ABSTRACT
In Mali, studies on arbuscular mycorrhizal fungi (AMF) characterization and diversity are still few, despite the important roles played by these microorganisms in plant growth and in degraded soils restoration. Sorghum is one of the staple food grains in Mali and as such plays a key role in food security in Mali. This study aims to determine diversity of AMF strains associated to sorghum in Sikasso region soils in Mali. For this, soils were sampled using sorghum plants in different fields of this region, which served as substrates for trapping AMF. These soils have been studied separately. Results gotten revealed 21 AMF morphotypes left between 6 genera (Glomus, Scutellospora, Gigaspora, Acaulospora, Entrophospora, Sclerocystis) of three families (Glomeracea, Gigasporacea, Scutellosporacea). Four of these morphotypes have been identified.
Key words: Diversity, arbuscular mycorrhizal fungi (AMF), Sikasso, Mali.
In Sub-Saharan Africa, climatic changes associated with anthropogenic pressure, have led to disappearance of woody plant cover. This degradation seriously affects diversity and abundance of symbiotic soil microflora (Diouf and Sougoufara, 2002). However, these symbiotic microorganisms play a fundamental role in soil fertility restoration and maintenance, in protection against certain telluric pathogens and in mineral nutrition of plants. In this area, mycorrhizal fungi, especially those with arbuscular mycorrhizal fungi (AMF), have been shown to be effective. AMF are naturally present in soils of natural ecosystems or agro-ecosystems (Oehl et al., 2004) and has established a compulsory symbiosis with more than 200.000 cultivated plants (Smith and Read, 2008). They have been widely described in tropics (Lambers et al., 2006) and have improved plant growth by better removal and transfer of nutrients, especially phosphorus (Finlay, 2008). Phosphorus is one of the most important elements that significantly affect plants growth and functioning and can represent up to 0.2% of its dry weight (Smith et al., 2011).
The decrease in soil fertility and consequently that in sorghum yields 0.2 to 1.1 tons per hectare in the Sahelian zone could be optimized to 3 or even 4 tons with fertilization (Vaksmann et al., 2008; Dembélé et al., 2011). Inoculation of plants with performing strains AMF (Fitter, 2012) has been considered to increase plant yield and reduce the use of chemical fertilizers that pollute the environment (Mummey et al., 2009).
In Mali, studies driven on AMF characterization and diversity remains scarcely used for microbial ecology. To our knowledge, the first investigations in this area were carried out in a study in the Sudanian zone in Mali on the positive influence of the age of fallow where the total number of mycorrhizal spores per unit of soil was noted (Sidibé and Yossi, 1997). However, it must be recognized that these fungi biology is complex and their identification represents a challenge for plant symbiosis. Despite this, knowledge accumulated in morphology and anatomy terms would allow a variety of AMF to be identified.
In the country, sorghum is one of the staple food grains in Mali (Bazile et al., 2004). These cereals occupy 75% of cultivated areas and the share of sorghum in the production of dry cereals, which mobilizes around 80% of rural populations, was 35% in 2003 (CILSS, 2002; Sarr, 2010). Moreover, Mali is the second country in the world to meet most of its food needs with sorghum and millet (Bazile et al., 2008). The objective this study was to determine diversity of AMF strains associated to sorghum in Sikasso region soils, Mali.
Study area
The study was conducted in Sikasso region, located in extreme south of Mali between 12°30 ′ north latitude and 8°45 ′ west longitudes. It covers an area of ??71790 km2 or 5.8% of national territory. It has 7 circles (Sikasso, Bougouni, Kadiolo, Kolondieba, Koutiala, Yanfolila, Yorosso), 3 urban communes (Sikasso, Bougouni, Koutiala), 144 rural communes, including Kolondieba and 1831 villages. An average annual temperature of 27°C is observed between April and May and an average of 24°C between December and January. These sites belong to the humid Sudanian zone in the North between the 750 mm isohyets in the North and 1150 mm in the South and the Guinean zone between the 1150 mm isohyets in the North and 1400 mm in the South (CSA., 2007) and the soil fertility leached tropical ferruginous soils, eroded ferralitic soils and mineral hydromorphic soils in lowlands (MATCL., 2012). This makes the region "the granary" of the country. Agricultural productions are indeed important, such as cereals and fruits (notably mangos). Cotton, which is the 1st export product of the country, is chiefly developed (National Institute of Statistics, 2010).
Study sites
The study comprises 12 sites: Keleya, Solo, Koumantou, Chobougou in Bougouni circle, Sinsimba, Boye, Kébila, Samba in Kolondièba circle, Guélébougoula, Dougoukolobougou, Titiéna and Niéna in Sikasso circle (Figure 1).
Soil sampling
At each site, 1 kg of soil was sampled during the dry season of 2014 at eight different points in surface horizons (0 to 30 cm), with an auger of 5 cm diameter in a sorghum field and a composite soil sample was constituted for each field. The composite samples have been put in bags and these bags were labeled, and then taken to the laboratory.
Trapping of arbuscular mycorrhizal fungal under sorghum plants
Trapping method (Walker, 1992) was used for the AMF diversity study in the different sites. It consisted of growing sorghum in polyethylene pots of 2.23 cm3, previously washed and disinfected with bleach, containing 2.1 kg of growing medium. Work has been done in greenhouse with one day/night temperature: 30°/25°C, photoperiod: 16 h. Three sorghum seeds were sown in each pot. Three pots have been considered by soil, making a total of thirty six pots. Plantations were daily watered with demineralized water at field capacity (14%, vol/weight). After 4 months cultivation, culture soils were removed for determination of AMF diversity.
Spore extraction
It was carried out according to wet sieving method described by Gerdemann and Nicholson (1963). It involved a sample of 100 g dry soil. Four repetitions have been considered for each soil.
Morpho-anatomical characterization of AMF spores
The morphology of the spores is the basis of their identification. For that, spores size, shape and color, their solitary or grouped nature, their clusters dissociable nature or not, presence or absence of sporogenic cell on spores were noted by discriminating spores extracted from each soil under microscopic. Data obtained were compared with original description by Schenck and Pérez (1990) and that of database of reference cultures published on the website (http: //invam.caf.wvu.edu, August 2016).
Preparation of the blades
Spores morphologically identical were removed using a pipette and then placed on a microscope slide containing each of these ends on a drop of distilled water. Two drops of PVLG solvent (polyvinyl alcohol, lactic acid, glycerol) (Koske and Testier, 1983) were placed at right end of the slide to allow observation of spore external morphology. Two drops of PVLG + Melzer v/v reagent (Morton, 1988) were placed at the left end to study membrane layers. At each end of slide containing drops of solvent, one to several similar spores were deposited and then gently covered with a coverslip.
Microscopic observation of spores
Spores were observed under an optical microscope with enlargement 10 (G X 10). After observing external morphology, using forceps, a pressure was gently applied above coverslip, covering PVLG solvent added to Melzer reagent pending bursting spores. Owing to the solvents, internal membranes of spores emerged after rupture.
Extracted AMF community structures
AMF spores morphology and anatomy observation enabled identification of 21 morphotypes for all 12 sites (Figure 2). These morphotypes belong respectively to six genera, Gigaspora, Scutellospora, Glomus, Sclerocystis, Entrophospora and Acaulospora; distributed in three families namely Gigasporaceae, Glomeraceae, and Acaulosporaceae. The comparison with the genera composition and the relative abundances of these genera in soils collected directly from the field shows the same genera composition in the soils before and after trapping. The structure of the different AMF communities also remains quite similar (Figure 3).
Relative abundance of different morphotypes from trapping soil
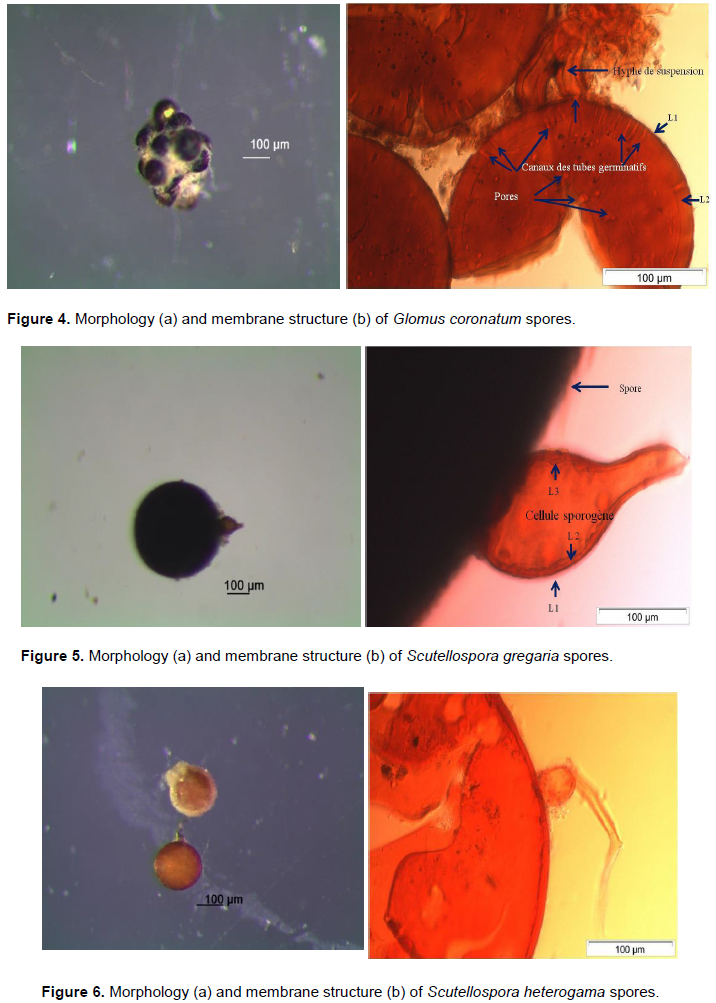
Glomus genus is most represented and most diversified with 15 morphotypes (65% of species observed). One of this genus morphotypes was identified using these morpho-anatomical characters as Glomus coronatum (Figure 4a and b), and the other fourteen unidentified were named Glomus spp. These are Glomus spp1 to Glomus spp14. The genera Scutellospora and Gigaspora are each represented by two morphotypes, 10% of each species were obtained. Two species of Scutellospora genus have been identified on account of their morpho-anatomical characters as Scutellospora gregaria and Scutellospora heterogama (Figure 5a, b and 6a, b). The morpho-anatomical characters of Gigaspora genus identified one of the two morphotype like Gigaspora rosea (Figure 7a and b). The other unidentified morphotype was named Gigaspora sp. Acaulospora, Entrophospora and Sclerocystis generas; each represented by a single morphotype or 5% of species. These generas have been respectively named Acaulospora sp., Entrophospora sp. and Sclerocystis sp.

This study showed a diversity of AMF associated with sorghum in the 12 soils of Sikasso in Mali. The genus Glomus is the most represented in all sites with 15 morphotypes (65%). Such a result has been noted in different natural and agro-ecological environments in West Africa as well as in Ivory Coast (Anguiby et al., 2019), in Togo (Gnamkoulamba et al., 2018), in Benin (Aguegue et al., 2017), in Senegal (Diop et al., 2015), and in India (Ajaz et al., 2017). Indeed, it has been reported that the genus Glomus predominates in terms of presence in ecosystems due to its adaptability and stability in tropical environments and various conditions (Lenoir et al., 2016b; Bencherif et al., 2015).
Spores are differentiated in the soil and in the roots (case of the genus Sclerocystis). They are used as a reference structure for the morpho-anatomical identification of species. These spores are generally round in shape, with a thick wall formed of several layers of different textures and connected to the filamentous network by a suspensory hypha.
The diversity observed in present study, comprising 21 morphotypes of 6 genera (Glomus, Gigaspora, Scutellospora, Acaulospora, Rhyzophagus and Entrophosphora) is greater than that of four genera (Glomus, Gigaspora, Scutellospora, and Acaulospora) obtained under the Cheese and Makorés in a Botanical garden in Ivory Coast (Anguiby et al., 2019) from 10 specimens, but lower than that of seven genera (Acaulospora, Claroideoglomus, Entrophospora, Funneliformis, Gigaspora, Glomus and Rhizoglomus) observed under different rice cropping systems in Togo (Gnamkoulamba et al., 2018) which brought in 25 species. With 15 morphotypes (Diop et al., 2015) also obtained in Senegal. These results are consistent with those of other authors regarding the existence of a diversity of AMF in soils of agro systems in West Africa. Indeed, the African west soils contain a big/great diversity AMF that can be identified, thanks to the morphology of their spores (Dalpé et al., 2000). In addition, these fungi are subservient to plants and do not exhibit host specificity; hence the diversity observed here in Mali and elsewhere in Sub-Saharan Africa.
In the Sikasso region of Mali, sorghum is naturally associated with a great diversity of arbuscular mycorrhizal fungi, identified in 21 morphotypes from six genera and three families. The genus Glomus was the most represented and diverse with fifteen morphotypes. Further work can be done by sequencing the strains in the collection.
The authors have not declared any conflict of interests.
REFERENCES
|
Aguegue RM, Noumavo PA, Gustave, Dagbenonbakin D, Baba Moussa L (2017). Arbuscular Mycorrhizal Fertilization of Corn (Zea mays L.) Cultivated on Ferrous Soil in Southern Benin. Journal of Agricultural Studies 5(3):99-115.
Crossref
|
|
|
|
Ajaz M, Mohammad YZ, Jagana CS (2017). Isolation, Identification and Characterization of Arbuscular Mycorrhizal Fungi in Apple (Malus Domestica Borkh) Growing Area of Kashmir Himalaya". International Journal of Current Microbiology and Applied Sciences 6(8):25-37.
Crossref
|
|
|
|
|
Anguiby BLA, Ouattara G, Bomisso EL, N'goran B, Ouattara B, Coulibaly SA, Aké S (2019). Assessment of the mycorrhizal status of trees of Ceiba pentandra (L), Gaertn and Tieghemella heckelii (A.Chev), Pierre, from the Botanical Garden of Bingerville in Ivory Coast. Journal of Applied Biosciences 138:14092-14105.
|
|
|
|
|
|
|
Bazile D, Dembélé S, Soumaré M, Dembélé D (2008). Use of the varietal diversity of sorghum to enhance the diversity of soils in Mali. Cahiers Agricultures vol. 17, n°2, Cirad/IER, Bamako / Mali.
Crossref
|
|
|
|
|
Bazile D, Soumaré M, Dembélé J (2004). Sorghum in Mali: Conserving agro-biodiversity for the stability of agricultural production. Sub-regional workshop on agricultural biodiversity in West Africa, December 15 to 19. GTZ / FAO, Bamako, Mali. Technical Sheet, Sorghum Program/CRRA/Sotuba-IER.16 p.
|
|
|
|
|
Bencherif K, Boutekrabt A, Fontaine J, Laruelle, F, Dalpè Y, Lounès-Hadj Sahraoui A (2015). Impact of soil salinity on arbuscular mycorrhizal fungi biodiversity and microflora biomass associated with Tamarix articulata Vahll rhizosphere in arid and semi-arid Algerian areas. Science of the Total Environment 533:488-494.
Crossref
|
|
|
|
|
Commission of Food Safety (CSA) (2007): Summary of municipal food security plans in the Sikasso region. PROMISAM /USAID-Mali 19p.
|
|
|
|
|
Dalpé Y, Diop TA, Plenchette C, Guèye M (2000). Glomales species associated with surface and deeprhizosphere of Faidherbia albida in Senegal. Mycorrhiza 10:125-129.
Crossref
|
|
|
|
|
Dembélé B, Diourté M, Traoré H, Wade M, Mourik TV, Macauley HR, Asiedu E, Koné AY, Kouamé KJ (2011). Promotion of striga-resistant sorghum varieties for the mitigation of food crises in the Senegal, Mali, Ghana and Burkina Faso. Global Food Security Response Initiative. Sorghum seed production training manual. CORAF/ WECARD. Food Crops Program 34:12-16.
|
|
|
|
|
Diop I, Ndoye F, Kane A, Krasova-Wade T, Pontiroli A, Do Rego FA, Noba K, Prin Y (2015). Arbuscular mycorrhizal fungi (AMF) communities associated with cowpea in two ecological site conditions in Senegal. African Journal of Microbiology Research 9(21):1409-1418.
Crossref
|
|
|
|
|
Diouf D, Sougoufara B (2002). "Reforestation in Senegal: assessment of achievements from 1993 to 1998." Rev. For. Fr. LIV: 227-238.
|
|
|
|
|
Finlay RD (2008). Ecological aspects of mycorrhizal symbiosis with special emphasis on the functional diversity of interactions involving the extraradical mycelium. Journal of Experimental Botany 59(5):1115-1126.
Crossref
|
|
|
|
|
Fitter A (2012). Why plant science matters. The New Phytologist 193(1):1-2.
Crossref
|
|
|
|
|
Gerdemann JW, Nicholson TH (1963). Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Transactions of the British Mycological Society 46(2):235-244.
Crossref
|
|
|
|
|
Gnamkoulamba A, Tounou AK, Tchabi A, Agboka K, Adjévi AKM, Batawila K (2018). Prevalence and diversity of spores of arbuscular mycorrhizal fungi in rice cultivation under different rice cropping systems in Togo. Journal of Applied Biosciences 126:12647-12664.
|
|
|
|
|
Permanent Interstate Drought Control Committee (CILSS) (2002). Strategic framework for sustainable food security in a perspective of poverty reduction in the Sahel. Niamey (Niger) 52:9-10.
|
|
|
|
|
Koske RE, Testier B (1983). A convenient, permanent slide mounting medium. Mycological Society of America Newsletter 34(2):59.
|
|
|
|
|
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas J (2006). Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98(4):693-713.
Crossref
|
|
|
|
|
Lenoir I, Fontaine J, Lounès-Hadj Sahraoui A (2016b). Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 123:4-15.
Crossref
|
|
|
|
|
Ministry of Territorial Administration and Local Authorities (MATCL) (2012). Diagnostic study of promising economic sectors and shared economic spaces in the Sikasso region. GERAD 100 p.
|
|
|
|
|
Morton JB (1988). Taxonomy of VA mycorrhizal fungi: Classification, nomenclature and identification. Mycotaxon 32:267-324.
|
|
|
|
|
Mummey DL, Antunes PM, Rillig MC (2009). Arbuscular mycorrhizal fungi pre-inoculant identity determines community composition in roots. Soil Biology & Biochemistry 41(6):1173-1179.
Crossref
|
|
|
|
|
National Institute of Statistics (2010). Provisional results of the General Census of Population and Housing.
|
|
|
|
|
Oehl F, Sieverding E, Mâder P, Dubois D, Ineichen K, Boller T, Wiemken A (2004). Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia 138(4):574-583.
Crossref
|
|
|
|
|
Sarr MB (2010). Interim report: Baseline study of the agro-food processing system in West Africa. Agri-business and Agro-Food Specialist 53 p.
|
|
|
|
|
Schenck NC, Pérez Y (1990). Manual for the Identification of VA Mycorrhizal Fungi. Synergistic-Publications, Gainesville, Florida.
|
|
|
|
|
Sidibé DK, Yossi H (1997). Effect of fallow age on the number of endomycorrhizal fungi spores in the Sudanian zone of Mali 1:6-10; Fallow Project. Forest Resources Program; IER; Bamako-Mali.
|
|
|
|
|
Smith SE, Jakobsen I, Grønlund M, Smith FA (2011). Roles of Arbuscular mycorrhizas in Plant Phosphorus Nutrition: Interactions between Pathways of Phosphorus Uptake in Arbuscular Mycorrhizal Roots Have Important Implications for Understanding and Manipulating Plant Phosphorus Acquisition. Plant Physiology 156(3):1050-1057.
Crossref
|
|
|
|
|
Smith SE, Read DJ (2008). Mycorrhizal symbiosis, 3rd edn. Academic Press Inc., London, 3rd eds.
|
|
|
|
|
Vaksmann M, Kouressy M, Chantereau J, Bazile D, Sagnard F, Touré A, Sanogo O, Diawara G, Dante A (2008). Use of the genetic diversity of local sorghums in Mali. Cahiers Agricultures 17:2, Cirad/IER, Bamako/Mali.
Crossref
|
|
|
|
|
Walker C (1992). Systematics and taxonomy of the Arbuscular endomycorrhizal fungi (Glomales)- a possible way forward Agronomy 12(10):887-897.
Crossref
|
|