Review
ABSTRACT
The review investigates phytoremediation as an alternative environmentally-friendly method of cleaning and restoring hydrocarbon contaminated soils. Phytoremediation is a ‘green’ technology that exploits the natural ability of green plants to remove, degrade or suppress contaminants in soils, sludges, sediments, surface-water and ground-water in an ecologically-friendly manner. Its processes are stimulated by sunlight and microbial biota in the contaminated medium. The use of various mechanisms of phytoremediation is reviewed along with the criteria used in the selection of plant species for phytoremediation exercises. The importance of soil amendments in phytoremediation experiments is also considered. The usefulness of phytoremediation as a viable tool for the remediation of contaminated soils in the crude oil bearing regions of the world, and particularly in Nigeria, have been assessed through the work of previous authors to verify its suitability for the remediation and restoration of contaminated soils.
Key words: Crude oil, phytoremediation, pollution, plant species, soil.
INTRODUCTION
Pollutants are often released into the environment (such as the atmosphere, soil and water) through human actions, such as agricultural and industrial activities, and these substances cause environmental pollution (Ikhuoria and Okieimen, 2000; Erakhrumen, 2007; Jadia and Fulekar, 2008; 2010; Oyedeji et al., 2015). The remediation of such environments after pollution incidents is often necessary and can be effectively achieved using phytoremediation (Erakhrumen, 2007). Phytoremediation is the methodology that exploits the natural ability of green plants to remove, degrade or suppress contaminants in soils, sludges, sediments, surface-water and ground-water in an ecologically-friendly manner and it is stimulated by sunlight. This technology operates on the concept of using ‘nature to cleanse nature’ following contamination of the environment (Osam et al., 2011).
Some plant species are capable of tolerating a wide range of environmental conditions and this ability can be used to modify these conditions (Susarla et al., 2002).
Phytoremediation technology enhances the amelioration and restoration of contaminated environments, such as soils and waters, by growing green plants that have the ability to tolerate and/or remove contaminating substances, thereby restoring the soil and its functions (Peer et al., 2006). It is a non-destructive, cost-effective in situ technology that utilizes plants and their associated micro-organisms to remediate contaminated soils. Cunningham et al. (1996) described it as an in situ use of plants and their associated micro-organisms to degrade, contain or render harmless, contaminants in soil or ground-water. The use of phytoremediation techniques can either be through naturally growing plants in the contaminated soil or by artificial cultivation of selected plant species (Erakhrumen, 2007) and has emerged as a viable option for the remediation of petroleum hydrocarbon polluted sites (Frick et al., 1999; Tanee and Kinako, 2008). A considerable number of regions in the global community continue to record substantial economic growth. In the clean-up of contamination by petroleum hydrocarbons, plants enhance the microbial degradation of contaminants in the rhizosphere (Merkl et al., 2004a, b, 2005; Atagana, 2011).
The use of phytoremediation as a remediation option may not only be to degrade contaminants but can also be used to enhance habitat recovery through the stimulation of vegetative plant growth. Plants can enhance bioremediation processes by absorbing, translocating or sequestering the organic contaminant and removing them from the soil system (Cunningham et al., 1995; Glick, 2003). In a situation where the contaminant, in its present concentration, is not phyto-toxic, the cultivation of plants can be a valuable tool in soil remediation. The mechanism and efficiency of phytoremediation technology depend on the type of contaminant, its bioavailability and the surrounding soil properties (Cunningham and Ow, 1996).
Although the phytoremediation of contaminated soil may be moderately slow, it is, however, environmentally- friendly, inexpensive, requires little equipment and or labour, is easy to perform, and has the benefit that the contaminated sites can be cleaned without removing the polluted soil. The key factor for successful phytoremediation practise is the identification of a plant species that is tolerant of the contaminated site, and can tolerate high concentrations of contaminant(s) in the polluted site. Bamidele and Agbogidi (2006) described an effective phytoremediation plant species as one that thrives well in a contaminated habitat. Some plant species of the families Poaceae, Brassicaceae, Fabaceae, Euphorbiaceae, Asteraceae and Lamiaceae have been identified, and considered as being able to remediate contaminants, due to their extensive root systems and presence of root nodules which house microbes that help in degrading hydrocarbons (Jadia and Fulekar, 2009; Hall et al., 2011).
Phytoremediation technology presents considerable potential for the treatment of contaminated soils and has proved successful in several studies. For instance, Merkl et al. (2004b) reported that the grass, Brachiaria brizantha (Hochst ex A. Rich.) Stapf. and the legumes Centrosema brasilianum (L.) Benth. and Calopogonium mucunoides Desv. are good plant species for phytoremediation because in crude oil contaminated soil they combined high seedling emergence with good biomass production.
White et al. (2006) investigated phytoremediation of alkylated polycyclic aromatic hydrocarbons in a crude oil- contaminated soil and reported that there was enhanced degradation of complex aromatic hydrocarbons attributable to the phytoremediation process. Agbogidi et al. (2007) investigated the use and effectiveness of Tectona grandis (Linn.) and Gmelina arborea (Roxb.) forest tree species of family Lamiaceae for phytoremediation of crude oil contaminated soils and reported that the two plant species are good candidates for phytoremediation, especially when the concentration of the crude oil is low in the contaminated soil. Atagana (2011) reported the bioremediation of co-contamination of crude oil and heavy metals in soil by phytoremediation using Chromolaena odorata (L) King & H.E. Robins of the family Asteraceae in a pot experiment. At the end of the experiment, crude oil was reduced in the soil and the reduction was attributed to natural attenuation and microbial action in the root system (rhizosphere) of the plant. It was also observed that C. odorata (L) has the capability of thriving and remediating crude oil contaminated soil.
Allowing polluted soil to undergo natural self- remediation takes some time (Kinako, 1981). Therefore, polluted soil needs human intervention to accelerate its recovery process after a pollution incident. Pollution is a serious environmental problem in the oil-bearing region of Nigeria (Figure 1). The practise of phytoremediation in developed and developing countries (such as Nigeria) could offer a feasible and economic alternative to achieve the remediation of petroleum hydrocarbon contaminated soils.
MECHANISMS OF PHYTOREMEDIATION
Remediation of soils contaminated with organic substances, including petroleum hydrocarbons, occurs through one, or more, of the following primary mechanisms: phytostabilization, phytoextraction, phytodegradation, phytovolatilization, and rhizodegradation (Figure 2).
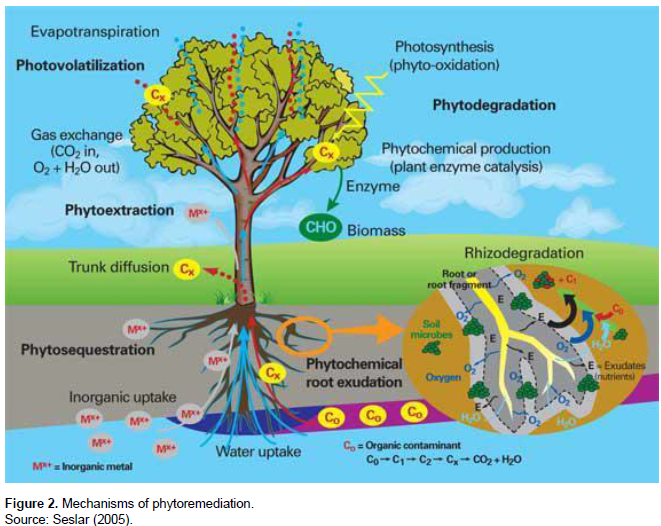
Phytostabilization
Phytostabilization is often referred to as the on-site activation of contaminants and is employed in the remediation of soil, sediment and sludges (Eapen and Dsouza, 2005; USEPA, 2000). In this process, plant roots limit contaminant mobility and availability within soils (Jadia and Fulekar, 2009; Mukhopadhyay and Maiti, 2010). The mechanisms involved may include absorption and accumulation by roots, adsorption onto root surfaces, or chemical precipitation within the root zone (Ghosh and Singh, 2005). Plant uptake and accumulation of petroleum hydrocarbons from contaminated soil, however, is generally quite small. Thus, in the case of petroleum hydrocarbons, phytostabilization may simply be involved in the establishment of vegetative cover to minimize potential migration of the contaminant through erosion, leaching or soil dispersion (Jadia and Fulekar, 2009; Raskin and Ensley, 2000). Plants (especially trees) can also act as organic pumps, transpiring water, and in turn retaining the contaminant in the root zone which helps prevent inter-site mobility (Berti and Cunningham, 2000). Phytostabilization has proved successful with low concentrations of contaminants in soil (Jadia and Fulenkar, 2009). It involves accumulation of the contaminants in the root zone. The plants harbour and tolerate the contaminants within the root system and this is one of the major advantages of this process (USEPA, 2000).
Phytovolatilization
Phytovolatilization refers to the use of plants for the uptake of contaminants. The contaminants are taken up by plants, converted into volatile, less chemically toxic substances and transpired into the atmosphere (Jabeen et al., 2009; Jadia and Fulekar, 2009) through the open stomata on the leaf surface and some radial diffusion from the stem tissues and plant bark (Kamath et al., 2004). Some plants have the ability to absorb heavy metals and convert them to a gaseous form in plant tissues and thereafter release them into the atmosphere (Ghosh and Singh, 2005).
Phytoextraction
Phytoextraction involves the extraction of contaminants by plants through their root system and its subsequent accumulation in the harvestable aerial parts of the plant (Erakhrumen, 2007). This is followed by harvesting and appropriate disposal of the plant biomass. The contaminant-accumulating plants are usually cultivated by agricultural practises (Jabeen et al., 2009). In the phytoextraction process, the roots of the cultivated plant species help absorb contaminants from the supporting soil, thereby reducing concentrations in the soil. With successive cropping and harvesting of plants from such contaminated soil, the concentration and level of the contaminants in the soil can be reduced (Vandenhove et al., 2001). It has been reported that the cost implication of a phytoextraction process is lower as compared to conventional soil remediation techniques.
Rhizofiltration
Rhizofiltration relies on the capability of the plant root system to take up and sequester contaminants, or nutrients, in excess quantities from aqueous waste streams (Erakhrumen, 2007). This process has the ability to remediate metals including lead (Pb), cadmium (Cd), nickel (Ni), vanadium (V) and chromium (Cr) (Jabeen et al., 2009). Plants suitable for this technique should produce extensive root systems, root biomass and surface area. The plant species should have the capability to accumulate and tolerate substantial amounts of contaminants (Dushenkov and Kapulnik, 2000). Terrestrial plants are very appropriate for rhizofiltration. Plants such as Helianthus annuus (L.) of the family Asteraceae, Brassica juncea (L.) (Brassicaceae), Nicotiana tabacum (L.) (Salicaceae), Spinacia oleracea (L.) (Amaranthaceae) and Zea mays (L.) (Poaceae) have been investigated for their suitability to remove pollutants (Raskin and Ensley, 2000). Rhizofiltration can also be conducted both in situ and ex situ to remediate contaminated water bodies. This method can be used to remediate many metal contaminants. Dushenkov et al. (1995) recommended its commercialization and public acceptance for phytoremediation works.
Phytodegradation (sometimes referred to as phytotransformation)
Phytodegradation involves the breakdown of contaminants either internally, through metabolic processes, or externally, through the release of plant-produced enzymes into the soil using the relationship between plants and their associated micro-organisms in the rhizosphere (Jabeen et al., 2009; Oyedeji, 2016). Some plants are capable of detoxifying contaminants (such as hydrocarbons) and transforming them into non- phytotoxic metabolites. These contaminants are detoxified in three phases: conversion, conjugation and compartmentalization (Kamath et al., 2004). Plants and micro-organisms are involved, both directly and/or indirectly, in the degradation of complex petroleum hydrocarbons into products that are generally simple, less toxic and less persistent in the environment than the parent compounds. Phytodegradation may occur internally in the rhizosphere (Mukhopadhyay and Maiti, 2010) and it is referred to as rhizodegradation or rhizoremediation. It is applied in the remediation of petroleum hydrocarbon contaminated soils.
Rhizodegradation or rhizoremediation
Rhizodegradation, otherwise referred to as rhizoremediation, is applied in the remediation of pollutants such as petroleum hydrocarbon contaminated soils. This process involves the use of tolerant plant species, and associated micro-organisms, in the rhizosphere to accelerate the remediation processes (Pajuelo et al., 2011). The root systems of plant species that are suitable for rhizodegradation support adequate microbial growth due to their ability to offer their root nodules as a habitat (for microbes, enzymes, nutrients and oxygen) as well as a large surface area for microbes to colonize in the soil layers (Anderson et al., 1993). The roots are capable of releasing ‘degradative enzymes’ to promote degradation of petroleum hydrocarbons (Wenzel, 2009). The root systems also play significant roles in transferring the contaminants to the degrading microbes and for the production of oxygen, either by transferring oxygen or creating a vacuum in the soil’s sub-surfaces that permit diffusion of atmospheric oxygen (Van Epps, 2006) to increase the degradation of contaminants in the remediation processes.
In the rhizosphere, a much higher microbial density (which could enhance rhizodegradation) is present in the surface soil layer than in deeper layers (Hinsinger et al., 2005, 2006) and this is associated with higher microbial numbers, diversity and bioactivity (Boopathy, 2000). Availability of numerous degrading microbes in the soil significantly determines their potential for remediation (Mikkonen et al., 2011). Bacteria in the soil rhizosphere are increased by organic contaminants (Chaineau et al., 2003; Chaudhary et al., 2012). This increased microbial population, and its availability, promote plant growth through the degradation of organic contaminants. The Rhizobium population helped increase the growth performance of Trifolium species (L.) Fabaceae on hydrocarbon contaminated soil (Chiapusio et al., 2007). Rhizoremediation can be employed in the treatment of soil contaminated by petroleum hydrocarbons, but the choice and tolerance of plant species also influence its effectiveness.
TOLERANCE MECHANISMS OF PLANTS AND THEIR SUITABILITY FOR REMEDIATION
The physiological and molecular mechanisms of a plant species determine the suitability of such plants for remediation processes. A plant’s tolerance to a particular contaminant is governed by its ability to tolerate an increasing level of the contaminant (Jabeen et al., 2009). Kamath et al. (2004) identified some criteria for selecting plant species. This should follow the needs of the application, the contaminants concerned and the potential of such plants to thrive well on contaminated soil. It is preferable to use native plant species for remediation purposes to support soil ecosystem restoration (Pilon-Smits and Freeman, 2006), as introduced, or exotic species, may become invasive during, or after, the clean-up exercise which give rise to other associated ecological problems.
SELECTION OF PLANT SPECIES FOR PHYTOREMEDIATION
Some researchers (Merkl et al., 2004a, b, 2005; White et al., 2006; Agbogidi et al., 2007; Atagana, 2011) have investigated and reported on the selection of plants for the remediation of soils contaminated with hydrocarbons. It was observed that there was enhanced degradation of complex hydrocarbons within the root rhizosphere (Merkl et al., 2004b; Atagana, 2011). This suggests that a good plant candidate for phytoremediation must have an extensive root system.
The selection of suitable plant species is a fundamental step to be considered in the phytoremediation processes. Some plants do not tolerate the presence of contamination, while others do and effectively enhance the remediation of hydrocarbons within soil. This may be due to variation in plant morphology (e.g. roots), physiology and biochemistry (e.g. root exudates) and interactions between microbes and the plants in the rhizosphere (Walker et al., 2003). Some grasses, herbs, shrubs and trees are good candidates for phytoremediation as listed (Table 1) and some of these plants have extensive branched fibrous roots that are more likely to provide large surface areas for interaction (Yateem et al., 2007). The rhizospheres of certain trees (e.g. Populus deltoides × nigra) have the capability to enrich hydrocarbon degrading micro-organisms more than the soil outside the root zone (Hutchinson et al., 2003). To achieve maximum hydrocarbon reduction in soil, and to successfully establish a stable vegetation cover, various criteria must be considered. Any ideal plant species candidate for the phytoremediation of hydrocarbon contaminated soil should be selected to provide a large surface area per unit volume of soil (Aprill and Sim, 1990; Smith et al., 2006) which thus permits rhizosphere-contaminant-microbe interactions. Due to the frequent poor nutrient availability in contaminated sites (Kirkpatrick et al., 2006; Wenzel, 2009), they should be able to tolerate and thrive with low nitrogen (N) and phosphorus (P) availability.
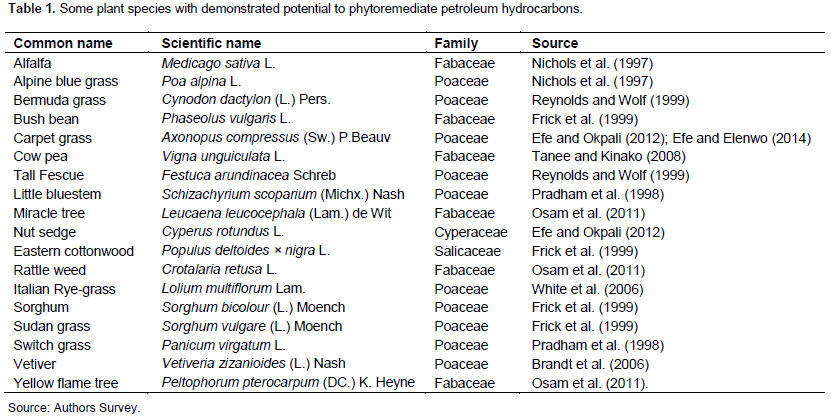
SUITABILITY OF FABACEAE FOR PHYTOREMEDIATION OF HYDROCARBON CONTAMINATED SOILS
The Fabaceae family is made up of plant species commonly referred to as legumes and consists of ~18,000 species across the world and grow in diverse terrestrial habitats. The potential and suitability of Fabaceae for phytoremediation of hydrocarbon polluted soil, with its unique adaptation and rhizodegradation mechanisms, is well known (Merkl et al., 2004b; Tanee and Akonye, 2009; Osam et al., 2011; Atagana, 2011; Hall et al., 2011). There are a number of reports on the use of legumes in hydrocarbon contaminated soils remediation and their ability to fix N (Nichols et al., 1997; Dzantor et al., 2000; Osam et al., 2011) has been known for some time.
Contaminated soils are usually particularly deficient in N and P (Wenzel, 2009) and competition for nutrients among the soil biota reduce nutrient availability. N fixing plant species, such as legumes, can be used in rhizoremediation work (Miller and Cramer, 2005). Microbes, such as Rhizobium species, can penetrate the root systems of leguminous plant species and form symbiotic interactions in their root nodules with which they are able to fix atmospheric N in the form of ammonium compounds (Suominen et al., 2000) and have also been found to increase potassium (K) and phosphorous (P) uptake in plants (Vershinina, 2012). Some of the common N-fixing microbes in soil include Azotobacter species, Azospirillum brasilense, Rhizobium spp. and Actinomycetes (Havlin et al., 2005) and these micro-organisms play vital roles in remediation work by degrading the contaminants. The amount of N fixation by microbes in plant root nodules is substantial, often >100 kg ha-1 year-1 (Vitosek et al., 2002). The interaction between microbes and leguminous plant species, such as alfalfa (Medicago sativa) and red clover (Trifolium pratense), have proven successful in the remediation of petroleum hydrocarbon contaminants (Frick et al., 1999). The use of woody leguminous plant species for phytoremediation in tropical areas is a reflection of their prevalence and abundance (Vitosek et al., 2002) and they can stimulate microbial growth, which increases oxidation of organic chemical substances (Peer et al., 2006).
THE USE OF PLANT SPECIES OF OTHER FAMILIES FOR REMEDIATION OF HYDROCARBON CONTAMINATED SOILS
Plant screening experiments have shown that some plant species, such as Lolium multiflorum Lam. and Festuca arundinacea Schreb are tolerant of hydrocarbon contamination (Frick et al., 1999). B. juncea L. is a useful plant species for phytoremediation and has been successfully used to remediate a 3 km Bulgarian ecological zone contaminated with Pb (Simeonova and Simeonov, 2006). Pb can be one of the impurities in crude oil. The results of their one season planting showed a decrease between 0 and 25.9% in the initial Pb concentrations at various sample locations.
Günther et al. (1996) found that soils planted with Italian rye-grass (L. multiflorum Lam.) had reduced hydrocarbons than soil that was unplanted. In their 22 weeks phytoremediation experiment, the initial extractable hydrocarbon concentration of 4330 mg total hydrocarbon/kg soil was decreased to <120 mg/kg soil (97% decrease) in planted soil.
The examination of the phytoremediation potential of two cold-hardy grass species, Tall fescue (F. arundinacea Schreb) and annual Italian rye grass (L. multiflorum Lam.) planted together in potted soils contaminated with crude oil, found that the contaminated soils had significantly lower concentrations of total petroleum hydrocarbon compared to unplanted controls (Reynolds and Wolf, 1999). The initial crude oil concentration for planted treatments and unplanted controls was ~6200 mg TPH per kg soil. After 640 days, crude oil-contaminated soils planted with both species had 1400 mg TPH per kg soil (77% decrease), while the unplanted control contained 2500 mg TPH per kg soil (60% decrease).
In a 6-month laboratory study, switch grass (Panicum virgatum) and little blue stem Schizachyrium scoparium (Michx.) Nash were capable of decreasing the concentration of total Petroleum Aromatic Hydrocarbons (PAHs) in contaminated soil collected from a manufacturing gas plant (Pradham et al., 1998). The initial soil concentration of total PAHs for the three plant treatments and an unplanted control was 184.5±14.0 mg total PAHs per kg of soil. After 6 months, the concentration in the unplanted control soil was 135.9±25.5 mg/kg, while the concentration in planted treatments was much lower (P. virgatum, 79.5±3.7 mg/kg and S. scoparium, 97.1±18.7 mg/kg).
THE ROLE OF SOIL AMENDMENTS IN PHYTOREMEDIATION PROCESSES
Fertilizers and natural zeolites can play vital roles in phytoremediation processes. White et al. (2003, 2006) and Tanee and Kinako (2008) reported the importance of fertilization in phytoremediation protocols. Chaineau et al. (2003) suggested that the addition of fertilizer (as a soil amendment) and periodic tillage are useful in the degradation of petroleum hydrocarbons in contaminated soil. However, excessive use of nitrogenous fertilizer can damage the environment and to avoid this problem, N-fixing plant species supplemented with soil amendments are encouraged in remediation work (Miller and Cramer, 2005). The environmental applications of natural zeolites such as clinoptilolite as a viable soil amendment has also been reported for contaminated soils (Ming and Allen, 2001; Bowman, 2003; Chmielewska, 2003; Tian et al., 2004; Englert and Rubio, 2005; Leggo et al., 2006; Misaelides, 2011) and for the restoration of soil nutritional qualities. Adebowale et al. (2005) reported environmental significance of Nigerian kaolin for adsorption and remediation of Pb2+, Cu2+, Zn2+ and Cd2+ metal ions in media. Trckova et al. (2004) affirmed that kaolin is effective in the amelioration of adverse effects of contaminants from both the living organisms and the environment.
COMPARISON OF PHYTOREMEDIATION WITH ALTERNATIVE REMEDIATION STRATEGIES
Phytoremediation has shown remarkable cost effectiveness and recent societal acceptance. Its advantages include low costs, according to various authors who have conducted a series of experiments (Frick et al., 1999; Macek et al., 2000; Glick 2003). Other advantages compared with other remediation processes include: Can be applied in situ; Cost-effective and, therefore, economically viable; Offers less disruption to the natural environment compared with mechanical methods; Avoids excavation and heavy damage to soils; Can be applied to large areas of terrestrial contamination; Relatively easy to apply; Preserves soil structure; Potentially quick to apply to the contaminated sites; No disposal site(s) is required; Can be applied to a diverse range of hazardous materials; Plants act as indicators of contamination; Plants help contain contaminants; Plants transfer oxygen and nutrients to the rhizosphere; Other additional advantages of providing plant cover are soil conservation, landscape aesthetics, improved habitat for fauna, carbon-sequestration, etc. (Frick et al., 1999; Oyedeji, 2016).
DISCUSSION
The use of phytoremediation as an alternative environmentally-friendly method of cleaning and restoring contaminated soils has been reviewed. The success of previous phytoremediation works conducted by different authors (Nichols et al., 1997; Dzantor et al., 2000; Tesar et al., 2002; Merkl et al., 2004b; Bamidele and Agbogidi, 2006; Osam et al., 2011; Atagana, 2011) using a range of plant species has shown that research on this emerging technology should be encouraged, strengthened and applied where applicable. This is especially the case in areas prone to hydrocarbon contamination such as the Niger Delta region of Nigeria. The findings of this work have revealed that phytoremediation, particularly rhizodegradation or rhizoremediation, could be employed in the remediation of hydrocarbon contaminated soils (Atagana, 2011; Osam et al., 2011; Oyedeji, 2016) and it also revealed that plants in the family Fabaceae have been used for phytoremediation and found to be good candidates because of their extensive root system and ability to fix N within their root nodules. It is, therefore, hoped that land owners, farmers and governments at all levels will gain awareness of this viable ecosystem remediation technology for our ecosystems and support research on phytoremediation as a practical and effective technology for soil remediation.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENT
The authors are grateful to the Petroleum Technology Development Fund (Abuja, Nigeria) for funding this research.
REFERENCES
|
Adebowale KO, Unuabonah IE, Olu-Owolabi BI (2005). Adsorption of some heavy metal ions on sulfate- and phosphate-modified kaolin Applied Clay Science 29:145-148. |
|
|
Agbogidi MO, Dolar ED, Okechukwu EM (2007). Evaluation of Tectona grandis (Linn) and Gmelina arborea (Roxb) for phytoremediation in crude oil contaminated soils Agriculturae Conspectus Scientificus 72(2):149-152. |
|
|
Anderson T, Guthrie E, Walton B (1993). Bioremediation in the rhizosphere Environmental Science and Technology 27:2630-2636. |
|
|
Aprill W, Sims RC (1990). Evaluation of the use of prairie grasses for stimulating polycyclic aromatic hydrocarbon treatment in soil Chemosphere 20(1):253-265. |
|
|
Atagana HI (2011). Bioremediation of co-contamination of crude oil and heavy metals in soil by phytoremediation using Chromolaena odorata (L) King & HE Robinson Water Air and Soil Pollution 215:261-271. |
|
|
Bamidele J, Agbogidi O (2006). The effect of soil pollution by crude oil on seedling growth of Machaerium lunatus (L) GFW Meg Discovery and Innovation 18(2):104-108. |
|
|
Berti WR, Cunningham SD (2000). Phytostabilization of metals - In: Raskin I Ensley B (eds) Phytoremediation of toxic metals: Using plants to clean up the environment Wiley Interscience New York pp. 71-88. |
|
|
Boopathy R (2000). Factors limiting bioremediation technologies Bioresource Technology 74(1):63-67. |
|
|
Bowman RS (2003). Applications of surfactant-modified zeolites to environmental remediation Microporous and Mesoporous Materials 61(1):43-56. |
|
|
Brandt R, Merkl N, Schultze-Kraft R, Infante C, Broll G (2006). Potential of Vetiver (Vetiveria zizanioides L Nash) for phytoremediation of petroleum hydrocarbon-contaminated soils in Venezuela. International Journal of Phytoremediation 8:273-284. |
|
|
Chaineau C, Yepremian C, Vidalie J, Ducreux J, Ballerini D (2003). Bioremediation of a crude oil-polluted soil: biodegradation leaching and toxicity assessments Water Air and Soil Pollution 144(1):419-440. |
|
|
Chaudhary P, Singh SB, Chaudhry S, Nain L (2012). Impact of PAH on biological health parameters of soils of an Indian refinery and adjoining agricultural area - a case study Environmental Monitoring and Assessment 184(2):1145-1156. |
|
|
Chiapusio G, Pujol S, Toussaint ML, Badot PM, Binet P (2007). Phenanthrene toxicity and dissipation in rhizosphere of grassland plants (Lolium perenne L and Trifolium pratense L) in three spiked soils Plant and Soil 294(1):103-112. |
|
|
Chmielewska E (2003). Remediation of specifically polluted waste effluents using natural zeolites Environment Protection Engineering 29(1):35-44. |
|
|
Cunningham SD, Ow DW (1996). Promises and prospects of phytoremediation Plant Physiology 110(3):715. |
|
|
Cunningham SD, Anderson TA, Schwab PA, Hsc FC (1996) Phytoremediation of soils contaminated with organic pollutants Advances in Agronomy 56:55-114. |
|
|
Cunningham SD, Berti WR, Huang JW (1995). Phytoremediation of contaminated soils Trends in Biotechnology 13(9):393-397. |
|
|
Dushenkov S, Kapulnik Y (2000). Phytofiltration of metals. In: Raskin I and Ensley BD (Eds) Phytoremediation of Toxic Metals: Using Plants to Clean-up the Environment New York: Wiley pp. 89-106. |
|
|
Dushenkov V, Kumar PBAN, Motto H, Raskin I (1995). Rhizofiltration: the use of plants to remove heavy metals from aqueous streams Environmental Science and Technology 29(5):1239-1245. |
|
|
Dzantor EK, Chekol T, Vough L (2000). Feasibility of using forage grasses and legumes for phytoremediation of organic pollutants Journal of Environmental Science and Health Part A 35(9):1645- 1661. |
|
|
Eapen S, D'Souza S (2005). Prospects of genetic engineering of plants for phytoremediation of toxic metals Biotechnology Advances 23(2):97-114. |
|
|
Efe SI, Okpali AE (2012). Management of petroleum impacted soil with phytoremediation and soil amendments in Ekpan Delta State Nigeria Journal of Environmental Protection 3(5):386-393. |
|
|
Efe SI, Elenwo EI (2014). Phytoremediation of crude oil contaminated soil with Axonopus compressus in the Niger Delta region of Nigeria Natural Resources 5:59-67. |
|
|
Englert A, Rubio J (2005). Characterization and environmental application of a Chilean natural zeolite International Journal of Mineral Processing 75(1):21-29. |
|
|
Erakhrumen AA (2007). Phytoremediation: an environmentally sound technology for pollution prevention control and remediation in developing countries Educational Research and Reviews 2(7):151-156. |
|
|
Frick CR, Farrell RE, Germida J (1999). Assessment of phytoremediation as an in situ technique for cleaning oil- contaminated sites Petroleum Technology Alliance of Canada (PTAC) Calgary pp. 1-88. |
|
|
Ghosh M, Singh S (2005). A review on phytoremediation of heavy metals and utilization of its by-products Applied Ecology and Environmental Research 3(1):1-18. |
|
|
Glick BR (2003). Phytoremediation: synergistic use of plants and bacteria to clean up the environment Biotechnology Advances 21(5):383-393. |
|
|
Günther T, Dornberger U, Fritsche W (1996). Effects of ryegrass on biodegradation of hydrocarbons in soil Chemosphere 33(2):203-215. |
|
|
Hall J, Soole K, Bentham R (2011). Hydrocarbon phytoremediation in the family Fabaceae- A review International Journal of Phytoremediation 13:317-332. |
|
|
Havlin JL, Beaton JD, Tisdale SL, Nelson WL (2005). Soil Fertility and Fertilizers: An Introduction to Nutrient Management Pearson Prentice Hall Upper Saddle River NJ. |
|
|
Hinsinger P, Gobran GR, Gregory PJ, Wenzel WW (2005). Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes New Phytologist 168(2): 293-303. |
|
|
Hinsinger P, Plassard C, Jaillard B (2006). Rhizosphere: A new frontier for soil biogeochemistry Journal of Geochemical Exploration 88(1):210-213. |
|
|
Hutchinson S, Schwab A, Banks M (2003). Biodegradation of petroleum hydrocarbons in the rhizosphere Phytoremediation: Transformation and Control of Contaminants 3:557-386. |
|
|
Ikhuoria EU, Okieimen F (2000). Scavenging cadmium copper lead nickel and zinc ions from aqueous solution by modified cellulosic sorbent International Journal of Environmental Studies 57(4):401-409. |
|
|
Jabeen R, Ahmad A, Iqbal M (2009). Phytoremediation of heavy metals: physiological and molecular mechanisms The Botanical Review 75(4):339-364. |
|
|
Jadia CD, Fulekar M (2008). Phytotoxicity and remediation of heavy metals by fibrous root grass (sorghum). Journal of Applied Biosciences 10:491-499. |
|
|
Jadia CD, Fulekar M (2009). Phytoremediation of heavy metals: Recent techniques African Journal of Biotechnology 8(6):921-928. |
|
|
Kamath R, Rentz J, Schnoor JL, Alvarez P (2004). Phytoremediation of hydrocarbon-contaminated soils: principles and a: lications Studies in Surface Science and Catalysis 151:447-478. |
|
|
Kinako PDS (1981). Short-term effects of oil pollution on species numbers and productivity of a simple terrestrial ecosystem Environmental Pollution Series A Ecological and Biological 26(2):87-91. |
|
|
Kirkpatrick WD, White P Jr., Wolf D, Thoma G, Reynolds C (2006). Selecting plants and nitrogen rates to vegetate crude oil- contaminated soil International Journal of Phytoremediation 8(4):285-297. |
|
|
Leggo PJ, Ledésert B, Christie G (2006). The role of clinoptilolite in organo-zeolitic-soil systems used for phytoremediation Science of the Total Environment 363(1):1-10. |
|
|
Macek T, Mackova M, Kas KJ (2000). Exploitation of plants for the removal of organics in environmental remediation Biotechnology Advances 18(1):23-34. |
|
|
Merkl N, Schultze-Kraft R, Infante C (2004a). Phytoremediation of petroleum-contaminated soils in the tropics: Pre-selection of plant species from eastern Venezuela Journal of Applied Botany and Food Quality 78(3):185-192. |
|
|
Merkl N, Schultze-Kraft R, Infante C (2004b). Phytoremediation of petroleum-contaminated soils in the tropics - The effect of crude oil on the growth of tropical plants Bioremediation Journal 8(3-4):177-184. |
|
|
Merkl N, Schultze-Kraft R, Infante C (2005). Assessment of tropical grasses and legumes for phytoremediation of petroleum- contaminated soils Water Air and Soil Pollution 165(1):195-209. |
|
|
Mikkonen A, Kondo E, Lappi K, Wallenius K, Lindström K, Hartikainen H, Suominen L (2011). Contaminant and plant-derived changes in soil chemical and microbiological indicators during fuel oil rhizoremediation with Galega orientalis Geoderma 160(3):336-346. |
|
|
Miller A, Cramer M (2005). Root nitrogen acquisition and assimilation Plant and Soil 274:1-36. |
|
|
Ming DW, Allen ER (2001). Use of natural zeolites in agronomy horticulture and environmental soil remediation Reviews in Mineralogy and Geochemistry 45(1):619-654. |
|
|
Misaelides P (2011). Application of natural zeolites in environmental remediation: A short review Microporous and Mesoporous Materials 144(1):15-18. |
|
|
Mukhopadhyay S, Maiti SK (2010). Phytoremediation of metal mine waste Applied Ecology and Environmental Research 8(3):207-222. |
|
|
Nichols T, Wolf D, Rogers H, Beyrouty C, Reynolds C (1997). Rhizosphere microbial populations in contaminated soils Water Air and Soil Pollution 95(1):165-178. |
|
|
Oyedeji AA (2016). Impacts of selected leguminous tree species and kaolinite pre-amendment on oil-contaminated soil for bioremediation in the oil-bearing region of Nigeria PhD thesis of the University of Wolverhampton Wolverhampton UK. |
|
|
Oyedeji AA, Kayode J, Besenyei L, Fullen MA (2015). Germination of seeds of leguminous tree species moistened with varying concentrations of crude oil-contaminated soil water extracts American Journal of Plant Sciences 6:1575-1580. |
|
|
Osam MU, Wegwu MO, Ayalogu EO (2011). Biochemical and physico- chemical assessment of the efficacy of some wild-type legumes in the remediation of crude-oil contaminated soils Archives of Applied Science Research 3(6):247-256. |
|
|
Pajuelo E, Rodríguez-Llorente ID, Lafuente A, Caviedes MÁ (2011). Legume-rhizobium symbioses as a tool for bioremediation of heavy metal polluted soils Biomanagement of Metal-Contaminated Soils Environmental Pollution 20:95-123. |
|
|
Peer W, Baxter I, Richards E, Freeman J, Murphy A (2006). Phytoremediation and hyperaccumulator plants Molecular Biology of Metal Homeostasis and Detoxification 14:299-340. |
|
|
Pilon-Smits EAH, Freeman JL (2006). Environmental cleanup using plants: biotechnological advances and ecological considerations Frontiers in Ecology and the Environment 4(4):203-210. |
|
|
Pradham SP, Conrad JR, Paterck JR, Srivastava VJ (1998). Potential of phytoremediation for treatment of PAHs in soil at MGP sites. Journal of Soil Contamination 7(4):467-480. |
|
|
Raskin I, Ensley BD (2000). Phytoremediation of toxic metals: using plants to clean up the environment John Wiley & Sons Incorporated, New York 352 p. |
|
|
Reynolds CM, Wolf DC (1999). Microbial based strategies for assessing rhizosphere enhanced phytoremediation Proceedings of the Pytoremediation Technology Seminar May 31-June 1 1999 Calgary Alberta Environment Canada: Ottawa pp. 125-135. |
|
|
Seslar T (2005). Natural remedies Frontiers: The BP Magazine of Technology and Innovation 14:18-21. |
|
|
Simeonova BG, Simeonov LI (2006). An application of a phytoremediation technology in Bulgaria-The Kremikovtzi Steel Works experiment Remediation Journal 16(2):113-123. |
|
|
Smith M, Flowers T, Duncan H, Alder J (2006). Effects of polycyclic aromatic hydrocarbons on germination and subsequent growth of grasses and legumes in freshly contaminated soil and soil with aged PAHs residues Environmental Pollution 141(3): 519-525. |
|
|
Suominen L, Jussila M, Mäkeläinen K, Romantschuk M, Lindström K (2000). Evaluation of the Galega-Rhizobium galegae system for the bioremediation of oil-contaminated soil Environmental Pollution 107(2):239-244. |
|
|
Susarla S, Medina VF, McCutcheon SC (2002). Phytoremediation: an ecological solution to organic chemical contamination Ecological Engineering 18(5):647-658. |
|
|
Tanee F, Kinako P (2008). Comparative studies of biostimulation and phytoremediation in the mitigation of crude oil toxicity in tropical soil Journal of Applied Sciences and Environmental Management 12(2):143-147. |
|
|
Tanee FBG, Akonye LA (2009). Effectiveness of Vigna unguiculata as a phytoremediation plant in the remediation of crude oil polluted soil for Cassava (Manihot esculenta Crantz) cultivation Journal of Applied Science and Environmental Management 13(1):43-47. |
|
|
Tesar M, Reichenauer TG, Sessitsch A (2002). Bacterial rhizosphere populations of black poplar and herbal plants to be used for phytoremediation of diesel fuel Soil Biology and Biochemistry 34(12):1883-1892. |
|
|
Tian W, Wen X, Qian Y (2004). Using a zeolite medium biofilter to remove organic pollutant and ammonia simultaneously Journal of Environmental Sciences 16(1):90-93. |
|
|
Trckova M, Matlova L, Dvorska L, Pavlik I (2004). Kaolin bentonite and zeolites as feed supplements for animals: health advantages and risks Vet Med-Czech 49(10):389-399. |
|
|
United States Environmental Protection Agency (USEPA) (2000) Introduction to phytoremediation EPA 600/R-99/107 US Environmental Protection Agency Office of Research and Development Cincinnati Ohio. |
|
|
Vandenhove H, Van Hees M, Van Winkel S (2001). Feasibility of phytoextraction to clean up low-level uranium-contaminated soil International Journal of Phytoremediation 3:301-320. |
|
|
Van Epps A (2006). Phytoremediation of petroleum hydrocarbons Technical publication report environmental careers organization for US Environmental Protection Agency Washington DC. |
|
|
Vershinina ZR, Baymiev AK, Blagova DK, Chubukova OV, Baymiev AK, Chemeris AV (2012). Artificial colonization of non-symbiotic plants roots with the use of lectins Symbiosis 56:25-33. |
|
|
Walker TS, Bais HP, Grotewold E, Vivanco JM (2003). Root exudation and rhizosphere biology Plant Physiology 132(1):44-51. |
|
|
Wenzel WW (2009). Rhizosphere processes and management in plant- assisted bioremediation (phytoremediation) of soils Plant and Soil 321(1):385-408. |
|
|
White PM Jr., Wolf DC, Thoma GJ, Reynolds CM (2003). Influence of organic and inorganic soil amendments on plant growth in crude oil- contaminated soil International Journal of Phytoremediation 5(4):381-397. |
|
|
White PM Jr., Wolf DC, Thoma GJ, Reynolds CM (2006). Phytoremediation of alkylated polycyclic aromatic hydrocarbons in a crude oil-contaminated soil Water Air and Soil Pollution 169(1):207- 220. |
|
|
Yateem A, Al-Sharrah T, Bin-Haji A (2007). Investigation of microbes in the rhizosphere of selected grasses for rhizoremediation of hydrocarbon-contaminated soils Soil and Sediment Contamination 16(3):269-280. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0