ABSTRACT
This study aimed to assess the extent of pollution of aquatic ecosystems by endocrine disrupting estrogens particularly the ethinylestradiol (EE2), estrone (E2) and estradiol (E1). The study was carried out in Morogoro urban and peri-urban areas. The main sources of fresh water for domestic uses, fishing and agricultural activities in the study areas including the Mindu dam catchment area, Ngerengere and Morogoro Rivers were assessed. The endocrine disrupting estrogens in water samples were identified and quantified using competitive Enzyme Linked Immunosorbent Assay (ELISA) kits. The recovery of estrogens in this study ranged from 65 to 90.22%, the range which is within the acceptable level. The levels of estrogens in Ngerengere River ranged from non-detectable levels to 0.68, 0.03 to 8.42 and 0.05 to 16.97 ng/L for EE2, E2 and E1, respectively. At Mindu Dam the levels ranged from 0.07 to 0.3 ng/L, 0.41 to 2.1 ng/L and 2.6 to 6.5 ng/L for EE2, E2 and E1 respectively. Furthermore, for Morogoro River the levels ranged from undetected to 0.92, 0.34 to 9.53 and 0.17 to 11.49 ng/L for EE2, E2 and E1 respectively. Mean concentrations in control samples and those in upstream and midstream of the rivers were comparable (p > 0.05). But the mean concentrations in downstream portions were significantly higher than those in control samples (p < 0.05). These concentrations however, were below those reported in other studies to cause harmful health effects. Hence, the extent of pollution was not significant enough to cause adverse health effects to aquatic organisms and human.
Key words: Ethinylestradiol, estradiol, estrone, micro pollutants, Ngerengere River, Morogoro River.
The aquatic ecosystems are the ultimate sink of most environmental pollutants originating from natural and anthropogenic sources such as industries, livestock farms, agricultural fields, hospital wastes, domestic wastes and municipal effluents. Several studies in Europe, Asia and USA have reported that sewage effluents are major contributors of manmade chemical pollution in rivers (Gomes et al., 2003; Mitani et al., 2005;
Huerta et al., 2016). On the other hand, runoff associated with waste from animal farming has been reported as another potential source of estrogens in the rivers (Williams et al., 2007; Kolok et al., 2007; Yuan et al., 2014; Huang et al., 2016).
Endocrine disrupting estrogens are among the emerging pollutants which end up in aquatic environment (Snyder et al., 2009). They are termed emerging because there are no established guidelines for environmental monitoring however, have adverse health effects to wildlife and human (Nosek et al., 2014). Estrogens are potent endocrine disruptors at concentrations frequently observed in surface water (Wedekind, 2014). They tend to bioaccumulation in aquatic organisms, such as algae which acts as scavenger or sinks for estrogens (Maes, 2011). In addition, food-web model predicted the bioaccumulation of estrogens in all organisms at low level (Lai et al., 2002).
Reproductive impairment have been reported in various species of fish in many countries due to exposure to estrogens (Jobling et al., 2003; Hinck et al., 2009; Ingram et al., 2011; Caldwell et al., 2012; Guellard and Soko, 2015; Huang et al., 2016). Both natural hormones (E1 and E2) and synthetic hormones (EE2) have endocrine disrupting effects such as reduced fertility and feminization of male fish (Tyler and Jobling, 2008; Bhandari et al., 2014; Iwanowicz et al., 2016). Exposure of male fish to estrogens can result in a range of effects from the complete sex reversal in most severe cases to different degrees of feminization including intersex and decreased expressions of secondary sex characteristics (Tabata et al., 2001; Gross-Sorokin et al., 2004; Lange et al., 2008; Länge et al., 2012).
Laboratory studies have shown that the estrogens have additive effects (Thorpe et al., 2003). Thus, even if the concentration of one of them is below the lowest observable effects, the combined effect can be significant. The EE2 is more potent in induction of reproductive abnormalities than the natural estrogens (Aris et al., 2014). It can induce vitellogenin formation in some male fish species at concentrations of as low as 1 ng/L and induce intersex of fish at 4 ng/L, whereas E2 can induce vitellogenin formation at 5 ng/L and induce intersex at 10 ng/L (Metcalfe et al., 2001; Thorpe et al., 2001).
The aquatic ecosystems in Morogoro Urban and Peri-urban areas include Mindu dam and its catchment, Ngerengere River, Morogoro River and other seasonal and permanent streams. Morogoro municipal effluents from wastewater stabilization ponds are discharged into Morogoro River, hence could be a potential source of estrogen pollution. In addition, within Morogoro urban there are several industries such as sisal, textile and leather. It has been observed that untreated or poorly treated effluents are being discharged into the Ngerengere and Morogoro rivers. Generally, the industrial development strategy in Tanzania was pursued without environmental regulation for a longtime and consequently many industries do not have waste treatment facilities (United Republic of Tanzania (URT), 2006). Furthermore, the land along the rivers and Mindu dam is used for agriculture, livestock breeding, residential, public and commercial purposes. Therefore, the ecosystems are prone to pollution from natural and anthropogenic sources.
Previous research in Morogoro aquatic ecosystems focused on pollution due to solid waste, nutrients, pesticides and heavy metals (Franks et al., 2005; Mdegela et al., 2009; Mero, 2011). On the other hand, another study dealt with interactive effects of mixed pollutants on biomarker responses in sewage wastewater and fresh water aquatic ecosystems in Morogoro (Mdegela et al., 2010). Generally, in Tanzania the research coverage on emerging pollutants such as endocrine disrupting estrogens are very limited (Miraji et al., 2016).
Despite the presence of endocrine disrupting estrogens in the environment, no guidelines have been established by Tanzania Bureau of Standards. The current guidelines TZS 860: 2005 with limits for municipal and industrial wastewaters includes chemical pollutants other than estrogens (TBS, 2005; EWURA, 2014). Furthermore, even World Health Organization (WHO), United Nations Environment Programme (UNEP), European countries United States Environmental Protection Agency (USA EPA) and Australian EPA are still collecting more research evidences so as to establish the guidelines for estrogens in the environment (WHO and UNEP, 2012). Therefore, it was necessary to carry out this study so as to establish a basis from which future researches on estrogens in Tanzania can rely on. In addition, the likely source and extent of pollution in aquatic ecosystem by endocrine disrupting estrogens needed to be assessed and mitigation measures be planned and implemented.
Description of the study area
Morogoro river originate from Uluguru mountains, it passes through Morogoro Urban eventually joins Ngerengere river between Kihonda and Tungi areas which are within Morogoro urban (Figure 1). There are seven tributaries which join Morogoro River. Those tributaries are Sole, Mwere, Kitundu, Mdirila, Mlali, Kikundi and Kilakala. Ngerengere River also originates from Uluguru Mountains, along with four other tributaries, namely Mzinga, Lukulunge, Mugera and Mlali.
Water from these tributaries is collected in the Mindu dam whose purpose is to supply drinking water to Morogoro urban area but also used for fishing activities. From the Mindu dam, the river passes through Morogoro urban towards the east (Figure 1). It finally joins the lower Ruvu River which discharges its water into Indian Ocean after passing through Coast region. The Ruvu River is the main source of domestic water supply to Dar es salaam city.
Chemicals and materials
Two standards ethinylestradiol (EE2) and β-estradiol (E2) hormones were supplied by Santa Cruz Biotechnology, Texas, USA. Other chemicals used were n-heptane (99%), methanol (99%), acetone (99.8%) and hydrochloric acid (37%, 1.18 M) supplied by Carlo Erba Reagenti and Sigma Aldrich, Germany. Glass fiber filter papers of MN 615, size Ǿ 150 mm and 2576 size; Ǿ 240 mm from Macherey-Nagel GmbH and Co.KG, Duren-Germany and Munktell and Filtrak GmbH, Barenstein Germany respectively, and solid phase extraction C-18 cartridges (130 mg, 3mL) by Varian and Chromabond.
Sampling of water samples
Purposive sampling strategy was adopted based on connections to perceived hotspots of estrogenic pollution. As described in Table 1, samples were drawn from each tributary which join Morogoro and Ngerengere rivers as well as from industrial wastewater and Municipal effluents getting into the rivers. Samples were also drawn from points of which the researchers hypothesized that pollution could be enhanced due to agricultural runoff as well as domestic wastes. Apart from those points also samples were drawn from point sources of the rivers where neither agricultural activities nor human settlement occurred. For Mindu dam, samples were drawn about 100 m from rivers entry points, three points were sampled and the fourth point was selected at mid of the dam.
Composite sampling being a technique that combines a number of discrete samples collected from a body of material into a single homogenized sample for the purpose of analysis (Australian, 2005) was adopted in this study. Composite sampling reduces costs of environmental and public health assessments, while maintaining and often increasing the precision of sample based inference (Patil, 2002). At each sampling point three samples each 500 ml were drawn and thoroughly mixed in glass bottle to make a 1.5 L of composite sample.
In addition, composite tape water sample was drawn immediately after Morogoro Urban Water and Sewarage Authority fresh water treatment unit. The pH of water samples was adjusted to about 3 by adding hydrochloric acid so as to fix the estrogens. The added acid suppressed microbial activity which could degrade the estrogens to some extent before analysis (Havens et al., 2010). Thereafter, the samples were carried in cool box packed with ice packs to the Ecotoxicology and Natural Products research Laboratory in the Faculty of Veterinary Medicine at Sokoine University of Agriculture, for pretreatment and solid phase extraction of estrogens that was done within 12 h after sample collection.
Extraction of estrogens from water samples
Extraction of estrogens from water samples was carried out according to the protocol described by Hansen et al. (2011) with some modifications customized to our laboratory settings. Each water sample (1.5 L) was first filtered twice using GFC filters papers to ensure removal of debris. Solid phase extraction was performed with C18 cartridges (Bond Elut 500 mg, 3cc reservoir, Varian Agilent Technologies, USA) and vacuum manifold. The C-18 cartridges were conditioned with 2×3 mL heptane, 3 mL acetone, and lastly with 3 ml of distilled water. After extraction the cartridges were dried in air using vacuum manifold for about half an hour, and then eluted using a mixture of 10 ml of heptanes and acetone (65:35). The eluate was then air dried at 30°C, and then reconstituted in 5 ml methanol. The samples were stored at -20°C before being analysed by enzyme linked immunosorbent (ELISA) competitive technique. The ELISA technique was used because it is cost effective method and has detection limit which is lower than the existing methods for screening estrogenicity (Mauricio et al., 2006; Pool, 2008).
Detection and quantification of estrogens by ELISA competitive technique
The detection and quantification of EE2, E2 and E1 was carried out using ELISA kit from Cloud-Clone Corp. 1304 Langham Creek Dr. Suite 226, Houston, TX 77084, USA. Manufacturer instructions were followed; immediately measurement on microplate reader was conducted at 450 nm.
Quantitative data analysis
The concentrations of the standards (2, 0.67, 0.22, 0.074 and 0.025 ng/ml) were transformed into natural logarithm to obtain linear calibration curve, in turn natural logarithm of concentration for each hormone was drawn against the respective absorbance. The linear equation obtained in the curve was used to interpolate the concentration of estrogens in samples.
Recovery studies
Four different concentrations (2, 1.33, 0.13 and 0.013 ng/ml) of mixture of standard EE2 and E2 each was made by dissolving in 1500 ml distilled water. The same pretreatment and analysis steps were followed as was done for water samples.
Statistical analysis
IBM SPSS version 20 was used for statistical analysis of the results; both descriptive and inferential statistics were carried out. For descriptive statistics means, standard deviation, median and range were calculated. Inferential statistics one way ANOVA with post hoc Tukey’s-b was employed for multiple comparisons of estrogens levels between sampling site clusters. Level of significance between groups was reported at p < 0.05.
Estrogens standard curves
Natural logarithms of standard concentrations were plotted against absorbance to obtain linear curves (Suppl Figure 1). The R2 for EE2, E2 and E1 was 0.9707, 0.9851 and 0.982 respectively. Hence, the linear equations were used to quantify the estrogens based on their respective absorbance.
Recovery results
The recovery of EE2 and E2 were assessed for solid phase extraction and ELISA technique analysis. The results are as shown in Suppl Table 1.
Identified and quantified endocrine disrupting estrogens in water sample from Morogoro River
Figure 2 shows the mean concentrations of identified and quantified natural and synthetic endocrine disrupting estrogens from Morogoro River. The results indicate that there were no significant difference in levels in upstream and midstream for all three estrogens (p ≥ 0.05). However, the downstream levels were significantly higher (p < 0.05) than those at midstream and upstream sampling points. The midstream and upstream levels were comparable (p ≥ 0.05) to those found in control samples. Hence, the extent of pollution at upstream and midstream was low.
At control site only natural estrogens (E2 and E1) were identified and quantified but at very low concentrations where E1 was found to be 0.17 ng/L and E2 was 0.34 ng/L. This implied that, those natural estrogens could be from animals dwelling in the forest. No traces of ethinylestradiol could be identified, therefore the results concur with the actual field situation in which no human settlement and activities were found. In upstream the levels of E1, E2 and EE2 ranged from 2.08 to 4.7, 0.48 to 2.17 and 0.019 to 0.22 ng/L, respectively. Whereas, in midstream ranged from 2.7 to 4.09, 1.13 to 4.91 and 0.21 to 0.3 ng/L for E1, E2 and EE2, respectively. Although the levels in midstream sites were relatively higher than those found at upstream, the difference was insignificant (p <0.05). The midstream sites were prone to more pollution owing to the intense human settlement and activities. For instance, a site named before Kikundi tributary ranged highest for all three estrogens due to pollution from domestic effluent, effluent from the bus terminal, as well as effluent leaking from hospitals. Essentially, low standard of sanitation and sewage in all of Tanzania's urban centres including Morogoro urban attributed to pollution (URT, 2006). In downstream sites, levels ranged from 7.37 to 11.49, 5.67 to 9.5 and 0.24 to 0.92 ng/L for E1, E2 and EE2, respectively. These sites received Morogoro Municipal effluent from wastewater stabilization ponds at Mafisa, also industrial effluent as well as waste from livestock farming. For instance, at Kichangani cattle farms were found near the river, hence could contribute to pollution of the river by estrogens. This observation is supported by finding reported by Williams et al. (2007), Kolok et al. (2007), Yuan et al. (2014) AND Huang et al. (2016), Animal farms are potential sources of natural and synthetic estrogens. In addition, several studies reported that WSPs are potential sources of estrogens pollution in rivers (Mitani et al., 2005; Gomes et al., 2003; Sim et al., 2011; Belhaj et al., 2014). The main source of EE2 in rivers could be the use of contraceptive by residents in domestic/commercial houses (Lei et al., 2009; Laurenson et al., 2014). In all samples the levels of EE2 were the lowest of all three estrogens, indicate that the extent of pollution by domestic waste was low or proportion of women who were using contraceptive pills in the study area was low.
Identified and quantified endocrine disrupting estrogens in water sample from Ngerengere River
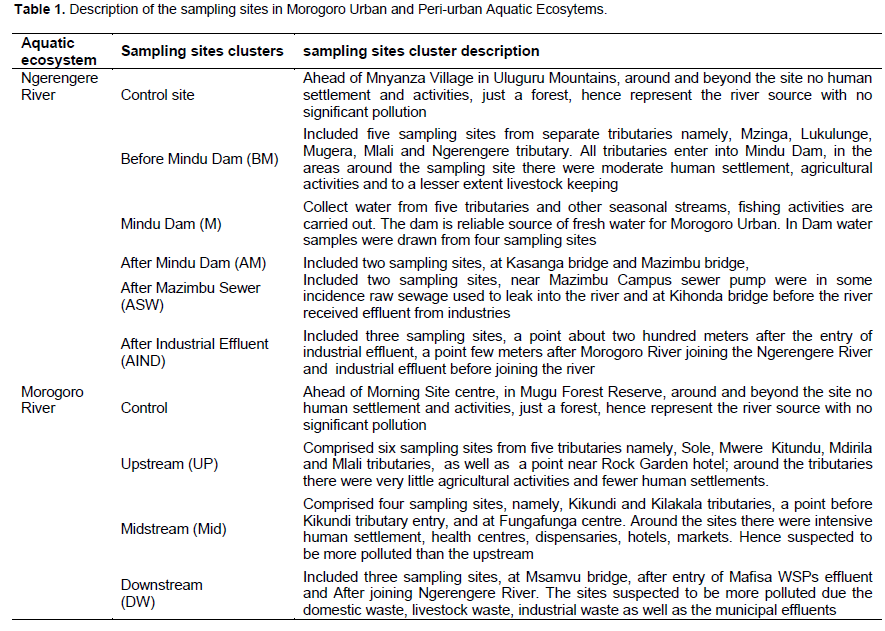
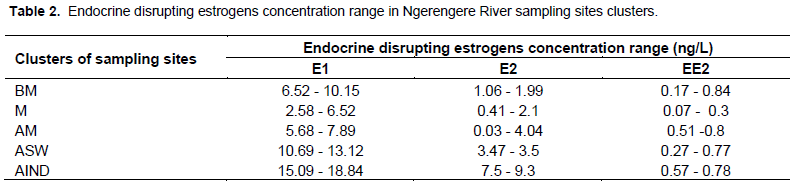
Figure 3 displays the mean concentrations of identified and quantified natural and synthetic endocrine disrupting estrogens in Ngerengere River. There were no significant difference between the control site samples and those collected Before Mindu (BM) and within Mindu dam sites for all of the three estrogens (p ≥0.05). In addition, mean concentrations for estradiol in samples collected After Mindu (AM) had no significant difference with the control samples. The results implied low extent of pollution, hence low health risks to aquatic organisms and human. However, considering levels at specific sites some had levels which could be enough to induce reproductive abnormalities in aquatic organisms. For instance samples from Mlali tributary contained 0.84 and 11.26 ng/L for EE2 and E1, respectively. The tributary received domestic effluent leaking from Changarawe Village. The Figure 3 shows that “After Industrial Effluent” (AIND) sampling cluster had significant higher mean concentration of the estrogens compared to other clusters. This observation is attributed by effluent from industries, Morogoro River which join Ngerengere River after receiving effluent from Morogoro Municipal WSPs as well as accumulation of domestic effluent and livestock waste. For estrone “After Mazimbu Sewer” (ASW) sampling cluster had significant higher level than other clusters except AIND which had statistically similar level to ASW. Table 2 displays the estrogens concentration range in Ngerengere River sampling sites clusters. The highest concentration of EE2, E2 and E1 were 0.84, 9.3 and 18.84 ng/L, respectively. The results imply low extent of pollution.
The results in this study show a similar trend to those reported by Kinoshita et al. (2010). It was observed that significant contamination of Thailand and Malaysia rivers with estrogens occurred in urban areas, contrary to remote areas where no detectable level was observed. Apart from that, the levels of estrogens in this study were lower than those reported by Lei et al. (2009), in which Dagu River, in China E1 ranged from 5 to 55.3 ng/L, E2 ranged 0.93 to 33.4 ng/L and EE2 ranged from not detected to 35.6. For Yongding New River in China ranged from 0.64 to 20.2 ng/L for E1, from non-detected to 13.6 ng/L for E2 and from non-detected to 12 ng/L for EE2. The third river named Beitang River in China E1 ranged from 4.29 to 49.8 ng/L, for E2 from 2.51 to 21.2 ng/L and EE2 was from 1.64 to 24.4 ng/L. In addition, Rao et al. (2013) reported that estrogens from three river water samples in Tianjin, China ranged from 0.64 to 50, 1.87 to 11.5 and 1.55 to 24.4 ng/L for E1, E2 and EE2 respectively. Furthermore, Rocha et al. (2016) reported an unexpectedly high level of estrogens in Mira River in Portuguese, obtained an annual average estrogen 57 ng/L.
Health implications
Estrogens even at low concentrations in the environment can have harmful effects on aquatic organisms and in humans, who might be consuming water or food contaminated with estrogens (Gustavo et al., 2014; Gross-Sorokin et al., 2004). Feminization or demasculinisations of molluscs, arthropods and fish have been reported in polluted lakes or rivers (Guillette et al., 2007; Krein et al., 2012). Effects shown in wildlife or experimental animals may also occur in humans if they are exposed to EDCs at a vulnerable time and at concentrations leading to alterations of endocrine regulation (UNEP and WHO, 2012; Bhandari et al., 2014). Concentrations which led to vitellogenin induction have been reported in previous studies as low as 5 ng/L for estradiol (Tabata et al., 2001), 3.2 ng/L for estrone and around 1 ng/L for ethinylestradiol (Fenske et al., 2001; Thorpe et al., 2001). The intersex condition has been recorded in various fish species following exposures to concentrations as low as 10 ng/L each for estradiol and estrone (Metcalfe et al., 2001; Tabata et al., 2001) and 4 ng/L for ethinylestradiol (Länge et al., 2001). Furthermore, Caldwell et al. (2012) reported that fish exposure to 17 beta-estradiol at concentration that exceeds 10 ng/L cause intersex in some species of male fish.
Based on previous other published studies on concentration of estrogens which lead to observable health defects to aquatic organisms, the concentration of estrogens obtained in this study have low health risks to aquatic organisms and humans. However, long-term exposure of aquatic organisms to such low concentrations can lead to significant health risks due to bioaccumulation (Lai et al., 2002). Therefore, measures should be taken to minimize pollution of the water bodies by endocrine disrupting estrogens.
The most potent estrogens namely ethinylestradiol, estrone and estradiol were identified and quantifies in aquatic ecosystems in Morogoro Urban and Peri-urbans areas. The results implied lower extent of pollution in the aquatic ecosystems by endocrine disrupting estrogens. However, a few sampling sites had significant higher concentration of estrogens, but dilution offset the impact. The sources of pollution mainly were industrial effluents, effluent from livestock farms, residential wastes, and effluents from wastewater stabilization ponds. Ethinylestradiol (EE2) which is the most potent estrogen, its concentrations was the lowest of all three estrogens in all samples. In addition, the EE2 concentrations were below (< 1 ng/L) to those reported in other studies that could bring observable health defects to aquatic organisms. Furthermore, the concentrations of estradiol and estrone in most samples could not cause observable health defects except in some sampling sites, could induce vitellogenin formation in male fish. No detectable estrogens were found in tape water. Therefore, the extent of pollution has low health risks to aquatic organisms and humans.
The authors have not declared any conflict of interests.
This research was funded by Danish Ministry of Foreign Affairs through Safe Water for Food (SaWaFo) project. The authors are very grateful to DANIDA for funding this research.
REFERENCES
|
Aris ZA, Shamsuddin SA, Praveena MS (2014). Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: a review. J. Environ. Int. 69:104-119.
Crossref
|
|
|
|
Australian EPA (2005). Composite Soil Sampling Guidelines. South Australian Environment Protection Authority (EPA). pp. 1-5.
|
|
|
|
|
Belhaj D, Turki N, Kallel M, Ayadi H, Zhou JL (2014). Comparison of estrogen compounds removal efficiency in sample and alternating anoxic / aerobic activated sludge process. J. Environ. Sci. Toxicol. Food Technol. 8(1):100-108.
|
|
|
|
|
Bhandari RK, Deem SL, Holliday DK, Jandegian CM, Kassotis CD, Nagel SC, Tillitt ED, vom Saal SF, Rosenfeld SC (2014). General and Comparative Endocrinology Effects of the environmental estrogenic contaminants bisphenol A and 17 a -ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. J. Gener. Comp. Endocrinol. 145:20-24.
Crossref
|
|
|
|
|
Caldwell DJ, Mastrocco F, Anderson PD, Lange R, Sumpter JP (2012). Predicted-no- effect concentrations for the steroid estrogens estrone, 17beta-estradiol, estriol, and 17alpha-ethinylestradiol. J. Environ. Toxicol. Chem. 31(6):1396-1406.
Crossref
|
|
|
|
|
EWURA (2014). Tanzania Water and Wastewater Quality Monitoring Guidelines for Water Utilities, December, 2014. pp. 1-29
|
|
|
|
|
Fenske M, van Aerle R, Brack S, Tyler CR, Segner H (2001). Development and validation of a homologous zebrafish (Danio rerio Hamilton-Buchanan) vitellogenin enzyme-linked immunosorbent assay (ELISA) and its application for studies on estrogenic chemicals. J. Comp. Biochem. Physiol. Toxicol. Pharmacol. 129:217-232.
Crossref
|
|
|
|
|
Franks P, Mansoor H, Meliyo J, Ruheza S (2005). Uluguru Mountains Environmental Management and Conservation Project (UMEMCP), Strategy for the Agriculture and Agroforestry Component, Care International, Tanzania.
|
|
|
|
|
Gomes RL, Scrimshaw MD, Lester J (2003). Determination of endocrine disrupters in sewage treatment and receiving waters. J. Trends Anal. Chem. 10:697-707.
Crossref
|
|
|
|
|
Gross-Sorokin MY, Roast SD, Brighty GC (2004). Causes and consequences of feminisation of male fish in English rivers. Scientific Report. Retrieved from www.environment-agency.gov.uk
|
|
|
|
|
Guellard T, Soko E (2015). First report on intersex in invasive round goby Neogobius melanostomus from the Baltic Sea (Gulf of Gda Å„ sk, Poland). J. Oceanol. 57:102-106.
Crossref
|
|
|
|
|
Guillette LJJ, Edwards TM, Moore BC (2007). Alligators, contaminants and steroid hormones. J. Environ. Sci. 14:331-347.
|
|
|
|
|
Gustavo G, Alberto MSF, Eugenia MSA, Anabella F, Martin ST, Vanesa MS, Aloranti C (2014). Comprehesive Assement of Estrogenic Contamination of Surface Waters of the River Basin Suquia. Euro. Sci. J. 3:297-302.
|
|
|
|
|
Hansen M, Jacobsen NW, Nielsen FK, Björklund E, Styrishave B, Halling-Sørensen B (2011). Determination of steroid hormones in blood by GC-MS/MS. J. Anal. Bioanal. Chem. 400(10):3409-3417.
Crossref
|
|
|
|
|
Havens MS, Hedman JC, Hemming CDJ, Mieritz GM, Shafer MM, Schauer J (2010). Stability, Preservation and Quantification of Hormones and Estrogenic and Androgenic Activities in Surface Water Runoff. J. Environ. Toxicol. Chem. 29(11): 2481-2490.
Crossref
|
|
|
|
|
Hinck JE, Blazer VS, Schmitt CJ, Papoulias DM, Tillitt DE (2009). Widespread occurrence of intersex in black basses (Micropterus spp.) from U.S. rivers, 1995-2004. J. Aquat. Toxicol. 95(1):60-70.
Crossref
|
|
|
|
|
Huang G, Liu Y, Chen X, Liang Y, Liu S (2016). Feminization and masculinization of western mosquitofish (Gambusia affinis) observed
Crossref
|
|
|
|
|
Huerta B, Rodriguez-mozaz S, Nannou C, Nakis L, Ruhí A, Acu-a V, Sabater S, Barcelo D (2016). Science of the Total Environment Determination of a broad spectrum of pharmaceuticals and endocrine disruptors in bio fi lm from a waste water treatment plant-impacted river. J. ScI. Total Environ. 540:241-249.
Crossref
|
|
|
|
|
Ingram RD, Miller LD, Ingram RT, Tannehill EJ (2011). Intersex Condition of Shoal Bass in the Flint River, Georgia. J. Aquat. Animal Health, 23:189-194.
Crossref
|
|
|
|
|
Iwanowicz LR, Blazer VS, Pinkney AE, Guy CP, Major AM, Munney K, Mierzykowskis S, Lingernfelser S, Second A, Patnode K, Kubiak JT, Stern C, Hahn MC, Iwanowicz DD, Walsh HI, Sperry A (2016). Evidence of estrogenic endocrine disruption in smallmouth and largemouth bass inhabiting Northeast U.S. national wildlife refuge waters: A reconnaissance study. J. Ecotoxicol. Environ. Saf. 124:50-59.
Crossref
|
|
|
|
|
Jobling S, Casey D, Rodgers-Gray T, Oehlmann J, Schulte-Oehlmann U, Pawlowski S, Tyler CR (2003). Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent. Aquat. Toxicol. 66: 207-222.
Crossref
|
|
|
|
|
Kinoshita M, Zakaria PM, Ismail A, Yusof S, Boonphakdee C, Boonphakdee T, Inoue K (2010). An attempt to detect contamination with estrogenic compounds in river water of urban area in Thailand and Malaysia using transgenic medaka. J. Coast. Mar. ScI. 34(1): 216–222.
|
|
|
|
|
Kolok AS, Snow DD, Sellin MK (2007). Occurrence and biological effect of exogenous steroids in the Elkhorn River, Nebraska, USA. J. ScI. Total Environ. 388:104-115.
Crossref
|
|
|
|
|
Krein A, Pailler J, Guignard C, Gutleb AC, Hoffmann L, Meyer B (2012). Determination of Estrogen Activity in River Waters and Wastewater in Luxembourg by Chemical Analysis and the Yeast Estrogen Screen Assay. J. Environ. Pollut. 1(2):86-96.
Crossref
|
|
|
|
|
Lai KM, Scrimshaw MD, Lester JN (2002). Prediction of the bioaccumulation factors and body burden of natural and synthetic estrogens in aquatic organisms in the river systems. J. Sc. Total Environ. 289(1):159-68.
Crossref
|
|
|
|
|
Länge R, Hutchinson TH, Croudace CP, Siegmund F, Schweinfurth H, Hampe P, Panter HG, Sumpter PJ (2001). Effects of the synthetic estrogen 17 alphaethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). J. Environ. Toxicol. Chem. 20:1216-1227.
Crossref
|
|
|
|
|
Länge A, Katsu MY, Miyagawa S, Ogino Y, Urushitani H, Kobayashi T, Hirai T, Shears JA, Nagae M, Yamamoto J, Ohnishi Y, Oka T, Tatarazoko N, Ohta Y, Tyler CR, Iguchi C R (2012). Comparative responsiveness to natural and synthetic estrogens of fish species commonly used in the Laboratory and field monitoring. J. Aquat. Toxicol. 109:250-258.
Crossref
|
|
|
|
|
Lange A, Paull CG, Tyler R (2008). Long-term exposure to environmentally relevant concentrations of ethinyloestradiol affects sexual differentiation and development in roach, Rutilus rutilus. Science Report SC030299/SR2 by Environmental Agency, Rio House, Waterside Drive, Aztec West, Almondsbury, Bristol pp.1-25.
|
|
|
|
|
Laurenson JP, Bloom RA, Page S, Sadrieh N (2014). Ethinyl Estradiol and Other Human Pharmaceutical Estrogens in the Aquatic Environment : A Review of Recent Risk Assessment Data, J. Am. Assoc. Pharm. Sci. 16(2):299-310.
Crossref
|
|
|
|
|
Lei B, Huang S, Zhou Y, Wang D, Wang Z (2009). Chemosphere Levels of six estrogens in water and sediment from three rivers in Tianjin area, China. J. Chem. 76(1):36-42.
|
|
|
|
|
Mauricio R, Diniz M, Petrovic M, Amaral L, Peres I, Barcelo D, Santana F (2006). A characterization of selected endocrine disruptor compounds in a Portuguese wastewater treatment plant. J. Environ. Monit. Assess. 118:75-87.
Crossref
|
|
|
|
|
Mdegela RH, Braathen M, Pereka AE, Mosha RD, Sandvik M, Skaare J (2009). Heavy Metals and Organochlorine Residues in Water, Sediments and Fish in Aquatic Ecosystems in Urban and Peri-Urban Areas in Tanzania. J. Water Air Soil Poll. 203: 369-379.
Crossref
|
|
|
|
|
Mdegela RH, Braathen M, Mosha RD, Skaare J U, Sandvik M (2010). Assessment of pollution in sewage ponds using biomarker responses in wild African sharptooth catfish (Clarias gariepinus) in Tanzania. J. Ecotoxicol. 19(4):722-734.
Crossref
|
|
|
|
|
Mero R (2011). Assessment of water quality and spatial distribution of the major pollutants in Ngerengere River catchment, Tanzania. Dissertation for Master of Science in Integrated Water Resources Management, University of Zimbabwe. pp. 1-83.
|
|
|
|
|
Metcalfe CD, Metcalfe TL, Kiparissis Y, Koenig BG, Khan C, Hughes RJ, Croley TR, March RE, Potter T (2001). Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes). J. Environ. Toxicol. Chem. 20(2): 297-308.
Crossref
|
|
|
|
|
Miraji H, Othman OC, Ngassapa FN, Mureithi EW (2016). Research Trends in Emerging Contaminants on the Aquatic Environments of Tanzania. J. Sci. pp. 1-6.
Crossref
|
|
|
|
|
Mitani K, Fujioka M, Kataoka H (2005). Fully automated analysis of estrogens in environmental water by in tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrophotometry. J. Chroma. A 1081:218-224.
Crossref
|
|
|
|
|
Nosek K, Styszko K, Golas J (2014). Combined method of solid phase extraction and GC-MS for determination of acidic, neutral, and basic emerging contaminants in wastewater (Poland). Int. J. Environ. Anal. Chem. 94(10):961-974.
Crossref
|
|
|
|
|
Patil P (2002). Composite sampling. Encyclopedia Environmetrics pp. 387-391.
|
|
|
|
|
Pool JE (2008). The Estrogenicity of Sewage Effluent Entering the Eerste-Kuils River Catchment System. South Africa, Water Research Commission (WRC) Report No 1590/1/08 pp. 1-29.
|
|
|
|
|
Rao K, Lei B, Li N, Ma M, Wang Z (2013). Determination of estrogens and estrogenic activities in water from three rivers in Tianjin, China. J. Environ. Sci. 25(6):1164-1171.
Crossref
|
|
|
|
|
Rocha JM, Cruzeiro C, Reis M, Pardal AM, Rocha E (2016). Pollution by oestrogenic endocrine disruptors and β-sitosterol in a south-western European river (Mira, Portugal). J. Environ. Monit. Assess. 188(4):1-15.
Crossref
|
|
|
|
|
Sim W, Lee WJ, Shin KS, Song BK, Oh E J (2011). Chemosphere Assessment of fates of estrogens in wastewater and sludge from various types of wastewater treatment plants. J.Chem. 82(10):1448-1453.
|
|
|
|
|
Snyder S, Lue-Hing C, Cotruvo J, Drewes JE, Eaton A, Pleus RC, Schlenk D (2009). Pharmaceuticals in the Water Environment. National Association of Clean Water Environment (NACWA) and Association of Metropolitan Water Agencies (AMWA).
|
|
|
|
|
Tabata A, Kashiwada S, Ohnishi Y, Ishikawa H, Miyamoto N, Itoh M, Magara Y (2001). Estrogenic influences of estradiol-17 beta, p-nonylphenol and bisphenol- A on Japanese Medaka (Oryzias latipes) at detected environmental concentrations. J. Water Sci. Technol. 43:109-116.
|
|
|
|
|
TBS (2005). Tanzania Bureau of Standards, National Environmental Standards
|
|
|
|
|
Thorpe KL, Hutchinson TH, Hetheridge MJ, Scholze M, Sumpter JP, Tyler RC (2001). Assessing the biological potency of binary mixtures of environmental estrogens using vitellogenin induction in juvenile rainbow trout (Oncorhynchus mykiss). J. Environ. Sci. Technol. 35: 2476-2481.
Crossref
|
|
|
|
|
Thorpe KL, Cummings RI, Hutchinson TH, Scholze M, Brighty G, Sumpter JP, Tyler CR (2003). Relative potencies and combination effects of steroidal estrogens in fish. J. Environ. Sci. Technol. 37:1142-1149.
Crossref
|
|
|
|
|
Tyler CR, Jobling S (2008). Roach, Sex, and Gender-Bending Chemicals: The Feminization of Wild Fish in English Rivers. J. Biosci. 58(11):1051-1059.
Crossref
|
|
|
|
|
UNEP, WHO (2012). State of the Science of Endocrine Disrupting Chemicals. Summary report for decision makers, United Nations Environment Programme and the World Health Organization. pp. 1-19.
|
|
|
|
|
URT (2006). The United Republic of Tanzania, State of Environment Report, Vice President's office- Division of Environment. pp. 1-157.
|
|
|
|
|
Wedekind C (2014). Fish populations surviving estrogen pollution. J. Biomed. Central 12(10):1-3.
Crossref
|
|
|
|
|
Williams M, Wood M, Kumar A, Ying GG, Shareef A, Karkkainen M, Kookana R (2007). Endocrine Disrupting Chemicals in the Australian Riverine Environment. A plot study on estrogenic compounds, Land and Water Australia Project Report. pp. 1-89.
|
|
|
|
|
Yuan X, Li T, Zhou L, Zhao X (2014). Characteristics and Risk Assessment of Estrogenic Compounds in Rivers of Southern Jiangsu Province, China. Euro. Instit. Int. Relations Procedia 9:176-184.
Crossref
|
|