Full Length Research Paper
ABSTRACT
The aim of this paper is to size the PFR reactor and to deduce the likely useful mass of the catalyst in the PBR for the production of hydrocarbons such as propane, butane and pentane from methanol via dimethyl ether (DME). The use of these reactors would require kinetic analysis, material balance, energy balance and then sizing. The interest in the chemical catalytic conversion of synthesis gas to gasoline suggests that the fuels industry may have come full circle from the time that synthesis gas from either natural gas or coal gasification were economic alternatives to crude oil. In this instance, cellulosic waste is the alternative raw materials that can be provided from DRCs logging waste and from agricultural activities. In the case of Muanda in the DRC, as in neighboring countries, the exploitation of black gold does not seem to have played any positive role in terms of development.
Key words: Packed bed reactor, plug flow reactor, modeling, simulation.
INTRODUCTION
Growth and development of population has led to increased demand for energy; forcing projected rise in overall energy demand by around 16% in the period 2015 - 2040, while gasoline demand is expected to grow by around 13%. Owing to its inherent contribution to a decrease in crude oil reserves, much focus has been on climate change; and its impact on greenhouse gas emissions in the environment, in particular CO2, has led to the development and increasing use of alternative sources of energies as well as synthesis of biofuels (Rojas, 2018) as a sustainable approach for producing gasoline-range hydrocarbon biofuels. It can also mitigate the challenges associated with crude oil, such as environmental pollution, scarcity and cost of fossil-derived fuels, as well as political instability in major crude oil-producing countries. MTG process begins with the production of syngas, followed by conversion of syngas to methanol and production of gasoline from methanol (Chakraborty et al., 2022).
As is often the case in oil extraction areas, crude oil leaks, gas flaring and toxic wastes are part of the daily life of Muanda's citizens. The authors of the report have gathered numerous textual and visual elements confirming the reality and extent of these pollution problems. Although residents have been complaining for many years about the severe degradation of all natural environments (fresh water, sea water, soil, air) caused by drilling, their accusations have been confirmed on several occasions by experts or local officials (https://multinationales.org).
Biofuels are produced by various means such as the FischerTropsch method or by methanol. This relates to the production of synthesis gas (mixture of CO and H2) from organic raw materials and its subsequent conversion to methanol, followed by dehydration to dimethyl ether and conversion to hydrocarbons. The final stage has attracted more attention in recent years, which is linked to the benefits that EMR comes from the upgrading of the feed stream of a low H2/CO ratio, either from biomass or coal or natural gas. The EMR has proven its applicability both as an intermediate and as a final product. Indeed, the direct use of DME as a transport fuel has been put into practice and proven (Rojas, 2018). Syngas, which is derived from wood, waste wood, cellulose or lignin is a renewable fuel type. Depending upon the feedstock type, it typically begins as a blend of hydrogen, carbon monoxide, carbon dioxide, methane and nitrogen. These components can be separated and the methane purified to create bioSNG. Both syngas and bioSNG can be used as a fuel in gas engines (https:www.clarke-energy.com/applications/renewable-gas/).
The Democratic Republic of Congo (DRC) is the second largest country in Africa and the third most populous. It has immense resources - forests, water, fertile soils and high rainfall, plus considerable mineral reserves: copper, cobalt, coltan, diamonds, gold, zinc and other base metals as well as oil.
Forests cover about 60% of the national territory (or some 134 million hectares), including most of the rainforest of the Congo Basin, which is the second largest complex of tropical forests in the world after the Amazon. These forests are crucial for the subsistence of around 40 million Congolese, to whom they provide food, medicine, energy, building materials and a source of income (https://reliefweb.int/report/democratic-republic-congo).
The aim of this work is to perform the sizing of reactors series for the production of hydrocarbons from methanol, with methanol based syngas and BioSNG derived from wood, waste wood, cellulose or lignin for the yield of a renewable fuel type, as well as gasoline based on methanol. With adequate conservation and management, the forests of the DRC could bring long-lasting multiple benefits to the country and the planet (https://reliefweb.int/report/democratic-republic-congo). Indeed, the reactor constitutes a main element in the design of the process which requires kinetic analysis, material balance, energy balance and then dimensioning.
METHODOLOGY
The different solutions are obtained by digital simulation and modeling by implementing the different values obtained, the results of which follow linear and exponential trend curves. The parameters related to the geometry and operation of series reactors in continuous mode, in particular the plug flow reactor and the packed bed reactor (fixed bed reactor), were simulated to obtain the best dimensions and optimize the process from the degree of conversion of methanol.
Mathematical model
Material and thermal balance of the PBR

The following assumptions were made for the development of the dimensioning for which the PFR performance of the reaction rate equations is of the first order (Figures 1 and 2).
The various pre-requisites are such that: i) the reactor operates in a state of equilibrium ii) the reaction is in the liquid phase with constant density, and iii) the pressure drop along the reactor is negligible (Couper, 2012).
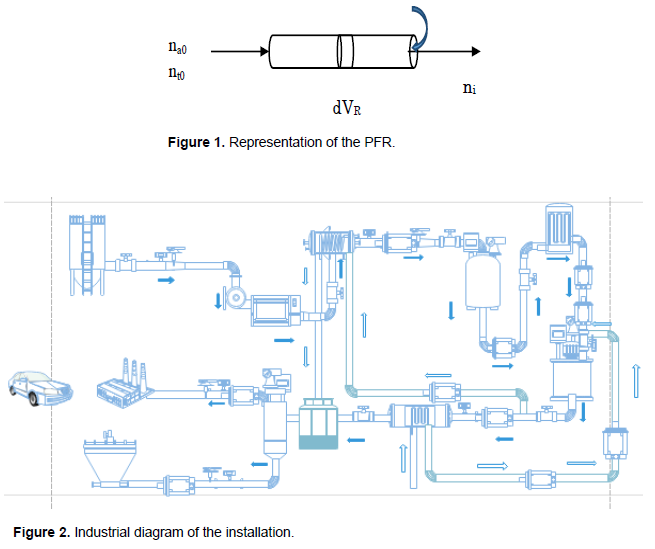
Modeling of PFR and PBR reactors in series and calculation of parameters
Considering the above relation, the equation giving the volume of the PFR is as follows (Diagbonia and Ehirim, 2019):

After adjusting the material balance from the differential elements to give an expression for the volume of the reactor, we have as follows (Couper, 2012):

Development of the dimensioning of the PBR
Firstly, for a heterogeneous reaction (e.g fluid-solid), the mass of the solid catalyst, W, is a bigger problem than that of determining the volume of the reactor (Rosen, 2014).
Thus, for the Packed Bed Reactor (PBR), if the pressure drop and the degradation of the catalyst are neglected for this purpose, its model will have been established (Rosen, 2014) (Figure 2).
Thus, the volume of the reactor depends only on the feed rate.
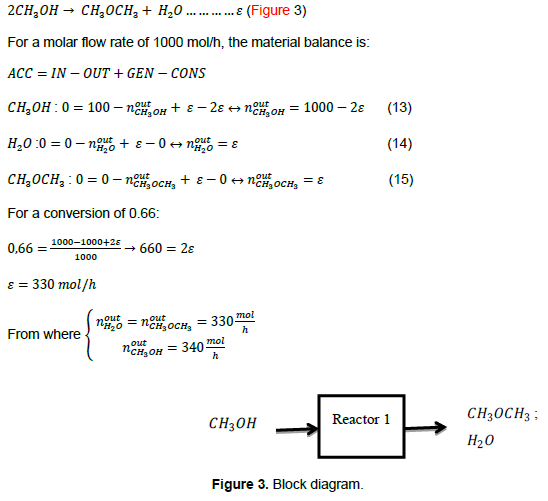
Calculation of the reaction rate (kinetic analysis), material balance, residence time, and PFR volume
Methoxymethane can be produced by the following reaction kinetics (Table 1):

RESULTS AND DISCUSSION
The material balance in the plug flow reactor (PFR) is as follows:
Block diagram
Equilibrium constant
The equilibrium constant is given as follows:
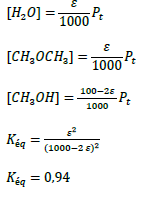
Determination of the temperature in the reactor
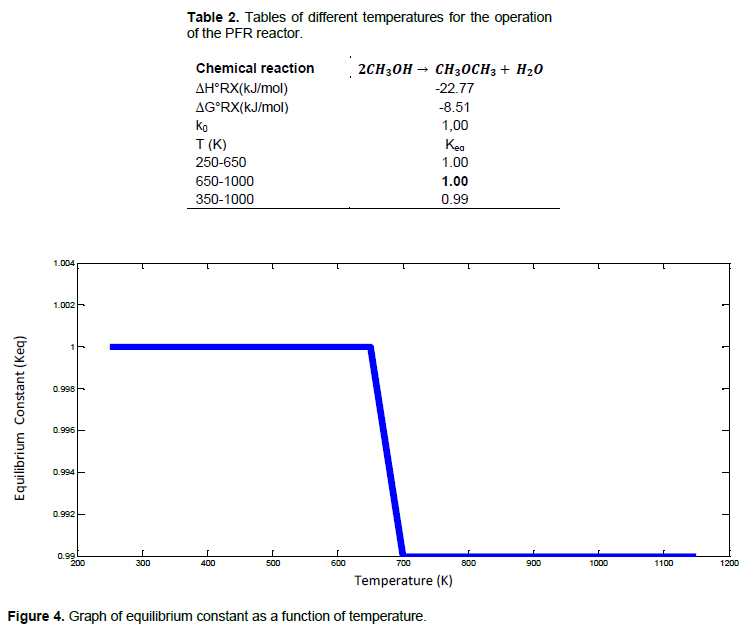
In chemistry, equilibrium constant characterizes the state of equilibrium of a chemical system, with the value of the latter dependent on the reaction considered and the temperature in order to predict the direction of evolution of the system (Table 2). It is noted that the progression of the reaction is effective at the temperature range going from 300 to 650K (Figure 4). Once exceeded, there is a regression of the reaction as indicated by the bending on the curve, which this time stabilizes and remains constant from 700K up to above 1000K.
Calculation of the volume of the PFR reactor
The volume of the reactor is obtained by the following relation:

Hence to calculate the residence time in the reactor, the following relation was used:

Having = 11.24 m3/s and the volume of the reactor, the residence time is = 0.22 s.
Calculation of the mass of the catalyst and the volume of the PBR
The mechanism involved in the methanol to gasoil process is quite complex. A simplified reaction scheme (proposed by Chang and Silvestri, 1977) is shown as follows:
Figure 5 shows the product selectivity measured as a function of a wide range of contact time. The latter measures the contact time between the catalyst and the reagent molecules. At the smallest contact time, that is, in the order of 10-3 hours, water and dimethyl ether are the main products obtained. When the contact time increases, the production of dimethyl ether finally reaches a maximum and then decreases. From the methyl ether, there is now a high probability of further dehydration to give the alken products. With further increase in contact time, alkanes / + alkenes and aromatics are obtained (Rosen, 2014) (Figure 6). Indeed
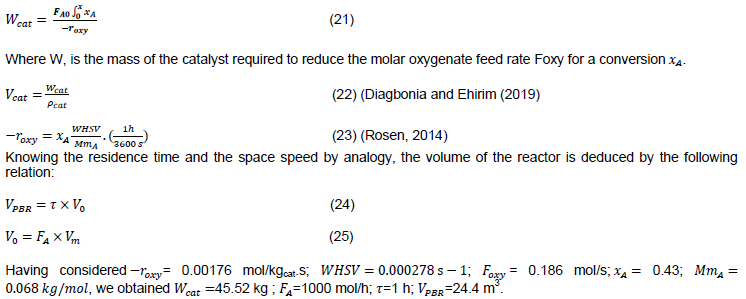
According to the graph in Figure 5, the hydrocarbons are obtained in a residence time of about one hour in the reactor from the experiments of Chang and Silvestri (1977).
Figure 7 gives the volume of the PFR reactor as a function of the fractional conversion of methanol. The volume of the reactor is proportional to the fractional conversion whereas its growth is linear for the PFR. With a fractional conversion = 0.90, the volume of the PFR reactor = 3.28 m3. However, for the feed equilibrium mixture, the desired fractional conversion = 0.67 and the reactor volume = 2.43 m3, for a pressure of 1346.868 Pa and a temperature of 600K.
Figure 8 gives the effect of the concentration of methanol on the volume of the PFR. The change in the volume of the reactor is linear; the greater the concentration of methanol, the greater is the volume of said reactor; it has an optimum concentration = 0.27 mole/m3 for which P = 1346.868 Pa and T = 600K; and the volume of the reactor is 2.43 m3.
Figure 9 gives the mass of the catalyst as a function of the conversion of dimethyl ether and methanol to hydrocarbons. The mass remains constant with the variation of the oxygenate conversion rate. For example, for a conversion of 0.43, the mass of the catalyst remains 45.52 kg. his is also the case for a constant reactor volume of 24.4 m3 and for a reaction rate of 0.00327 mol/kgcat.s.
As regards PBR where the reactions are complex with several kinds of mechanisms established by different authors, however, the model established by Rojas (2018) made the task simpler in terms of the reaction kinetics related to our second reactor.
The physicochemical analysis of the inputs of the PFR and the PBR provided several elements sufficiently necessary in the meticulous approach of the dimensioning, starting in fact, by the establishment of the material balance around the PFR. However, the fractional conversion of methanol into DME can reach 0.9, the feed into the PBR required equilibrium mixing of methanol/ DME/water since for an infinitely small residence time the conversion of unconverted methanol into PFR continued in the PBR until an almost complete conversion is reached (Figure 5). Thus, for this process, therefore knowing the course in the reactors, the fractional conversion established was therefore 0.67 (which can also reach 0.75 for other authors) in the PFR reactor.

Here the considered fuel to the gasifier is of recent biological origin, such as wood or organic waste; the gas produced by the gasifier is a renewable fuel and the power produced by its combustion is renewable. When the fuel to the gasifier is a waste stream, its conversion to power in this manner has the combined benefit of the conversion of this waste into useful products as biofuel route of methanol to gasoline process (https:www.clarke-energy.com/applications/renewable-gas/ visited 04 march 2022 at 15h 57'). The wealth of the DRC in natural resources offers a striking contrast with the poverty of its population (https://reliefweb.int/report/democratic-republic-congo). With those DRC's forests, there is a big potential to improve social and human health by renewable energies industries that will contribute to enhancing the local economic situation.

CONCLUSION
The objective of this work was to size the reactors series (plug flow reactor and packed bed reactor) for the production of gasoline (hydrocarbons). He revealed the importance of the process and various advantages of the latter, mainly from a technical point of view; in fact, the development of a process requires several prerequisites for highlighting the process from design to synthesis (Complete process). This approach helped to orient in a more objective sense what has been the aim of this work.
Determination of the volume of the PBR revealed that the latter depends on the molar feed rate when the molar volume is considered at the standard condition of temperature and pressure (CSTP.) The biggest problem was determining the reaction rate for the methanol / DME mixture conversion to hydrocarbons, in order to deduce the mass of the catalyst. The latter has been shown, to be independent of methanol / DME conversion.
The yield of the process was 43% of the methanol / DME mixture mass entering the PBR. Different topological optimization experiments could improve this efficiency since it is proportional to the conversion of the methanol / DME mixture.
Therefore, the narrow coastal strip of the Democratic Republic of Congo (DRC), at the mouth of the Congo River, is an area of ??oil extraction – as is off neighboring Angola, Congo-Brazzaville and Gabon, although on a smaller scale. In the DRC as in neighboring countries, the exploitation of black gold does not seem to have played any positive role in terms of development. On the contrary perhaps according to a report recently published by a group of French and Congolese non-governmental organizations, these extractive activities have a considerable environmental and social cost for local populations. In Muanda, a coastal town that is home to the deposits, oil exploitation is clearly not synonymous with wealth, but, according to these NGOs, with human rights violations (https://multinationales.org/Perenco-en-RDC-quand-le-petrole).
Modeling and simulation of reactors in PFR and PBR series for the conversion of methanol into hydrocarbons is a route studied in this manuscript for solving this pollution, economic, social and human health problems as is often the case in oil extraction areas. Crude oil leaks, gas flaring and toxic wastes are part of the daily life of Muanda’s citizens (https://multinationales.org/Perenco-en-RDC-quand-le-petrole).
Furthermore, Primus Green Energy who have constructed a 100,000-gallon demonstration plant at Hillsborough, NJ contemplates a variation of the ExxonMobil MTG process. The synthesis gas to be used is a combination of municipal solid waste and natural gas. Similarly, the proposed MTG plant of Sundrop Fuels will be based on gasification of forest wastes supplemented with hydrogen from natural gas. The interest in the chemical catalytic conversion of synthesis gas to gasoline suggests that the fuels industry may have come full circle from the time that synthesis gas from either natural gas or coal gasification were economic alternatives to crude oil. In this instance, cellulosic waste is the alternative raw materials which can be provided from DRCs agricultural activities and logging waste (https://www.sciencedirect.com/topic-engineering-methanol-gasoline-process/).
Finally, all the results shown in this paper had matched with others researches results that dimethyl ether is a building block for the production of green fuels of Ortega Rojas. This manuscript confirm also by sizing, modeling and simulation of PFR and PBR, the prefeasibility to use this one process among economical alternatives to reach that goal or aim of the research, with particular attention on using the different basics reactors PFR and PBR.
NOMENCLATURE

CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Chakraborty JP, Singh S, Maity SK (2022). Advances in the conversion of methanol to gasoline. In Hydrocarbon Biorefinery (pp. 177-200). Elsevier. |
|
|
Chang CD, Silvestri AJ (1977). The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts. Journal of Catalysis 47(2):249-259. |
|
|
Couper JR (2012). Chemical process equipment selection and design, third edition. |
|
|
Diagbonia P, Ehirim OE (2019). Design flow reactors for production of Dimethyl ether. American Journal of Engineering Research 8(8):70-83. |
|
|
Keraron M (2010). Conversion du méthanol en oléfines sur SAPO-34: modélisation cinétique et design de réacteur. Ecole Polytechnique, Montreal (Canada). |
|
|
Rojas CEO (2018). Dimethyl ether as building block for the production of green fuels. |
|
|
Rosen A (2014). Reactor Design. TUFTS Chemical Engineering Review Guide Available at: |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0