biosorption of Pb2+ by Freezdried Biomass of Pseudomonas aeruginosa. Egyptian Journal of Microbiology 53(1):37-48. |
|
|
Reffas A, Bernardet V, David B, Reinerta L, Lehocine MB, Dubois M, Batisse N, Declaux L (2010). Carbon prepared from coffee grounds by H3PO4 activation: characterization and adsorption of methylene blue and Nylosan Red N-2RBL. Journal of Hazardous Material 175(1-3):779-788. |
|
|
Sugumaran P, Priya SV, Ravichandran P, Seshadri S (2012). Production and characterization of activated carbon from banana empty fruit bunch and delonix regia fruit pod. Journal of Sustainable Energy and Environment 3(3):125-132. |
|
|
Udeagbara SG, Isehunwa SO, Okereke NU, Oguamah IU (2021). Treatment of produced water from Niger Delta oil fields using a simultaneous mixture of local materials. Journal of Petroleum Exploration and Production 11(1):289-302. |
|
|
United Nations (UN) (2015). Transforming our world: The 2030 Agenda for Sustainable Development. New York: UN Publishing. |
|
|
USEPA (2018). Effluent guidelines planning review report supporting the final 2016 effluent guidelines program plan. Washington D.C. (January). EPA-821-R-18-002. EPA-HQ-OW-2015-0665. DCN 08318. |
|
|
Zi?zio M, Charmas B, Jedynak K, Hawryluk M, Kucio K (2020). Preparation and characterization of activated carbons obtained from the waste materials impregnated with phosphoric acid (V). Applied Nanoscience 10:4703-4716. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0