Full Length Research Paper
ABSTRACT
Majority of people in rural areas of Uganda and other malaria-endemic parts of the world use medicinal plants to treat the disease. This study documented medicinal plants used to treat malaria around Kilembe copper mines and assessed the presence of essential and potentially toxic elements. Household surveys and key informant interviews were carried out while anti-malarial plants were sampled, prepared and concentrations of Fe, Mn, Zn, Co, Cu and Ni determined by atomic absorption spectrometry. It was established that Vernonia amygdalina (40%), Ocimum suave (35%), Justicia betonica (32%) and Aloe felox (20%) were the most used plants to treat malaria. Leaves were the most commonly used plant part (83%) while decoctions were reported by 51% of respondents. Concentration of trace elements (mg/kg) in the four plant species ranged from 50.4-422 (Mn), 16.7-202 (Fe), and 19.6-198 (Zn) and from 1.6-44.1, 0-7, and 0.1-31.5 for Cu, Co and Ni, respectively. Fe, Cu and Ni exceeded the recommended thresholds in almost all Kilembe mine samples as well as controls while Mn, Zn and Co exceeded thresholds in more than 25% of the samples. Remediation of Kilembe catchment soils as well as public sensitisation on the safety of medicinal plants is recommended.
Key words: Malaria, medicinal plants, trace elements, Kilembe mine.
INTRODUCTION
The increasing environmental pollution especially soil contamination with heavy metals such as Lead (Pb), Mercury (Hg), Cadmium (Cd), Chromium (Cr), Arsenic (As), and Nickel (Ni), has led to their consumption by human beings through food chains (Kulhari et al., 2013). Heavy metals can be emitted into the environment by natural causes associated with weathering of rocks and minerals, erosion and flooding or anthropogenic causes associated with industrialization, mining, waste disposal, urban effluent discharge, vehicle exhaust fumes, fertilizer and sewage sludge application in agriculture land all of which influence the uptake, accumulatio and concentration of trace elements in plants (Zhao et al., 2007).
Environmental pollution by heavy metals is very prominent in areas of mining and old mine sites and pollution reduces with increasing distance away from mining sites (Peplow, 1999). Peplow (1999) reported that hard rock mines operate from 5 to 15 years until the minerals are depleted, but metal contamination that occurs as a consequence of hard rock mining persist for hundreds of years after the cessation of mining operations.
Kilembe copper mine was opened in 1959 and operated until 1979 when mining and mineral processing ceased due to civil unrest and fall in copper prices on the world market. Up to 15 Mt. of rock waste was generated during the processing of the copper-cobaltiferous pyrite ores (Owor et al., 2007). Several studies have drawn attention to the serious environmental consequences of copper mining pollution of Kilembe area and the surrounding environment (Mwesigye et al., 2016; Mwesigye and Tumwebaze, 2017). Mwesigye et al. (2019) reported wide scale soil, water and food contamination with trace metals which exposed local residents to trace metal toxicity.
Malaria remains one of the most important global health issue accounting for more than 1 million deaths per year (Greenwood et al., 2005). Medicinal plants are traditionally used to treat diseases ranging from common colds to malaria, arthritis and ulcers among others (Radulescu et al., 2013). In Uganda, medicinal plants have contributed significantly to current malaria therapy (Katuura et al., 2007a; Stangeland, 2011). According to WHO (2013), 80% of the people living in malaria-endemic parts of Africa depend on medicinal herbs to treat the disease. The affordability of most traditional medicines makes them more attractive at a time of soaring health-care costs and nearly universal austerity (WHO, 2013). In Uganda, over 90% of the rural population rely on herbal medicine for their health care because it is easily accessible and effective (Orem and Zikusooka, 2010). Trace elements in medicinal plants can have negative health effects on consumers if they exceeded recommended thresholds (Szentmihályi et al., 2006). This study was conducted to assess the levels of trace elements and safety of commonly used medicinal plants around Kilembe mine catchment.
MATERIALS AND METHODS
Study area
The study area covered Kilembe Mine (0°12' N 30°0E), located in Western Uganda, 10 km west of Kasese town on the slopes of Rwenzori mountain range (Figure 1). The valley area is bisected by River Nyamwamba and is occupied by copper mining infrastructure such as the Old Mine complex, the waste tailings dams, and Kilembe mine housing estates. This study was undertaken to document plants used locally in treatment of malaria by communities around Kilembe copper mines in Kasese and to assess the presence of essential and potential toxic elements in the most commonly used plants, namely: Vernonia amygdalina Delile (Asteraceae) (NS01), Ocimum sauve L (Lamiaceae) (NS10), Aloe ferox (Aloaceae) (NS04), and Justicia betonica L (Acanthaceae) (NS08).

Field survey
A survey was conducted targeting traditional health practitioners (THPs), the elderly and mothers who are the custodians of indigenous knowledge in management of various diseases (Katuura et al., 2007b). Qualitative data was obtained using questionnaires. A total of 103 consenting adults were interviewed, and comprised THPs (n=21), herbal medicine collectors/vendors (n=10), Traditional Birth Attendants (TBAs) (n=5), and the elderly residents (n=67).
Medicinal plants sampling
About 200 g of fresh leaves of medicinal plants at different maturity stages were randomly collected from mature disease free medicinal plants growing within the same plots within a radius of 50 m between January and April 2019. The medicinal plant species targeted included V. amygdalina, O. suave, A. ferox, and J. betonica, growing within the contaminated soils (Mwesigye et al., 2016) close to the defunct ore processing site and mine tailing dumps. The four plant species were purposively selected because they are the commonly used in the treatment of malaria. Where more than one plant of the same species was found in one place or within 20 m range, leaves were randomly picked from each plant and mixed up to form a homogenized sample.
Five composite control samples representing each of the four plant species under study were randomly collected from 4 to 7 different and well established medicinal plants growing upstream of Kilembe mine area, approximately 3 km from the Kilembe copper mine site. All samples were collected using a stainless-steel knife and first rinsed with tap water followed by distilled and deionized water. Samples were packed in polythene bags before transportation to Makerere University laboratory for further preparation and analysis.
Sample preparation and analysis
In the laboratory, the samples were oven dried at 70°C for 48 h to remove all moisture. The cut pieces were carefully ground in a ceramic mortar, sieved through nylon mesh (2 mm) and the resultant powder packed and sealed in plastic ziplock bags. At the Department of Chemistry, Makerere University, the powdered samples were further ground using a Phillips blender (Model: HR 2058) and sieved through 0.053-micron sieve. Approximately, 0.5 g from each sample was then weighed into Pyrex conical flask. Up to 30 ml of aquaregia solution was added to the samples in the flasks and heated slowly at a low temperature of 100°C using a hotplate for about 50 min until digestion was completed. The resultant solution was filtered using Whatman filter paper (40 mm × 100 mm) to remove residues. Trace elements in the medicinal plants were analysed using Atomic Absorption Spectrophotometer (Agilent 200AA Series).
Quality control
For quality control, all samples were prepared and analyzed in duplicates. Blanks were used during digestion and analytical processes. The reagents used for sample preparations were of analytical grade (AR) or trace analytical grade (TAG).
Statistical analysis
Survey data was cleaned and entered into SPSS Version16 to generate descriptive statistics. Data for elemental concentrations in the leaf samples of different medicinal plants were analyzed using Pearson’s correlation to determine whether there was any linear association between the elements. A two-sample t-test was conducted using Mintab version 18 to assess if there were significant differences in elemental concentrations of medicinal plants growing in contaminated and control sites. All statistical tests were conducted at 5% significance level.
RESULTS AND DISCUSSION
Socio-demographic factors associated with use of medicinal plants
Majority of medicinal plant users were females (65%), aged between 35 and 55 years (48%), mostly with only the seven-year primary level education (38%). Majority of the respondents were peasant farmers (57%) with low income levels and depended on subsistence farming for their livelihood.
Thirty seven plants species distributed among 27 families were mentioned by respondents to be used traditionally in treatment of malaria. Out of these, 29 were scientifically identified to species level. Most of the plants were from the family of Fabaceae, Asteraceae and Myrsinaceae, respectively. Results of the study revealed that V. amygdalina was the most frequently used medicinal plant to treat malaria (40%), followed by O. suave (35%), J. betonica (32%) and A. felox (20%), respectively. Earlier studies (Katuura et al., 2007; Stangeland et al., 2011) also reported V. amydalina, J. betonica and A. ferox as the most commonly used plants to treat malaria in Mbarara district in Western Uganda. Twenty four plants reported in this study were found to be documented to treat malaria in other parts of Uganda as well as in different countries in Africa and across Asia and Latin America (Bhat and Surolia, 2001; Willcox and Bodeker, 2004). The use of the same plant species in malaria treatment across different traditions strongly suggests that these species may be highly effective in treating malaria (Orwa and Pharm, 2002). According to various studies (Challand and Willcox, 2009; Kumar et al., 2017; Anywar et al., 2020), extracts of A. ferox and V. amygdalina have good anti plasmodial activity against chloroquine sensitive strain of Plasmodium falciparum (MRC-2) in vivo and are effective in treating malaria in adult patients.
Concentrations of essential elements of Fe, Zn, Mn in four selected medicinal plants
Table 1 shows the concentration of Fe, Zn and Mn found in the leaves of V. amygdaline, O. suave, J. betonica and A. ferox on dry weight basis.
Iron (Fe) had the highest concentrations across all the four plant species sampled, followed by Mn and Zn. However, there was no correlation among the three elements, suggesting no common uptake and transportation mechanism. O. suave had the highest mean concentrations of Fe followed by V. amygdalina, J. betonica and A. forex at 501±504, 218±1201, 157±7.7 and 111±67, respectively. Iron concentrations in V. amygdalina were significantly higher than the control samples (P=0.04), whereas Mn concentrations in O. suave were significantly higher than in the control sample (P= 0.03). Iron concentration in J. betonica was higher in the control than in the treatment, suggesting that area mineralogy and geological could be key in influencing trace element in soils, besides past mining activities. Iron concentrations in all the four different species exceeded the WHO/FAO permissible levels in medicinal plants of 20 mg/kg (WHO/FAO, 1998). The concentrations of Fe (111- 501 mg/kg), Zn (29.6-83 mg/kg) and Mn (105.9-245.3 mg/kg) observed in this study were higher than those reported for medicinal plants from Khetri copper mines in India of Fe (228-461 mg kg-1), Zn (24.9-49.9 mg kg−1) and Mn (37.0-57.1 mg kg-1) (Maharia et al., 2010). This can be attributed to the differences in the geochemical soils characteristics, geographic region and the ability of different plant species to selectively accumulate these elements (Korfali et al., 2013). Iron has several key functions in human body including oxygen supply, energy production, immunity and its deficiency may result in anaemia. However, excessive iron intake is associated with symptoms of vomiting and nausea, dizziness, diarrhoea, liver damage and joint pain. Continuous intake of excess Fe may damage the mucosal cells which may result in hematemesis (Obi et al., 2006).
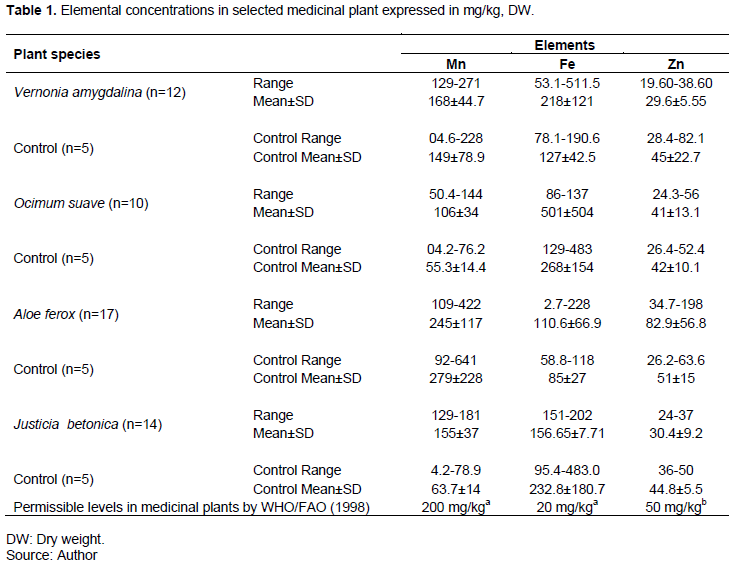
The observed mean concentrations for Mn were 245±117, 168±45, 155±37 and 106±34 for A. forex, V. amygdalina, J. betonica and O. suave, respectively. Mean concentration of Mn in A. ferox was higher in the controls. For Zn, A. ferox had the highest mean concentration at 83±57; followed by O. suave, J. betonica and V. amygdalina at 41±13, 30.40±9.2 and 29.6±5.6, respectively. Results of this study showed higher ranges of Mn compared to the selected medicinal herbs of Kenya which ranged from 3.2 to 17.3 mg/kg, and were considered safe (Maobe et al., 2012). High Mn concentrations were also observed in the medicinal plants of Egypt (Khan et al., 2008)and selected medicinal plants commonly used in Tanzania which ranged from 44.6 to 339 ppm and 12.07 to 317.23 ppm, respectively (Nkuba et al., 2017). High Mn concentrations in medicinal plants could be due to high Mn concentrations in the soils in which the plants are growing since the main route of uptake in plants is via roots (Kabata-Pendias, 2011). Manganese is an essential trace element known to be less toxic than any other metals and acts as co-factor for many enzymes. However, continuous exposure to concentrations of more than 5 mg/kg can cause severe health problems including neurological disorders (Kulhari et al., 2013).
From the elemental concentrations observed in different medicinal plants, it can be noted that the four medicinal plants sampled have different metal uptake and bioaccumulation potentials, due to differences in physiology. The tailings from Kilembe cooper mine have been reported to have high concentration of Fe, Zn and Mn (Mwesigye et al., 2016) and this implies that plants growing in soils contaminated by tailing are likely to have these minerals in high concentration following root uptake from the soil solution (Gajalakshmi et al., 2012).
Concentration of potentially toxic trace elements of Cu, Co, and Ni in four selected medicinal plant samples
The concentrations of Cu, Co and Ni in the four medicinal plants sampled, on dry weight basis are presented in Table 2. Copper was the most accumulated trace element across the four sampled medicinal plants, followed by Ni and Co. The concentration of Cu in J. betonica and O. suave were higher in control samples than Kilembe catchment soils, suggesting that area geology and mineralogy could be a key factor in soil elemental concentrations (Owor et al., 2007). There was no correlation among the elements suggesting lack of a common uptake and transportation mechanism. O. suave had the highest observed mean concentration of Cu (mg kg-1) at 19.38±8.85, followed by V. amygdalina (16.36±6.35) and A. ferox (12.11±14.42), while J. betonica had the lowest (4.6±4.24). Further still, V. amygdalina had the highest observed mean concentration of Ni at 7.82±8.05, while O. suave had a mean concentration of 5.2 ±4.9. J. betonica and A. ferox had 6.60±1.56 and 6.02±3.24 mg/kg, respectively. Cobalt showed the lowest mean concentrations observed in the four medical plant species sampled. The highest was 5.2±2.4 mg/kg for A. ferox followed by 3.8±2.2 mg/kg for O. suave and 3.±2.6 mg/kg for V. amygdalina. J. betonica had the lowest Co concentrations observed at 2.5 mg/kg. Cobalt concentrations in J. betonica (n=14) were significantly higher in the control samples (p= 0.03). Copper concentration in V. amygdalina, A. ferox and O. suave were all above the 10 mg/kg WHO threshold in medicinal plants implying that these medicinal plants could potentially negatively affect consumers. Only J. betonica had Cu concentration of 4.6±4.24, which was below the 10 mg/kg threshold (WHO, 1998). In related studies, Maharia et al. (2010) found high accumulation of Cu in the leaves of all medicinal plants growing in contaminated soils around Khetri copper mine in India. However, the Cu concentration levels observed in the present study which ranged from 4.60 to19.38 mg/kg, were significantly lower than the levels recorded in medicinal plants from Khetri, which ranged from 31.6 and 76.5 mg kg−1. As Kabata-Pendias (2011) noted, the common route for trace elements in plants is through root uptake and therefore the differences in plant trace element uptake and accumulation could be associated with soil elemental concentrations.
Copper is essential to the human body in very minute amount, since it forms a component of many enzyme systems, such as cytochrome oxidase, lysyl oxidase and ceruloplasmin, an iron-oxidizing enzyme in blood (Nkuba et al., 2017). However, high Cu accumulation levels in these medicinal plants are not only a danger to plants but pose negative human health effects due to copper toxicity. The Cu ion is said to aid production of reactive oxygen species, hence causing oxidative damage in human system (Maharia et al., 2010). Copper toxicity is also associated with Wilson’s disease, liver damage and jaundice, which may lead to death in absence of treatment (Murray et al., 2000; Soetan et al., 2010).
Nickel concentration in all the four plant species sampled exceeded the WHO/FAO (1998) permissible limit of 1.5 mg/kg and in A. ferox, concentration was slightly higher in the control samples (P=0.055). The concentration of Ni in all the four medicinal plants was higher in controls than in the treatments. This could possibly suggest that Ni concentrations are not only higher in contaminated soils of Kilembe as reported by Mwesigye et al. (2016) but also higher in the surrounding hills of Kilembe where the controls were sampled. Nickel concentration observed in O. sauve was lower than that of the same plant from Khetri soils from India (Maharia et al., 2010). High Ni concentrations were also reported in different herbal remedies used in Nigeria (Obi et al., 2006). Nickel is a known carcinogen reported to adversely affect lungs and nasal cavities (Nkuba et al., 2017). It has also been reported to be toxic to human reproductive system (Obi et al., 2006). According to Annan et al. (2010), Ni is also associated with dose-related decreases in bone marrow cellularity and in granulocyte macrophage and pluripotent stem cell proliferative responses. The results of the present study reveal high accumulated levels of Ni above the WHO/FAO (1998) permissible levels of 1.5 mg/kg in medicinal plants, which suggest possible health risks for consumers.
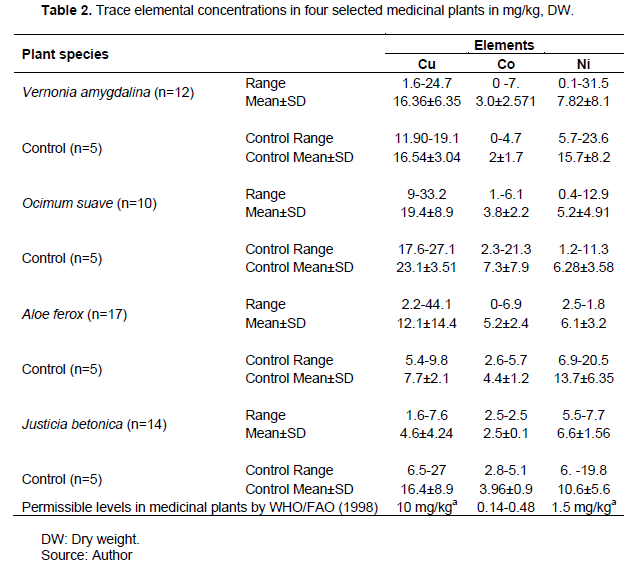
Cobalt was recorded to have the lowest mean concentration levels in all the four plant species sampled. The Co concentrations observed in the present study ranged from 0 to 7 mg/kg. This was lower than the range of Co concentrations reported in the selected common medicinal herbs of Haripur, Pakistan where the minimum Co concentration was 3.41 and the maximum was 11.26 mg/kg (Jabeen et al., 2010). However, Co concentration observed in this study are much higher than those observed from medicinal plants collected from the contaminated soil of Khetri copper mine in India where the highest Co mean concentration was 1.80±0.12 mg/kg as compared to 5.2±2.4mg/kg observed in the current study. Even in the controls, the Co concentrations in the present study are higher (7.25±7.92 mg/kg) than those from Haridwar in India which was equally far away from the copper mine where the highest concentration level was 1.09±0.1 mg/kg (Maharia et al., 2010). The wide variations in metal concentrations in the analysed medicinal plants could possibly be attributed to differences in the plant metal uptake and translocation capabilities. In human body, Co is required as constituent of vitamin B12 in minute amounts (Soetan et a.l, 2010). However, human toxicity symptoms associated with excessive Co intake include goitre, hypothyroidism and heart failure (Murray et al., 2000). The high concentration of Cu, Co, and Ni observed in Kilembe mine medicinal plants can be attributed to high concentrations of these elements in the soils around Kilembe copper mine which were reported to be above the world average crust as a result of erosion and contamination from the mine tailings (Mwesigye et al., 2016). Since controls also exhibited high elemental Co concentrations, geological sources of Co in soils are also highly likely.
CONCLUSION
The study established that there is wide scale use of traditional herbal medicine within Kilembe mine area and surroundings. However, it also confirmed the presence and varying concentrations of essential and potentially toxic trace elements in the four selected medicinal plants. Iron, Ni and Cu concentrations exceeded thresholds in all samples of different medicinal plant species collected while Zn, Mn and Co were also significantly higher and exceeding thresholds in over 20% of the medicinal plant species sampled. The high concentrations of essential and potentially toxic elements in medicinal plants could pause health risks to the users of anti-malarial herbal medicines from Kilembe mine area. Also, contrary to the common belief among community members that traditional herbal medicine is safe and free of side effects, the results of this study indicate that herbal medicines may contain potentially toxic elements when grown in soils that are highly contaminated with trace elements from anthropogenic or geological sources. The high concentration of trace elements in Kilembe medicinal plants calls for urgent efforts to remediate the polluted soils. Equally important is the need to create awareness among the communities of the potential risks associated with use of herb medicines collected from Kilembe catchment soils.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The authors appreciate the Departments of Chemistry and Plant Science, Microbiology and Biotechnology, Makerere University for the Laboratory analysis and technical expertise rendered during the study.
REFERENCES
|
Anywar G, Kakudidi E, Byamukama R, Mukonzo J, Schubert A, Oryem-Origa H (2020). Indigenous traditional knowledge of medicinal plants used by herbalists in treating opportunistic infections among people living with HIV/AIDS in Uganda. Journal of Ethnopharmacology 10:246. |
|
|
Annan K, Kojo A, Cindy A, Samuel AN, Tunkumgnen B (2010). Profile of heavy metals in some medicinal plants from Ghana commonly used as components of herbal formulations. Pharmacognosy Research 2(1):41-44. |
|
|
Bhat GP, Surolia N (2001). In vitro antimalarial activity of extracts of three plants used in the traditional medicine of India. American Journal of Tropical Medicine and Hygiene 65(4):304-308. |
|
|
Challand S, Willcox M (2009). A clinical trial of the traditional medicine Vernonia amygdalina in the treatment of uncomplicated malaria. Journal of Alternative and Complementary Medicine 15(11):1231-1237. |
|
|
Greenwood B, Bojang K, Whitty C, Targett C (2005). Malaria Lancet [Internet]. The Lancet pp. 1487-1498. Available from: |
|
|
Jabeen S, Shah MT, XKhan S, Hayat MQ (2010). Determination of major and trace elements in ten important folk therapeutic plants of Haripur basin, Pakistan. Journal of Medicinal Plants Research 4(7):559-66. |
|
|
Kabata-Pendias A (2011). Trace elements in soils and plants. CRC Press, Taylor and Francis group. Boca Raton (FL). |
|
|
Katuura E, Waako P, Tabuti JRS, Bukenya-Ziraba R, Ogwal-Okeng J (2007a). Antiplasmodial activity of extracts of selected medicinal plants used by local communities in western Uganda for treatment of malaria. In: African Journal of Ecology pp. 94-98. |
|
|
Katuura E, Waako P, Ogwal-Okeng J, Bukenya-Ziraba R (2007b). Traditional treatment of malaria in Mbarara District, western Uganda. African Journal of Ecology 45:48-51. |
|
|
Khan SA, Khan L, Hussain I, Marwat KB, Akhtar N (2008). Profile of heavy metals in selected medicinal plants. Journal of Research in Weed Science 14(12):101-110. |
|
|
Korfali SI, Mroueh M, Al-Zein M, Salem R (2013). Metal Concentration in Commonly Used Medicinal Herbs and Infusion by Lebanese Population: Health Impact. Journal of Food Research 2(2):70. |
|
|
Kulhari A, Sheorayan A, Bajar S, Sarkar S, Chaudhury A, Kalia RK (2013). Investigation of heavy metals in frequently utilized medicinal plants collected from environmentally diverse locations of north western India. Springerplus 2(1):1-9. |
|
|
Kumar S, Yadav M, Yadav A, Rohilla P, Yadav JP (2017). Antiplasmodial potential and quantification of aloin and aloe-emodin in Aloe vera collected from different climatic regions of India. BMC Complementary and Alternative Medicine 17(1):1-10. |
|
|
Maharia RS, Dutta RK, Acharya R, Reddy AVR (2010). Heavy metal bioaccumulation in selected medicinal plants collected from Khetri copper mines and comparison with those collected from fertile soil in Haridwar, India. Journal of Environmental Science and Health Part B 45(2):174-181. |
|
|
Maobe M, Gatebe E, Gitu L, Rotich H (2012). Profile of heavy metals in selected medicinal plants used for the treatment of diabetes, malaria and pneumonia in Kisii Region, Southwest Kenya. Global Journal of Pharmacology 6(3):245-251. |
|
|
Murray RK, Granner DK, Mayes PA, Rodwell V (2000). Harper's biochemistry / Robert K. Murray ... [et al.]. 25th Edition. McGraw-Hill, Health Profession Division, USA. Prentice Hall International. |
|
|
Mwesigye AR, Young SD, Bailey EH, Tumwebaze SB (2016). Population exposure to trace elements in the Kilembe copper mine area, Western Uganda: A pilot study. Science of the Total Environment 573:366-375. |
|
|
Mwesigye AR, Young SD, Bailey EH, Tumwebaze SB (2019). Uptake of trace elements by food crops grown within the Kilembe copper mine catchment, Western Uganda. Journal of Geochemical Exploration 207:106377. |
|
|
Mwesigye AR, Tumwebaze SB (2017). Water contamination with heavy metals and trace elements from Kilembe copper mine and tailing sites in Western Uganda: implications for domestic water quality. Chemosphere 169:281-287. |
|
|
Nkuba LL, Mohammed NK (2017). Heavy metals and essential elements in selected medicinal plants commonly used for medicine in Tanzania. Chemical Science International Journal 19(2):1-11. |
|
|
Obi E, Akunyili DN, Ekpo B, Orisakwe OE (2006). Heavy metal hazards of Nigerian herbal remedies. Science of the Total Environment 369(1-3):35-41. |
|
|
Orem JN, Zikusooka CM (2010). Health financing reform in Uganda: How equitable is the proposed national health insurance scheme? International Journal for Equity in Health 9 p. |
|
|
Orwa JA, Pharm B (2002). Herbal medicine in Kenya: Evidence of safety and efficacy. East African Medical Journal 79(7):341-342. |
|
|
Owor M, Hartwig T, Muwanga A, Zachmann D, Pohl W (2007). Impact of tailings from the Kilembe copper mining district on Lake George, Uganda. Environmental Geology 51(6):1065-1075. |
|
|
Peplow D (1999). Environmental Impacts of Mining in Eastern Washington. Center for Water and Watershed Studies Fact Sheet. University of Washington, Seattle. |
|
|
Radulescu C, Stihi C, Popescu IV, Ionita I, Dulama ID, Chilian A, Let D (2013) Assessment of heavy metals level in some perennial medicinal plants by flame atomic absorption Spectrometry. Romanian Reports in Physics 65(1):246-260. |
|
|
Soetan KO, Olaiya CO, Oyewole OE (2010). The importance of mineral elements for humans, domestic animals and plants: A review, African Journal of Food Science 4(5):200-222. |
|
|
Stangeland T, Alele PE, Katuura E, Lye KA (2011). Plants used to treat malaria in Nyakayojo sub-county, western Uganda. Journal of Ethnopharmacology 137(1):154-166. |
|
|
Szentmihályi K, Marczal G, Then M (2006): Medicinal plants in view of trace elements. Thaiszia - Journal of Botany 16:99-107. |
|
|
Willcox ML, Bodeker G (2004) Traditional herbal medicines for malaria. British Medical Journal 329(7475):1156-1159. |
|
|
World Health Organization (WHO) (2013). WHO Traditional Medicine Strategy 2014-2023. 16 p. |
|
|
WHO/FAO (1998). Quality Control Methods for Medicinal Plant Material. Geneva, Switzerland. |
|
|
Zhao YF, Shi XZ, Huang B, Yu DS, Wang HJ, Sun WX (2007). Spatial Distribution of Heavy Metals in Agricultural Soils of an Industry-Based Peri-Urban Area in Wuxi, China1 1 Project supported by the RURBIFARM (Sustainable Farming at the Rural-Urban Interface) project of the European Union (No. ICA4-CT-2002-10021). Pedosphere 17(1):44-51. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0