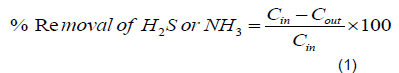ABSTRACT
The presence of hydrogen sulfide (H2S) and ammonia (NH3) in biogas pose serious human health and environmental challenges. In this study, H2S and NH3 were successfully removed from biogas using water hyacinth-derived carbon (WHC) nanomaterials. Carbonization temperature, biogas flow rate, mass of the adsorbent and activating agent (KOH/water hyacinth (WH)) ratio were found to greatly influence the efficiency of the H2S and NH3 removal. The adsorption capacity of both H2S and NH3 was found to increase with the carbonization temperature as carbon materials prepared at 450, 550, and 650°C afforded removal efficiencies of 22, 30, and 51% for H2S and 42, 50, and 74% for NH3, respectively, after contact time of 2 h. Similarly, the KOH/WHC ratio showed huge impact on the adsorptive removal of the two species. WH materials carbonized at 650°C and activated at 700°C using 1:4, 1:2, and 1:1 KOH/WHC ratios showed removal efficiencies of 80, 84, and 93% for H2S and 100, 100, and 100% for NH3, correspondingly after 2 h contact time. The adsorption capacity of NH3 increased with the decrease in flow rate from 83 to 100% at flow rates of 0.11 and 0.024 m3/h, respectively, while that of H2S increased from 22 to 93% with flow rate 0.11 and 0.024 m3/h, respectively. The removal of H2S and NH3 increased with adsorbent mass loading. With the 0.05, 0.1, 0.2, and 0.3 g of the adsorbent, the adsorption of H2S after 1.5 h contact time was 63, 93, 93, and 95%, respectively while that of NH3 was 100% for all the adsorbent masses.
Key words: Waste water, KOH, activation ratio, carbonization temperature, flow rate.
The sustainability of fossil fuels is currently a growing concern as more countries strive for independence and security (Florin and Harris, 2008). In addition, global warming, that is strongly linked to the carbon dioxide from fossil fuels, is at the top of the rapidly growing list of global issues that require urgent and concerted efforts.
In the light of these challenges, a number of clean and sustainable sources of energy, among them biogas, have attracted huge research interest that has already translated into tangible alternative energy technologies for power generation. Biogas technology represents one of a number of feasible village-scale alternative energy technologies with great promise in rural energy program in Africa. Unlike other renewable energy sources, biogas technology can be deployed at small and large scales in urban or very remote rural locations with little or no geographical limitations. Moreover, biogas has multi-pronged benefits since in addition to generating energy, the technology helps in waste management which turn mitigates public-health and environmental problems while the solid residue from biogas plants is used as fertilizer (Amigun and von Blottnitz, 2007).
Biogas comes from a variety of sources including waste from wastewater treatment facilities, slaughterhouses, and farms. By composition, biogas contains from 40 to 60% methane, 35 to 50% carbon dioxide, 0 to 20% nitrogen, 0 to 1% oxygen, and the remainder is a mixture of noxious compounds such as hydrogen sulfide, siloxanes, carbonyl sulfide, carbon disulfide, methyl and dimethyl sulfides and other halogenated compounds (Rasi et al., 2014). Due to its low caloric value and potentially harmful sulfur, nitrogen, halogen, and silicon-based fractions, biogas has to be cleaned before it is utilized in cooking and power generation.
Hydrogen sulfide is considered a broad-spectrum poison that poisons a number of systems in the body (Lindenmann et al., 2010). As such, there is need to remove it from biogas. In addition, combustion of unclean biogas as in cooking stoves and steam turbines generates pollutants such as SOx and NOx gases, which exceeds pollution limits in many parts of the world including Africa. SOx and NOx gases are very corrosive; as a result, they wear down the economy through frequent repairs of the infrastructure and expenditure on respiratory related illnesses. On the other hand, siloxanes in biogas leads to silicon dioxide (SiO2) build up in stove burners and pipes. SiO2 build up could potentially lead to blockage with further risks.
The current techniques for biogas upgrading involve desulfurization of biogases carried out with various physical, chemical, or biological processes. Conventional physico-chemical techniques such as LOC CAT process (wet scrubbing redox system that utilizes chelated iron to transform H2S to elemental sulfur), and hydro-desulfurization that are often used for bulk.
H2S removal from natural gas and biogas (Wachs, 2008) are costly and thus unviable for rural settings. Commercial activated carbon impregnated with potassium hydroxide has been reported for substantial removal of bulk H2S from biogas (Choo et al., 2013; Sitthikhankaew et al., 2014). However, commercial activated carbon materials are expensive and are usually inefficient in removing organic sulfur compounds such as dimethyl sulfides (Cui et al., 2009). To significantly remove organic sulfur compounds with impregnated activated carbon, frequent recharge of the adsorbent materials is required which is costly. Some bacteria, for instance, Azospirillum and Thiobacillus are capable of oxidizing organic sulfur, however, they are usually slow and can only be active in liquid phase (da Silva et al., 2014). Moreover, single metal oxides such as ZnO and mixed metal oxide systems such as mixtures of vanadium, titanium, cerium or molybdenum oxides have been used for catalytic oxidative desulfurization (Sahle-Demessie and Devulapelli, 2009). This catalytic process is extremely costly not only because expensive metals are involved but also due to the fact that the oxidative reaction occurs at high temperatures. This represents a major setback to the use of this approach in decentralized small-scale biogas plants.
Scrubbers such as water and polyethylene glycol have been used in removing H2S and NH3 where the gas stream is passed through the scrubbing agent (Eze and Agbo, 2010). Since H2S and NH3 are soluble in water, their removal in aqueous chemical scrubbers becomes effective up to 99% (Munoz et al., 2013; Lien et al., 2014) but a large amount of scrubber is needed making it expensive for small scale application. Moreover chemicals such as chlorinated compounds leads to the production of secondary pollutants, which necessitates for treatment of the spent scrubbers before their disposal (Moussavi et al., 2008).
Biological methods have shown good efficiency to about 99% (Gadre, 1989; Zhao et al., 2010) as the bacteria in the biofilter biodegrade hydrogen sulfide to elemental sulphur. Despite of their efficiency, biological methods are very slow, sensitive to temperature, and need time to stabilize (Rattanapan and Ounsaneha, 2011; Soroushian et al., 2006; Seredych et al., 2008). As such, they are not suitable for large-scale purification and need considerable attention.
In addition, materials and chemicals such as ZnO, FeCl3 and FeO have been used to reduce H2S to elemental sulphur (Kapdi et al., 2005). The use of nanoZnO has achieved up to 100% catalysis (Sayyadnejad et al., 2008) but the reaction is water sensitive therefore the presence of water vapour in the biogas favour backward reaction (Novochinskii et al., 2004).
The use of AC impregnated with different chemical species such as hydroxides, carbonates
and chlorides of earth and alkaline earth metals has afforded promising results on adsorption of H2S and NH3 (Gao et al., 2013).
Different carbon precursors have been utilized as source for AC; these include graphene,
aerogel and agricultural waste such corncobs, coconut shells banana peels. The use of biomass is attractive because it is cheap and readily available and does not pose food security issues (Sodeinde, 2012; Sirichote et al., 2002; Alyani and Amin, 2008).
With the growing number of biogas plants, there is urgent need to develop cheap and effective materials for sulfur sequestration from biogas room temperature. In this study, water hyacinth derived carbon materials with very high sorption capacity for both hydrogen sulfide and ammonia were successfully prepared.
Adsorbent preparation
Water hyacinth stems and leaves were collected from Lake Victoria Mwanza, Tanzania, washed, and dried in sunlight for one week. The dried precursor was then chipped into small pieces and powdered. Carbonization was then performed on the powdered samples at 450, 550 and 650°C in a carbolite horizontal tubular furnace (CTF 12/65/550) for 1 h under constant flow of nitrogen gas. The carbonized materials were activated at 700°C using KOH under inert nitrogen atmosphere. The activation was done by separately mixing 1 g of carbon samples suspended in 10 ml of distilled water with 1, 0.5, and 0.25 g of KOH getting 1:1, 1:2, and 1:4 KOH:WHC ratio. The KOH/carbon mixture was then stirred for 30 min at 80°C. The resultant homogenous mixture was dried in an oven at 100°C and then activated at 700ºC for 30 min in an inert atmosphere (constant flow of nitrogen gas). The heating rate for carbonization and activation was 10°C/min. The activated carbon materials were washed with 10 ml of 0.1 M HCl followed by distilled water till neutral pH was attained after which they were dried in an oven under 100°C for 12 h. The dried samples stored in a dry place before being tested for H2S and NH3 removal. The prepared samples were named as WHC-T and WHAC-X-T, where T represents carbonization temperature while X represents KOH/WHC ratio. X also s tands for flow rate or mass adsorbent during adsorption process.
Characterization
SEM
The morphology of the products was studied by field emission scanning electron microscopy (FESEM) using a Zeiss DSM 982 Gemini instrument equipped with a Schottky emitter at an accelerating voltage of 2.0 kV and a beam current of 1.0 mA. The FESEM samples were prepared by dispersing the powdered samples in absolute ethanol. The suspension was then sonicated for 35 min, and a drop was loaded on Au Pd-coated silicon glass chips mounted onto aluminum stubs with a two-sided carbon tape. The samples were dried by vacuum desiccation prior to SEM analysis.
H2S and NH3 adsorption set-up
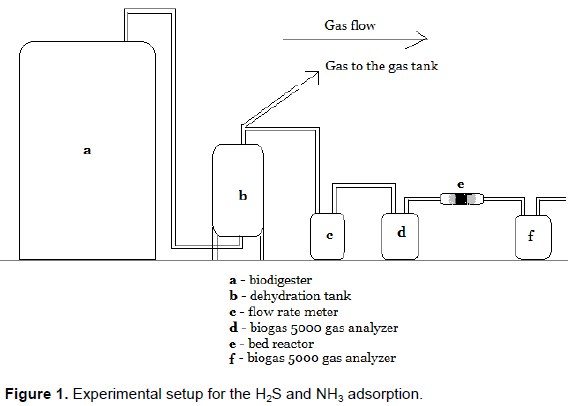
The H2S and NH3 adsorption tests were carried out at Banana Investment Co. Ltd in Arusha, Tanzania where the biogas is produced from industrial waste. The company produces 60 m3 of industrial waste water daily and the amount of biogas produced from the Up flow Anaerobic Sludge Blanket averages to about 100 m3 daily. The composition of the gas produced is 84.3 to 89.8% CH4, 12 to 14% CO2, Ë‚1% O2, 0.005 to 0.027 mg/g NH3, and 0.02 to 0.056 mg/g H2S as analyzed by the biogas 5000 gas analyzer.
Biogas was passed through a fixed bed column made of plastic tube, 5 cm long and 1 cm diameter, packed with the water hyacinth-derived carbon adsorbent. A given mass of adsorbent was placed in the tube and then supported by cotton wool on both sides of the tube. Then biogas was allowed to flow through the column with a flow rate 0.024, 0.04, 0.07 and 0.11 m3/h at room temperature as illustrated in Figure 1. The flow rate of the biogas was monitored and controlled using flow meter, model JBD2.5-SA. The concentration of H2S and NH3 was recorded before and after the adsorption process. The measurements for both H2S and NH3 during the adsorption studies were taken at time interval of 5, 10, 20, 40, 60, 90, and 120 min. The breakthrough time was recorded when the outlet concentration of H2S and NH3 reached 40% of the incoming concentration. These adsorption tests were repeated three times for each sample tested.
Morphological studies
The surface morphology of the water hyacinth-derived carbon materials obtained via carbonization at 650°C for 1 h and activated at 700°C for 30 min using different KOH to WHC ratios are shown in SEM images in Figure 2. The morphology of inactivated WHC is as shown in Figure 2a, while Figure 2b, c, and d are morphologies of WHAC activated at 1:4, 1:2, and 1:1 KOH to WHC ratios, respectively. The morphology of inactivated WHC shows microsphere-like clusters with smooth surfaces. The microsphere transformed into smaller irregular particles with rough surfaces upon activation with KOH, as indicated in SEM images in Figure 2b to d. This was due to the etching effect of the KOH, as a result, the surface area is increased (Tseng et al., 2006). This morphology is similar to that observed by Kurniawan et al. (2015) for carbon materials derived from water hyacinth.
H2S and NH3 adsorption studies
Effect of carbonization temperature
The water hyacinth derived carbons were tested on the removal of H2S and NH3 from biogas produced from winery effluent at Banana Investment Ltd. The performance of the materials was evaluated based on percentage removal. The percent removal was calculated using Equation 1:
For both gases, the adsorption capacity increased with carbonization temperature as shown in Figure 3a and b. WHC-650 sample afforded 51% removal after 2 h followed by WHC-550 and WHC-450 which removed 30 and 22% of the H2S, respectively. The performance of these samples on NH3 followed the same trend where 74, 50, and 42% of ammonia was adsorbed by the same adsorbents, respectively. The increase in adsorption capacity with temperature was due to the fact that at 650°C more organic matter volatilized from the water hyacinth compared to the low temperatures thus creating enough pores for adsorption of H2S and NH3 (Ioannidou and Zabaniotou, 2007).
Effect of biogas flow rate
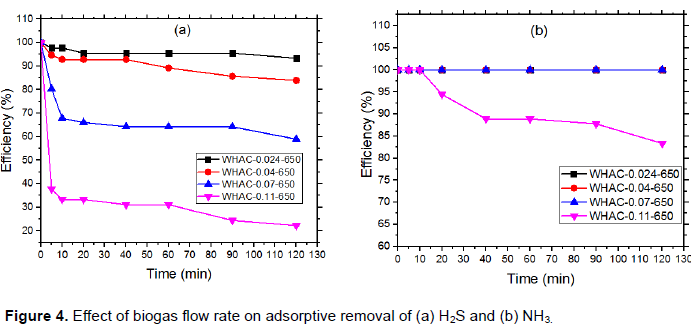
Figure 4 shows the effect of flow rate on H2S and NH3 adsorption. It was observed that the capacity of the materials in removing H2S and NH3 was different at different flow rates. With WHAC-1:1-650 at flow rates of 0.024, 0.04, 0.07, and 0.11 m3/h, the adsorption of H2S removing was 93, 93, 59, and 22%, respectively after 2 h. At the same flow rates and contact time, the efficiency of NH3 removal was 100, 100,100 and 83.3%, respectively. This indicates the high effectiveness of the materials prepared for the ammonia removal. High adsorption of H2S and NH3 was observed at low flow rates since biogas had enough time to contact with the adsorbent. At high flow rates, the contact time between the gas stream and the adsorbent decreased and thus the amount of H2S and NH3 molecules passing through the adsorbent without being adsorbed increased.
Effect of adsorbent mass
The effect of the adsorbent mass on the adsorption of H2S and NH3 was studied using WHAC-1:1-650 sample and results given in Figure 5. The removal of H2S was found to increase with increase in mass of adsorbent. When 0.05, 0.1, 0.2 and 0.3 g of adsorbent were used, 63, 93, 93, and 95% H2S removal was obtained after 1.5 h contact time (Figure 5a). On the other hand, 100% NH3 removal was obtained with all the adsorbent masses including 0.05 g (Figure 5b). The increase of adsorbent mass increased the number of adsorption sites in WHAC materials and hence increased removal of H2S and NH3.
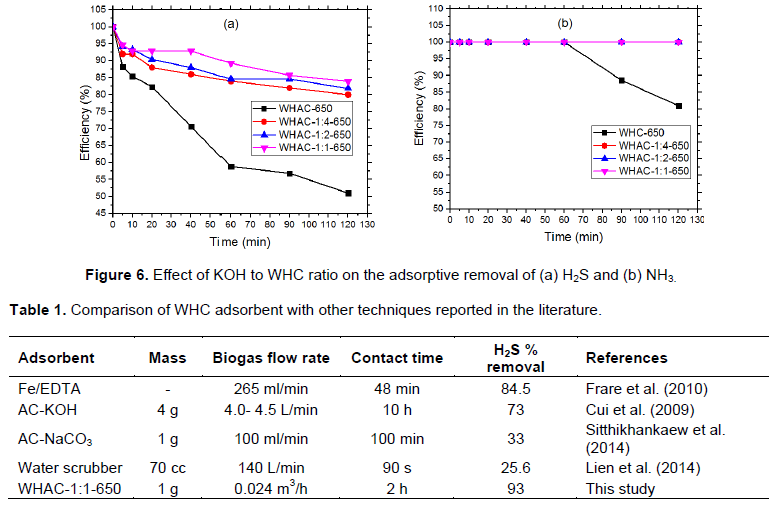
Effect of WHC to KOH ratio
The activation ratio has been reported as the most important observed parameter in synthesizing porous carbon. Figure 6 shows that the increase in KOH in impregnation increases the adsorption of H2S and NH3. The KOH assists in the formation and widening of the pores through arching (Lozano-Castello et al., 2001). At all ratios used for carbonization, the 1:1 KOH: WHC ratio had the best results for both NH3 and H2S removal. With NH3, the adsorption is more efficient as it was at 100% with the ratio 1:2 and all ratios for 650°C activated samples. The highest adsorption of H2S was 93% achieved by WHAC-1:1-650 samples after 2 h. Washing of the WHAC samples with HCl assisted the removal of the KOH used in impregnation process resulting in the increase of adsorption sites. The comparison of this technique with others is described in Table 1.
Sorption capacity (SC) of the WHC samples was determined using Equation 2, where WHSV is the space hourly velocity in mLh-1g-1, Vmol is the molar volume in Lmol-1, M is the atomic weight of sulphur at standard conditions, Cin and Cout are the H2S concentration before and after adsorption in ppm, respectively, and t is the breakthrough time (BT) (Garces, et al., 2012). BT was taken when the H2S concentration of the treated gas was 40%.

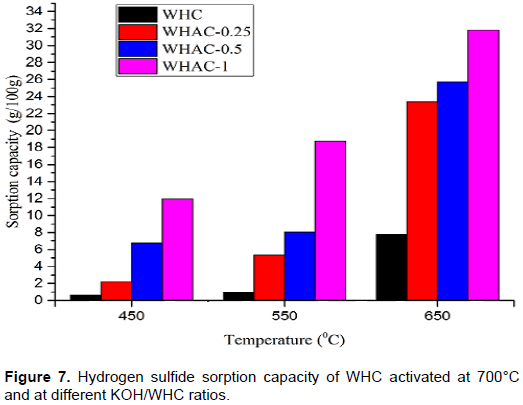
For this study, BT was defined as the time when the H2S concentration adsorption decreased to 40% of the total. SC of the carbon samples increased with carbonization temperature and KOH/WHC ratio as shown in Figure 7. WHC-450, WHC-550 and WHC-650 gave SC of 0.63, 1, and 7.8 g/100 g, respectively, while WHAC-1:4-650, WHAC-1:2-650, and WHAC-1:1-650 gave SC of 23.4, 25.7, and 31.85 g/100 g, respectively. These results agreed with the percentage removal data where WHC-450, 550, and 650°C samples showed 22, 30, and 51% H2S removal efficiencies, while WHAC-1:4-650, WHAC-1:2-650, and WHAC-1:1-650 samples showed 80, 84, and 93% H2S removal efficiencies after 2 h, respectively. The breakthrough time (BT) increased as the carbonization temperature and the activation ratio increased. WHC-450, WHC-550 WHC-650 had a BT of 5, 10, and 60 min, respectively and WHAC-1:4-650, WHAC-1:2-650, and WHAC-1:1-650 had the BT of 180, 180, and 210 min, respectively as shown in Table 2.
In this study, water hyacinth-derived carbon materials were successfully synthesized and their performance in NH3 and H2S removal from biogas tested. Morphology studies showed a decrease in particle sizes of WHC with KOH amount. The WHAC samples prepared at 650°C and those activated at the highest ratio (1:1) showed superior hydrogen sulfide and ammonia removal capacity of 31.85 g/100 g and longer breakthrough time of 3.5 h. Furthermore, high hydrogen sulfide removal efficiencies of 95% were obtained with 0.3 g of the adsorbent. On the other hand, low biogas flow rate of 0.024 m3/h afforded 93% H2S removal.
The authors have not declared any conflict of interests.
REFERENCES
|
Alyani Z, Amin M (2008). Agricultural residues as Precursors for activated carbon production (coconut waste).
|
|
|
|
Amigun B, von Blottnitz H (2007). Investigation of scale economies for African biogas installations. Energy Convers. Manag. 48(12):3090-3094.
Crossref
|
|
|
|
|
Choo HS, Lau LC, Mohamed AR, Lee KT (2013). Hydrogen sulfide adsorption by alkaline impregnated co-â€conut shell activated carbon. J. Eng. Sci. Technol. 8:741-753.
|
|
|
|
|
Cui H, Turn SQ, Reese MA (2009). Removal of sulfur compounds from utility pipelined synthetic natural gas using modified activated carbons. Catalysis Today 139(4):274-279.
Crossref
|
|
|
|
|
da Silva ML, Mezzari MP, Ibelli AM, Gregory KB (2014). Sulfide removal from livestock biogas by Azospirillum-like anaerobic phototrophic bacteria consortium. Int. Biodeterior. Biodegr. 86:248-251.
Crossref
|
|
|
|
|
Eze J, Agbo K (2010). Maximizing the potentials of biogas through upgrading. Am. J. Sci. Ind. Res. 1(3):604-609.
Crossref
|
|
|
|
|
Florin NH, Harris AT (2008). Enhanced hydrogen production from biomass with in situ carbon dioxide capture using calcium oxide sorbents. Chem. Eng. Sci. 63(2):287-316.
Crossref
|
|
|
|
|
Frare LM, Vieira MG, Silva MG, Pereira NC, Gimenes ML (2010). Hydrogen sulfide removal from biogas using Fe/EDTA solution: gas/liquid contacting and sulfur formation. Environ. Progress Sustain. Energy 29(1):34-41.
|
|
|
|
|
Gadre R (1989). Removal of hydrogen sulfide from biogas by chemoautotrophic fixedâ€film bioreactor. Biotechnol. Bioeng. 34(3):410-414.
Crossref
|
|
|
|
|
Gao Y, Zhou YS, Qian M, He XN (2013). Chemical activation of carbon nano-onions for high-rate supercapacitor electrodes. Carbon 51:52-58.
Crossref
|
|
|
|
|
Garces HF, Espinal AE, Suib SL (2012). Tunable shape microwave synthesis of zinc oxide nanospheres and their desulfurization performance compared with nanorods and platelet-like morphologies for the removal of hydrogen sulfide. J. Phys. Chem. C 116(15):8465-8474.
Crossref
|
|
|
|
|
Ioannidou O, Zabaniotou A (2007). Agricultural residues as precursors for activated carbon production - a review. Renew. Sustain. Energy Rev. 11(9):1966-2005.
Crossref
|
|
|
|
|
Kapdi SS, Vijay VK, Rajesh SK, Prasad R (2005). Biogas scrubbing, compression and storage: perspective and prospectus in Indian context. Renew. Energy 30(8):1195-1202.
Crossref
|
|
|
|
|
Kurniawan F, Wongso M, Ayucitra A, Soetaredjo FE, Angkawijaya AE, Ju YH, Ismadji S (2015) not cited. Carbon microsphere from water hyacinth for supercapacitor electrode. J. Taiwan Institute Chem. Eng. 47:197-201.
Crossref
|
|
|
|
|
Lien CC., Lin JL, Ting CH (2014). Water Scrubbing for Removal of Hydrogen Sulfide (H2S) Inbiogas from Hog Farms. J. Agric. Chem. Environ. 3(02):1.
|
|
|
|
|
Lindenmann J, Matzi V, Neuboeck N, Ratzenhofer-Komenda B, Maier A, Smolle-Juettner FM (2010). Severe hydrogen sulphide poisoning treated with 4-dimethylaminophenol and hyperbaric oxygen.
|
|
|
|
|
Lozano-Castello D, Lillo-Rodenas MA, Cazorla-Amorós D, Linares-Solano A (2001). Preparation of activated carbons from Spanish anthracite: I. Activation by KOH. Carbon 39(5):741-749.
Crossref
|
|
|
|
|
Moussavi G, Naddafi K, Medaghinia A, Mohseni M (2008). Effectiveness of hydrogen peroxide in H2S removal by a packed high specific surface area bed scrubber. Chem. Biochem. Eng. Q. 22(1):9-14.
|
|
|
|
|
Mu-oz R, Estrada JM, Lebrero R, Quijano G, Kraakman NJ (2013). Strategies for Odour Control. In odour impact assessment handbook. John Wiley & Sons New Jersey. pp. 85-124.
|
|
|
|
|
Novochinskii II, Song C, Ma X, Liu X, Shore L, Lampert J, Farrauto RJ (2004). Low-temperature H2S removal from steam-containing gas mixtures with ZnO for fuel cell application. 1. ZnO particles and extrudates. Energy Fuels 18(2):576-583.
Crossref
|
|
|
|
|
Rasi S, Läntelä J, Rintala J (2014). Upgrading landfill gas using a high pressure water absorption process. Fuel 115:539-543.
Crossref
|
|
|
|
|
Rattanapan C, Ounsaneha W (2011). Removal of Hydrogen Sulfide Gas using Biofiltration-a Review. Walailak J. Sci. Technol. 9(1):9-18.
|
|
|
|
|
Sahle-Demessie E, Devulapelli VG (2009). Oxidation of methanol and total reduced sulfur compounds with ozone over V 2 O 5/TiO 2 catalyst: effect of humidity. Appl. Catalysis A: General 361(1):72-80.
Crossref
|
|
|
|
|
Sayyadnejad M, Ghaffarian H, Saeidi M (2008). Removal of hydrogen sulfide by zinc oxide nanoparticles in drilling fluid. Int. J. Environ. Sci. Technol. 5(4):565-569.
Crossref
|
|
|
|
|
Seredych M, Strydom C, Bandosz TJ (2008). Effect of fly ash addition on the removal of hydrogen sulfide from biogas and air on sewage sludge-based composite adsorbents. Waste Manage. 28(10):1983-1992.
Crossref
|
|
|
|
|
Sirichote O, Innajitara W, Chuenchom L, Chunchit D, Naweekan K (2002). Adsorption of iron (III) ion on activated carbons obtained from bagasse, pericarp of rubber fruit and coconut shell. Songklanakarin J. Sci. Technol. 24:235-242.
|
|
|
|
|
Sitthikhankaew R, Chadwick D, Assabumrungrat S, Laosiripojana N (2014). Performance of Sodium-Impregnated Activated Carbons toward Low and High Temperature H2S Adsorption. Chem. Eng. Commun. 201(2):257-271.
Crossref
|
|
|
|
|
Sodeinde O (2012). Preparation of a Locally Produced Activated Carbon from coconut shells and its use in reducing Hexamine Cobalt (III). Int. J. Chem. Eng. Appl. 3(1):67-68.
Crossref
|
|
|
|
|
Soroushian F, Shang Y, Whitman EJ, Garza G, Zhang Z (2006). Development and application of biological H2S scrubbers for treatment of digester gas. Proceed. Water Environ.Federation 9:3541-3547.
Crossref
|
|
|
|
|
Tseng RL, Tseng SK, Wu FC (2006). Preparation of high surface area carbons from Corncob with KOH etching plus CO 2 gasification for the adsorption of dyes and phenols from water. Colloids and Surfaces A: Physicochem. Eng. Aspects 279(1):69-78.
Crossref
|
|
|
|
|
Wachs IE (2008). Oxidative desulfurization of sulfur-containing hydrocarbons. Google Patents.
|
|
|
|
|
Zhao Q, Leonhardt E, MacConnell C, Frear C, Chen S (2010). Purification technologies for biogas generated by anaerobic digestion. Climate Friendly Farming. Available at:
View
|
|