ABSTRACT
Aquatic macrophytes and benthos are unchangeable biological filters and they carry out purification of the water bodies by accumulating dissolved metals and toxins in their tissues. In view of their potential to entrap several toxic heavy metals, 3 groups of benthos and 6 macrophytes (submerged species: Potamogeton pectinatus, Ceratophyllum demersum and Najas armata); (floating species: Lemna gibba and Eichhornia crassipes root and shoot) and (emergent species: Phragmites australis shoot) were collected from 15 different locations on Lake Burullus and analyzed for determination of 6 heavy metals (Fe, Zn, Ni, Cu, Pb and Cd) contenets. The study was aimed at understanding the importance of these benthos and macrophytes in accumulation of toxic metals and suggesting preservation and restoration of Lake Burullus ecosystem. The distribution of the investigated metals in water, sediments, benthos and aquatic plants of the lake showed that, the eastern and eastern southern parts of the lake have generally higher concentrations of heavy metals than the western and middle one. Potamogeton pectinatus showed high contents of Pb, Cd and Zn respectively. On the other hands Eichhornia crassipes showed high level of copper while in Ceratophyllum demersum high concentration of Iron was detected. The present study reveals that the aquatic macrophytes and benthos play a very significant role in removing of the different metals from the aquatic environments and they probably reduced the effect of high concentrations of these metals on the lake ecosystem. Bioaccumulation factor values showed that the trend of accumulation of most metals in the benthos was as follows: Mullusca > Arthropoda > Annelida > and in aquatic plants as: Lemna gibba> Potamogeton pectinatus > Ceratophyllum demersum > Eichhornia crassipes Root > Najas armata>phragmites australis shoot> Eichhornia crassipes shoot, which make them suitable candidates to be used in biomonitoring surveys as a good tools for heavy metal pollution markers, in the biological treatment of the polluted water and in sustainable development of Lake Burullus.
Key words: Biofilter, bioaccumulation, sustainable developments, biological treatment, macro benthos, aquatic plants, sustainable management.
The term heavy metal refers to any metallic chemical element that has a relatively high density and is highly toxic or poisonous at low concentrations (Harris and Santos, 2000). Macrophytes are aquatic plants, growing in/or near water that are emergent, submerged or floating. Macrophytes are considered as important component of the aquatic ecosystem not only as food source for aquatic invertebrates, but also act as an efficient accumulator of heavy metals (Devlin, 1967; Chung and Jeng, 1974). They are unchangeable biological filters that play an important role in the maintenance of aquatic ecosystem.
Some sources of heavy metals are industry, municipal wastewater, atmospheric pollution, urban runoff, river dumping, and shore erosion. High levels of Cd, Cu, Pb, and Fe can act as ecological toxins in aquatic and terrestrial ecosystems. Some of these metals (Cu, Ni, Cr and Zn) are essential trace metals to living organisms, but become toxic at higher concentrations. Others, such as Pb and Cd have no known biological function but are toxic elements (Guilizzoni, 1991; Balsberg-Påhlsson, 1989). Many of the aquatic macrophytes and benthos are found to be the potential scavengers of heavy metals from water and wetlands sediments (Gulati et al., 1979). The present investigation was planned and executed considering the potentials of benthos and macrophytes as biological filters for metals that become bound to living materials.
Biomonitoring of pollutants using accumulator species is based on the capacity which has some plants and animal taxa have to accumulate relatively large amounts of certain pollutants by concentration many times higher than those of the surrounding waters, (Nafea, 2005). In addition, the pollutants concentration in sediments and the organisms are the result of the past as well as the recent pollution level of the environment in which the organism lives, while the pollutant concentrations in the water only indicate the situation at the time or seasons of sampling (Ravera et al., 2003).
Although, Lake Burullus attracts attention of many authors because of its economic and scientifically importance to study its unique ecosystem but, the studies dealing with the accumulation of heavy metals in different ecosystem components are still scarce except few studies (Elsaraf, 1995a; Radwan and Lotfy, 2002; Nafea, 2005). The present study deals with the aquatic plants and macro benthos as biomarkers and bio accumulators for heavy metals, in order to use these aquatic plants and benthos in sustainable development and management of Lake Burullus.
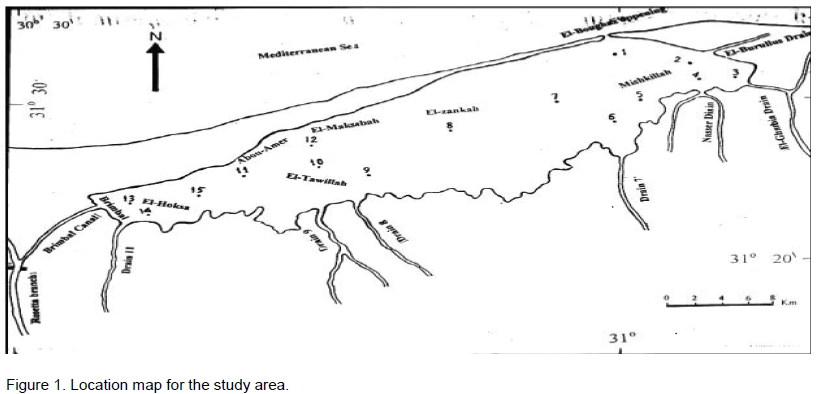
The study area
Al Burullus Lake is situated along the Mediterranean coast. It occupies more or less, a central position between the two branches of the Nile and extends between 31° 22' - 31° 26' N and 30° 33' - 31° 07' E. It’s a shallow brackish lake connected with the sea by a small outlet (Boughaz), about 44 m width and 150 m length. The length of the lake was about 65 km, and its width varies between 6 and 16 km, with an average of about 11 km. The depth of the lake anges between 0.42 and 2.07 m. The eastern sector of the lake is the shallowest, showing an average depth of 0.8 m. The present area of the lake is about 410 km2 (100,000 Feddan), of which 370 km2 is open water. The capacity of the lake is about 330 million cubic meters. The eastern and southern parts of the lake receives agricultural sewage drainage water through 8 drains and one brackish water canal, while saline water enters the lake from the sea through El-Boughaz (Figure 1).
Methods
The study focused primarily on metal investigation in water, sediments, benthos and aquatic plants. The sampling program was carried out in the summer and winter of 2013. Aquatic plants and benthos were collected from the 15 sites and five samples were prepared for each species at every sampling site. At the same time water and sediment samples were collected at the corresponding sampling locations. The collected water was filtered through a Whatman glass-fiber filter (0.45 μm). The filtered water was stored in a 0.5-L polypropylene bottle to avoid any adsorption of metals on the wall of the sample bottles; the filtered water was preserved by acidification with 1.0 ml concentrated nitric acid. Water analysis for heavy metals was according to Solvent extraction method (APHA, 1992). The sediment samples were air dried at room temperature (25°C) for 10-15 days, then ground in a mortar and sieved in 0.5 nm sieve, the samples were then finally stored for analysis according to Moore and Chapman (1986).
Five plant samples were mixed with each other's and analyzed for heavy metals where the dry samples of Macrophytes were wet-digested in a mixture of concentrated nitric acid and perchloric acid (4:1 v/v) (Sawicka-Kapusta, 1978). Samples were analyzed with a Perkin Elmer model 2380 atomic adsorption spectrophotometer (A.A.S.). Bottom fauna were classified to three main groups (Mullusca, arthropoda and Annelida), and digested after drying according to Metcalfe-Smith (1994). Method metals concentrations were determined using atomic absorption (Perkin Elemer Model 3700) with flameless graphite furnace (GA-2). The bioaccumulation factor was calculated according to Klavinš et al. (1998) as follow: BAF = M_tissue / M_water or sediments where M_tissue is metal concentration in plant tissue and M_water is the metal concentration in water or sediments.
Water and sediments
Copper content in water of Lake Burullus ranged between19.7 μg/l in the western parts and 35.8 μg/l in the eastern part. Its values in sediment ranged from 19.4 (μg/g) dry wt and 47.9 μg/g dry wt. in the western and eastern sites, respectively. Iron content in water ranged between 25.3 μg/l in station 8 and 60.4 μg/l in station 4. Its Iron values in sediments ranged between 42.4 μg/g dry wt.in site 15 and 97.5 μg/g dry wt in site 4. Cadmium contents ranged between 2.9 μg/l in station10 and 8.5 μg/l in station 4 while in sediments ranged between 3.2 μg/g in station 10 and 8.5 μg/g in station 6. Zinc contents
in water ranged between 20.6 μg/l in station15 and 55.3 μg/l in station 4 while in sediments it ranged between 24.2 μg/g in station15 and 97.2 μg/g in station 4.
Lead content showed its high values in water at site 4 (10.1 μg/l) and low value (4.5 μg/l) in station 15 while in sediments high value was 27.5 μg/g in station 1 and 6.5 μg/g in station15. Nickel showed its high value in water (10.3 μg/l) in station 2 and the low one (4.3 μg/l) in station 6 while in sediments high value recorded 19.7 μg/g in station 2 and 7.1 μg/g in station 15. The metals concen-trations are given as shown in Tables 1 and 2.
Aquatic plants
Copper contents in the aquatic plants showed high range of variation which ranged between 13.9 μg/g in Eichhornia root in eastern site and 5.1 μg/g in Ceratophyllum in western site. Iron contents in aquatic plants ranged between 107 μg/g in potamogeton and 50 μg/g in Najas, while cadmium content ranged between 1.0 μg/g in Ceratophyllum and 4.7 μg/g in potamogeton. On the other hand nickel ranged between 15.3 μg/g in Lemna and 5.8 μg/g in Najas. High value of Lead observed at potamogeton 15.4 μg/g and low value 5.5 μg/g at Eichhornia shoot. Zinc contents ranged between 98 μg/g in Lemna and 43 μg/g in Eichhornia shoot as shown in Table 3.
Benthos mullusca
Benthos mullusca showed high ranges of metal content in their bodies and tissues more than Arthropoda and Annelida as shown in Table 4.
Bioaccumulation factor
The bioaccumulation values of heavy metal by aquatic plants in relation to water ranged between ( 260-548, 1256-5370, 200-913,1111-2392,1171-2722 and 850-2220 for Cu, Fe, Cd, Zn, Pb and Ni, respectively (Table 3), while in benthos in relation to sediments ranged between 0.59-0.69, 0.96-1.3, 0.32-0.62, 1.1-1.44, 0.63-0.75 and 0.93-1.2 for Cu, Fe, Cd, Zn, Pb and Ni respectively (Table 4).
Generally the high concentrations of heavy metals were measured in the sediments, benthos and macrophytes in the eastern sites of the lake, while the low concentrations were detected in the middle and western sites of the lake. The concentration of lead varied from site to site where the high concentrations were detected in water, sediments, Mullusca and Potamogeton pectinatus in the eastern site (8.06 μg/l, 15, 15 and 13 μg/g dry wt) respectively while the low concentrations were measured in the western parts of the lake (6.1 μg/l, 8.5,10 and 5.5 μg/g dry wt) respectively (Tables 1, 2, 3 and 4). The variation of lead content in benthos sediments and macrophytes depends on the inflow of many sources of pollution from sewage, agricultural and industrial wastes into the lake. El-Sarraf (1995a) mentioned that in Lake Manzala P. pectinatus had high lead concentration (26 .6 μg/g dry wt) and thus it is a good lead contamination indicator. This agrees with the conclusion of Abo-Rady (1977) that P. pectinatus may be considered as an indicator for lead in Lake Manzala. Our results in burullus confirm this conclusion (Radwan and shokier, 2005).
High levels of cadmium contents were found in L. gibba and Eichhornia root, Mullusca and sediments of the eastern parts (4.5, 6.5 and 4.2 μg/g dry wt.) while the lowest concentrations of cadmium were observed in C. demersum (1 μg/g dry wt). El-Sarraf (1995b) mentioned that there was high significant correlation between lead and cadmium concentration in aquatic plants which is probably attributed to their association in the same phase during assimilation (Tables 2, 3 and 4).
The macrophytes and benthos have different levels of zinc concentrations in their organs where the high levels were recorded in L. gibba, P. pectinatus and Mullusca (83, 77.6, 107 and 104 μg/g dry wt.) respectively, Alloway and Davis, (1971); while the low concentrations were found in N. armata, E. crassipes shoot and Annelida (50, 46 and 49 μ g/g dry wt.) respectively. Heydt (1977) found that P. pectinatus has high zinc content which ranged between 16.5 and 517μ g/g dry. For weight in Elsenz River, Bauda et al. (1981) recorded that in Lake Manzala the mean concentration of Zinc level in the same species is 168 μg/g dry wt. El-Sarraf (1995a) found that the concentration of Zinc in P. pectinatus was 117 μg/g dry weights whereas Abo-Rady (1977) found that the zinc content of P. pectinatus ranged between 137 and 213 μg/g dry weight in Leine River.
The copper concentrations fluctuated between 5.4 and 35 μg/g dry wt. in plants, benthos and sediments. The copper content in Lake Manzala varied from 5.0 to 37.6 μg/g wt. in potamogeton pectinatus (Boudo et al. 1988). On the other hand El-Sarraf (1995a) found that copper content showed a small range of fluctuation with irregular concentration in aquatic plants. In Ceratophyllum demersum the highest level was 18.5 μg/g dry wt. The positive correlation between Cu and Zn was attributed to the same biological behaviors during the assimilation in macrophytes (Alloway and Davis, 1971; El-Sarraf, 1995b).
The concentrations of trace metals (Cu, Zn, Pb, Fe, Ni and Cd) in the aquatic macrophytes, benthos and sediments varied according to their locations at the different parts of the lake; this depends on the source of pollution invading the lake from several directions. Seidal (1996) and Ozimek (1978) recorded high contents of trace metals in the macrophytes growing in habitats affected by industrials effluents and effect of sewage and industrial wastes on the chemical composition of aquatic macrophytes is very obvious. The magnitude of aquatic plants and benthos to assimilate heavy metals would be largely dependent upon the levels of these metals in the water and sediment. The removal of certain mineral from water reservoirs by submerged macrophytes and benthos is observed as practical methods for water purification (Hillman and Cully, 1978). The high variations found in the element content of the aquatic macrophytes both between species and within species were related to different location. Crowder and Painter (1991) inferred that the variation of metals content in macrophytes is not necessarily bioaccumulations or biomagnified these metals from the sediment and it may be attributed to site-specific and species specific differences in metals uptake. From this hypothesis it is important to mention that the non-essential trace metals such as lead and cadmium were highly concentrated in Lake Burullus eastern side (Nafea, 2005) and the industrial wastes may also be responsible for the elevation of the Pb and Cd in Lake Burullus. The order of abundance of the trace metals in the macrophytes and benthos of Lake Burullus were:
(1) Lead: Lemna > Potamogeton > Eichh. Root > Ceratophyllum > phragmites sh. >Najas > Eichh. Sh., Mullusca>Arthropoda> Annelida.
(2) Cadmium: Lemna > potamogeton > Najas>phragmites > Eichhornia Root > Eichh. Shoot > Ceratophyllum.
And in benthos: Mullusca>arthropoda> Annelida
(3) Zinc: Lemna > Ceratophyllum > Potamogeton > Eichh. Root >phragmites shoot > Eichh. Shoot > Najas
And in benthos: Mullusca>arthropoda> Annelida
(4) Copper: Lemna > Eichh. Root > Najas >phrag. shoot >Eichh.Shoot >Ceratophyllum.> Potamogeton
And in benthos: Mullusca > Annelida> arthropoda.
(5)Nickel: Lemna > Potamogeton > Ceratophyllum > Eichh. Root > Najas > phrag. Shoot > Eichh. Shoot and in benthos: Mullusca > arthropoda > Annelida.
(6)Iron: Lemna > Ceratophyllum >Eichh. Root > Najas > phrag.shoot> Potamogeton > Eichh. Shoot and in benthos: Mullusca>arthropoda> Annelida.
Lemna gibba, potamogeton pectinatus and Mullusca showed the higher capacity of heavy metal accumulation than the other aquatic plants and benthos groups. Aquatic macrophytes and benthos can be used as bio-indicator and biomarkers for water and sediment pollution as they can trap micro- and macro-elements (inorganic pollutions) as investigated by Fayed and Abdel-Shafy (1985). El-Khatib and Sawaf (1998) reported that the concentrations of heavy metals in macrophytes were positively related to the concentration in the environment and the macrophytes have high potential power for pollution monitoring (Yamanowska et al., 1999).
Depending on the heavy metals concentration in the aquatic macrophytes and benthos it can be concluded that the aquatic macrophytes and benthos can accumulate heavy metals and have a restricted role in the treatment and control of pollution of the aquatic ecosystems. Accordingly, the macrophytes and benthos can be considerable as reliable way for Biomonitoring the heavy metals contamination in Lake Burullus. Trace metals concentration in macrophytes and benthos species widely differ. This can confirmed if a species is used for heavy metals monitoring within one or different areas. Ghobrial (2000b) reported that Ceratophyllum demersum can accumulate zinc more than Cu, Pb and Cd and acts as a potential biological filter for trace metals removal from domestic effluents and has a capacity to retain heavy metals in its tissues.
High range of the heavy metals concentration in the studied aquatic plants and benthos indicates different extent of pollution; this high variability is associated with the different absorption rate for the heavy metals by the aquatic plants and benthos (Pajevi? et al., 2003; Klink, 2004; Maria et al., 2006).
Recently, there has been growing interest in the use of metal-accumulating plants or benthos for the removal of heavy metals from contaminated aqueous streams, in the biological purification of waste water and in Biomonitoring of heavy metals pollution in the Egyptian lakes (Nafea, 2005).
We conclude that there is a uniform pattern of heavy metal variation in the macrophytes, sediments and benthos of Lake Burullus. In general, values of some metals like iron, zinc and copper are higher in almost all the specimens. This shows the universal importance of these macrophytes, sediments and benthos in cleaning up of the aquatic environment. The results presented here could be very useful for environmental monitoring and checking the health of the water body. The aquatic macrophytes and benthos were found to be the potential source for accumulation of heavy metals from water and sediments and act as biofilters for metals. Accordingly they could be used in sustainable development, management and pollution assessment program in the northern deltaic lakes of Egypt especially lake burullus.
The author(s) have not declared any conflict of interests.
REFERENCES
|
Abo-Rady MD (1977). Die Belastungder oberch Leine mits chwermetallen. Durc Kommunate and. Industrielle Abwasser, ermittelt and hand von wasser, sediment, F.shared prlanzenunt, enchungen. Dess. Univ. Gyttingen (FRG) 120. |
|
|
Alloway, B.J., Davis, B.E (1971). Heavy metal content of plants growing on soils contaminated by lead mining. J. Agric. Sci. Camb. 76:321-323.
Crossref |
|
|
|
APHA (1992). Standard methods for the examination of water and waste water, Washington, 18 Ed. |
|
|
|
Bauda R, Galanti P, Guilizzoni P, Varini P (1981). Relationship between heavy metals and aquatic organisms in Lake Manzala hydrograph systems (Northern Italy): Mem. IST. Ital. Hdrobiol. 39:203-225. |
|
|
|
Chung IH, Jeng SS (1974). Heavy metal pollution of Ta-Tu River. Bull. Inst. Zool. Acad. Sci.13:69-73. |
|
|
Crowder A, Painter P (1991). Submerged macrophytes in Lake Ontario: current know edge, importance, threats to stability and needed studies. Can. J. fish. Aquat. Sci. 48:1539-1545.
Crossref |
|
|
|
Devlin RM (1967). Plant Physiology. Reinhold, New York. p. 564. |
|
|
|
El-Khatib A, El-Sawaf N (1998). Differential trapping of heavy metals by macrophytes in different water bodies near Sohag, Upper Egypt. Acta Hydrobiologica 40:67-73. |
|
|
|
El-Sarraf WM (1995a). Trace metals concentration of aquatic Macrophytes in Lake Manzalah, Egypt. Bull. Nat. Inst. Ocean. and Fish. A.R.E. 21(1):171-181 |
|
|
|
El-Sarraf, W.M. (1995b): Chemical analysis of Some macrophytes in Maruit and Idku Lakes, Egypt. Alex. J. Agr. Res.40 (1): 255-271. |
|
|
|
EPA (1983). Methods of chemical analysis of water and waste water USA. Environmental Protection Agency (EPA) 690:4-79. |
|
|
Fayed S, Abd-El-Shafy H (1985). Accumulation of Cu, Zn, Cd and Pb by aquatic Macrophytes. Environ. Int. 11(1):77-88.
Crossref |
|
|
Gbaruko, B. C.and. Friday, O.U. (2007): Bioaccumulation of heavy metals in some fauna and flora Int. J. Environ. Sci. Tech., 4 (2): 197-202,
Crossref |
|
|
|
Ghobrial MG (2000b). Treatment of Cadmium, Copper, Zinc and Iron in waste water by the Horn wort Ceratophyllum demerssum. Egypt. J. Aquat. Biol. Fish. 4(1):35-46. |
|
|
Guilizzoni P (1991). The role of heavy metals and toxic materials in thePhysiological ecology of submersed macrophytes. Aquat. Bot. 41:87-109.
Crossref |
|
|
|
Gulati KL, Nagpaul KK, Bukhari SS (1979). Uranium, boron, nitrogen, phosphorus and potassium in leaves of mangroves, Mahasagar Bull. Nat. Inst. Oceangra. 12:183-186. |
|
|
Harris RR, Santos MCF (2000) Heavy metal contamination and physiological variability in the Brazilian mangrove crabs Ucides cordatus and Callinectes danae (Crustacea: Decapoda). Mar. Biol. 137:691-703.
Crossref |
|
|
|
Heydt G (1977). Schwerrnetallg chalteven Wasser, wasser.p franzen, chironomikae and Mullusca der. Elsenz. Dipl. Arbeituciv, Heidelberg. 143. |
|
|
|
Hillman WS, Culley DD (1978). The used of Duck weed. Am. Sci. 66:442-451. |
|
|
Klavinš M, Briede A, Parele E, Rodinov V, Klavina I (1998). Metal accumulation in sediments and benthic invertebrates in Lakes of Latvia. Chemosphere 36 (15): 3043-3053.
Crossref |
|
|
Metcalfe-Smith JL (1994). Influence of species and sex on metal residues in fresh water mussels (family Unionidae) from the St. Lawrence River, with implications for biomonitoring programs. Environ. Toxicol. Chem. 13: 1433-1443.
Crossref |
|
|
|
Moore P, Chapman S (1986): Methods in plant ecology. Second edition, Blackwell Scientific Publication. |
|
|
|
Nafea EMA (2005). On the ecology and sustainable development of the northern delta lakes, Egypt. PhD Thesis, Mansoura University Faculty of Science. |
|
|
|
Radwan AR, Lotfy IH (2002). On the pollution of Burullus Lake water and sediments by heavy metals. Egypt. J. Aquat. Biol. Fish 6(4):147-164. |
|
|
|
Radwan, A., shokier, L. (2005): study on the heavy metals in some fishes and aquatic plants of Burullus Lake Bull. Nat. Inst. Ocean. Fish. AR.E, 32: 215-231. |
|
|
|
Sawicka- Kapusta K (1978). Estimation of the content of heavy metals in atlas of rae-deer from silesian Woods. Arch. Ochr. Sord. 1:107-121. |
|
|
|
Seidal K (1966). Biologischer seenschutzpfan zer wasser filter-foederation Europaischer Gewasser schutz. Symp. 76: 357 - 195. |