ABSTRACT
Recently, increasing attention has been paid on Leuconostoc lactis as a promising bioactive organism against food-borne and spoilage bacteria. A total of nine strains, including six different species of the genus Lactobacillus and three species of the genus Leuconostoc, were isolated from chicken carcasses (n=60) collected from wholesale poultry markets located at Al-Riyadh city, Saudi Arabia in 2016 and identified by API 50 CHL assays. L. lactis isolates were resistant to bile salts and vancomycin. The autolytic phenotype of L. lactis was evaluated under starvation conditions in the presence of potassium phosphate buffer. The strains tested showed partial autolysis of approximately 18% after 7 h of starvation at 37°C at the end of the exponential phase. The inhibitory activity of whole-protein extracts of L. lactis against the foodborne bacteria, Listeria monocytogenes, Bacillus cereus, Bacillus subtilis, Staphylococcus aureus and Micrococcus luteus was evaluated by renaturing sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). The major activity of the total protein appeared as two clear bands on the SDS-PAGE, of approximately 57 and 42 kDa against L. monocytogenes, B. cereus and B. subtilis. No active band was shown against S. aureus and M. luteus.
Key words: Bacteriocins, biopreservation, lactic acid bacteria, pathogens, poultry.
Abbreviation:
Analytical profile index; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis; kDa, kilo Dalton; OD, optical density; IU, international Unit; EDTA, ethylenediaminetetraacetic
acid; TES buffer, Tris EDTA buffer; UV, ultra violet.
Leuconostoc is a genus of Gram-positive bacteria, placed within the family of Leuconostocaceae. They are generally ovoid cocci often forming chains. Leuconostoc spp. are resistant to vancomycin and are catalase-negative, which differentiates them from staphylococci (catalase-positive). Leuconostoc bacteria are used as starter culture bacteria, and are similar to other lactic acid bacteria (LAB), because their fermentation of some vegetables and milk products results in a sour odour that is used as an indicator of freshness. Although, some species of the genus are capable of causing infections in humans (Coovadia et al., 1987; Coovadia et al., 1988), Leuconostoc may be used to improve food hygiene, safety and shelf-life by producing antimicrobial substances such as bacteriocins, lactic acid and hydrogen peroxidase (Holzapfel et al., 1995; Jay 1996).
Bacteriocins are active against some Gram-positive bacteria and foodborne pathogens, such as Listeria monocytogenes, Clostridium botulinum and Staphylococcus aureus, and have been proposed to serve as bio-preservatives in foods (Franz et al., 2007). Probiotics are defined as live microorganisms that provide health benefits to the host (FAO/WHO, 2002). Some lactic acid bacteria, including those belonging to the groups Lactobacillus, Streptococcus, Pediococcus and Leuconostoc, have been reported to possess active probiotic capabilities (Fuller, 1991; Ogunshe 2008).
Leuconostoc spp. are inhibitory to other bacteria because they produce organic acids and hydrogen peroxide, thereby altering the pH. Bacteriocin is produced during bacterial growth in a suitable medium. As shown by BAuthor%D&cauthor=true&cauthor_uid=7814741"Stiles (1996), although bacteriocin production by Leuconostoc spp. was first observed in the 1950s, more extensive studies of bacteriocins produced by Leuconostoc spp. were not conducted until 1984, when the antagonistic activity of Leuconostoc spp. was reported. Since then, the following bacteriocins have been identified: Mesentericin Y105 produced by Leuconostoc mesenteroides; leucocin A-UAL 187 produced by Leuconostoc gelidum; carnosine 44A produced by Leuconostoc carnosum; and leuconocin S produced by Leuconostoc paramesenteroides. D&cauthor=true&cauthor_uid=7814741"Stiles (1996) reported that bacteriocins produced by Leuconostoc may or may not be active against other lactic acid bacteria but exhibit varied effects against Listeria. Mesentericin Y105 is active against Listeria spp., and the amino acid sequences for leucocin A and mesentericin Y105 have been determined. Only two amino acids in the antibacterial protein sequences of these two bacteriocins were found to be different.
The aim of the present work was to identify and characterize commensal bacteria isolated from chicken carcasses, focusing especially on Leuconostoc lactis and its activity against some Gram-positive foodborne bacteria.
Whole chicken carcasses (n = 60) were obtained from a wholesale poultry market located in Saudi Arabia. Chicken carcasses were approximately of 1.5 kg ± 150 g weight and transported under controlled cooling temperatures (2°C) at the Food Microbiology Laboratory, Department of Food Science and Nutrition, College of Food and Agricultural Sciences, King Saud University, until further use.
Microbiological analysis
The isolation of lactic acid bacteria from chicken carcasses was performed by routine microbiological isolation procedures with serial dilutions on selective media of Man Rogosa Sharpe (MRS) agar (Oxoid CM115, Basingstoke, Hampshire, UK) supplemented with 0.25% (w/v) L-cysteine CHR (Fluka AG, Buchs SG- K595-/88/4./8). Whole chickens were washed in 0.1% lactose broth medium (Oxoid CM0137), shaken manually for 1 min and then serially diluted at 1/10 proportions with 0.1% lactose broth. One millilitre of each sample dilution was plated onto Petri dishes and covered with MRS agar that had been sterilized and cooled to 50°C. The plates were incubated for 72 h at 37°C in an anaerobic system (BBL Gas Pak, Becton Dickinson and Co., Cockeysville, MD 21030, USA). Suspected colonies were selected from MRS agar plates of the 10-5 dilution (5 log CFU). Pure colonies were also randomly selected from the primary plates after incubation and preserved in 15% glycerin at -20°C for stock and bench cultures. Colonies were identified using the API 50 CHL kit (bioMerieux, Marcy l’Etoile, France).
Biochemical tests
Biochemical tests were performed using API 50 CH and API CHL test kits (Bio-merieux), according to the manufacturer’s instructions. The ability of a strain to ferment 49 carbohydrates after 18 h of growth in MRS broth and change the colour of the bromocresol purple indicator from purple to yellow indicated a positive result. Susceptibility to vancomycin was evaluated by the disc diffusion method on Mueller-Hinton agar (CM 0337) with antibiotic discs containing 30 µg of vancomycin (Oxoid CT00588), ampicillin 10 µg (CT 0003B as a positive control and according to the National Committee for Clinical Laboratory Standards (NCCLS) (2002).
In the present study, in order to examine the possibility of L. lactis to be a Gram-positive pathogen, apart from the API 20 STREP and API 50 CHL kits (bioMerieux, Marcy l’Etoile, France) that led to the identification of vancomycin-resistant, catalase-negative Gram- positive species, additional phenotypic assays, with assistance of a genotypic confirmation were run, confirming its non-pathogenic character (results not shown). However, molecular identification data on L. lactis are not provided in the present study.
Assessment of antimicrobial activity by well diffusion assay
The antimicrobial activity of L. lactis (whole crude protein of the cells) against L. monocytogenes, Bacillus cereus and Bacillus subtilis, S. aureus and Micrococcus luteus was determined through the well diffusion assay. All the tested bacteria were incubated in nutrient broth at 37°C for 24 h. Twenty millilitres of Muller-Hinton agar were poured into Petri dishes and inoculated with 0.1 mL of the broth from a 24 h culture containing the target bacteria. After solidification, the dishes were stored at 4°C for 2 h. Two wells were made in each dish and filled with 50 μL of different concentrations of cell-free Leuconostoc filtrates. The Petri dishes were then incubated at 37°C for 24 h. The antimicrobial activity was detected by the presence of a clear zone around the wells. Similarly, the effect of 50 μL of filtered, crude protein from the Leuconostoc cultures after sterilization at 121°C for 15 min was tested on each target bacteria.
Analysis of bile salt tolerance
The effect of bile salt on growth rate of L. lactis was evaluated according to the method developed by Walker and Gilliland (1993). The cultures were evaluated for growth in MRS broth supplemented with 0.1, 0.15, 0.20, 0.25 and 0.30% bile salt (Difco, 0129-02), with the acidity adjusted to a pH of 3 using 0.1 N HCl. Fresh cultures of Le. lactis were inoculated into each MRS broth containing different bile salt concentrations and were incubated at 37°C in a water bath. The growth was monitored hourly for a period of 11 h using a spectrometer at an optical density (OD) of 620 nm.
Effect of bile salts and pH on the growth rate of L. lactis
The growth rate of L. lactis in tubes containing 10 mL of MRS with different concentrations of bile salts (0.1, 0.2, and 0.3%) and the following two pH conditions were determined: one set of tubes was adjusted to pH 3.0 and the other was adjusted to pH 6.0 with 0.1 N of HCl. All the solutions were autoclaved at 121°C for 15 min, cooled and then inoculated with 100 µL of fresh cultures that had been grown overnight. The MRS inoculations were then incubated at 37°C for 24, 48 and 72 h. Each treatment was tested in triplicate. The turbidity, as an indication of growth or no growth, was monitored hourly at an OD of 620 nm using a spectrophotometer.
Effect of Tween 80 (1%) on the tolerance of L. lactis at different concentrations of bile salts
Different concentrations of bile salts (0.1, 0.2 and 0.3%) were added to test tubes of MRS broth containing 1% Tween 80 (v/v). The pH was adjusted to 3, and the tubes were then autoclaved at 121°C for 15 min. Each tube was inoculated with 100 µL of broth cultured overnight and incubated at 37°C for 6, 12, 24, 48 and 72 h. The bacterial growth rate was determined at an OD of 620 nm using a spectrophotometer.
Autolysis of whole cells in buffer solution
L. lactis cells in the exponential phase of growth (OD at 620 nm = 1±1.5) were centrifuged at 3000 g and 4°C for 10 min. The cells were harvested and washed in potassium phosphate buffer (50 mM at pH 6.5). The cells were then suspended in the same buffer to an OD at 620 nm of 0.6±0.8 and incubated at 30°C. The percent decrease in the OD of the cells was expressed as the extent of autolysis after 7 h of incubation in a water bath at 30°C: lysis (%) = 100 - (A1/A2) x 100, where A1 is the lowest OD value and A2 is the maximal OD value measured during the incubation period (Cibik, 2010).
Preparation of cell extracts and polyacrylamide gel electrophoresis (PAGE)
Cell extracts from bacterial cultures of L. lactis were prepared after 24 h of growth in fresh liquid MRS medium, according to the method described by Yehia and Al-Dagal (2014). One hundred microliters of the overnight cultures were inoculated into 10 mL of fresh liquid MRS medium, and the cells were then grown for 3 to 4 h until they reached an OD at 620 nm of 0.6. The cells were collected, weighed (250 mg) and then suspended in 100 µL of TES buffer (50 mM Tris, 1 mM EDTA, and 25% sucrose, pH 8). Mutanolysin (5000 IU/mL) and 20 µL of lysozyme (50 mg/mL) were added to the suspension cells in TES buffer, and the cells were then incubated at 37°C for 30 min. Ten microliters of 20% SDS was added, and the contents were mixed until the solution was clear. Forty microliters of total protein extract was loaded onto SDS-PAGE gels. The SDS-PAGE of the isolates and the running of the samples were performed using a 12% polyacrylamide running gel and a 4% stacking gel with a 0.025 M Tris/0.19 M glycine buffer at pH 8.3 and 100 μL of a sucrose buffer (50 mM Tris-HCl, pH 8; 40 mM EDTA, pH 8; 0.75 M sucrose). Using a vertical tank apparatus, electrophoresis was performed at 25°C with a constant voltage power supply until the bromophenol blue tracking dye reached the bottom of the gel. The gels were stained with 0.25% Coomassie Brilliant Blue R-250 (Bio-Rad, Marnesla-Coquette, France) in a ratio of water:methanol:acetic acid 6.5:2.5:1 for 18 h at room temperature. Gel destaining was performed by continuous agitation in a ratio of methanol:acetic acid:water 20:10:70 v:v:v solvent until obvious protein bands were obtained.
Renaturing SDS-PAGE (zymogram)
SDS-PAGE electrophoresis renaturation was performed as described by Potvin et al. (1988) and Lepeuple et al. (1998b) with 12.5% (w/v) polyacrylamide separating gels in a Mini-Protean I cell. Autoclaved cells (120°C for 15 min) of L. monocytogenes, B. cereus, B. subtilis, S. aureus and M. luteus were incorporated separately into polyacrylamide gels at a concentration of 0.2 to 0.4% (w/v) and used to test the bacteriolytic activity of L. lactis. The polyacrylamide gels were soaked in 250 mL of distilled water for 30 min at room temperature under gentle agitation. The gels were then transferred to 200 mL of renaturing buffer (50 mM of 1X potassium phosphate buffer at pH 6.5 with 0.1% Triton X-100) and incubated at 37°C with gentle agitation for 16 h. The gels were stained in 0.01% KOH containing 0.1% methylene blue for 2 h and destained in distilled water. Clear zones of lytic bands appeared in the opaque background. The molecular weights of the lytic bands were determined in comparison with standards run on the same gel.
Statistical analysis
Experiments were replicated twice (n=2) on different occasions with different chicken samples (total number of chicken samples = 60). In each of the two experiments, 30 chicken samples were analysed in triplicate for each replicate. Results are reported as mean values ± standard deviation (S. D.). Data were analysed using the software Stat graphics (Statistical Graphics Corp., Rockville, MD, USA).
The results presented in Table 1 show the types of bacterial isolates isolated from chicken carcasses. According to the API 50 CHL assays, these isolates belonged to five different species of the genus Lactobacillus and one isolate belonged to the genus, Leuconostoc. The percentage of each species identified, out of the total number of species examined, varied as follows: L. lactis presented the highest ratio (33.33%), followed by Lactobacillus salivarius (22.22%), and the lowest ratios were obtained for Lactobacillus delbrueckii, Lactobacillus acidophilus, Lactobacillus fermentum and Lactobacillus brevis (each at 11.11%).
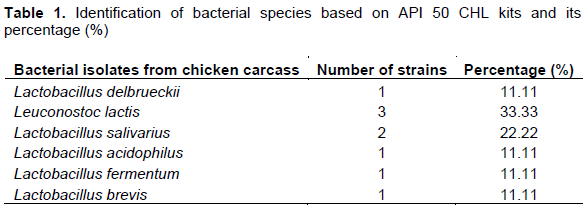
Figure 1 depicts an example of L. lactis species resistant to 30 µg of vancomycin, and sensitive to ampicillin 10 µg, as determined through the disc diffusion method with Mueller-Hinton agar. The additional identification of this strain as catalase-negative was detected by adding one drop of H2O2. Figure 2 shows effect of the whole crude protein extracted of L. lactis cells against the target bacterial species, B. subtilis, B. cereus and L. monocytogenes. Pronounced inhibition zones were observed for Leuconostoc cells against all three of these bacteria. The addition of methylene blue clarified these zones. Zhang et al. (2013) reported that L. lactis isolated from the intestine of black porgy fish was most effective against the growth of Escherichia coli strain O157:H7, Salmonella typhimurium, B. subtilis, Proteus vulgaris, Vibrio parahaemolyticus, Vibrio alginolyticus, Vibrio harveyi and Shigella, but showed low inhibition towards S. aureus and no inhibition towards L. monocytogenes, L. lactis subsp. cremoris and Aspergillus niger.
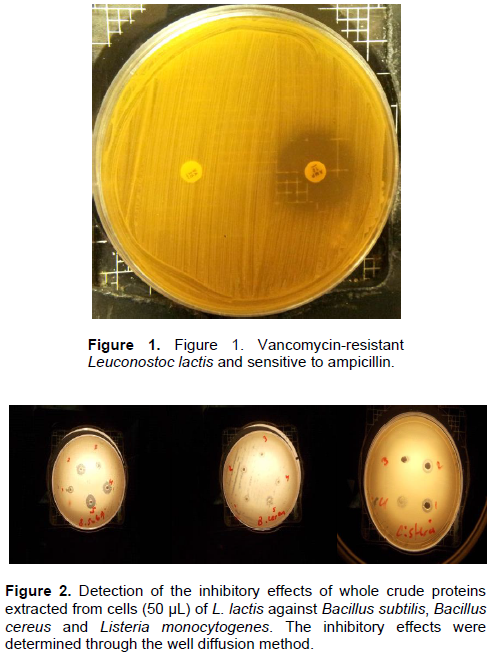
The most interesting finding in the present study is shown in Figure 3, which shows that the crude protein extracts of the cells were also effective against the three tested bacteria after sterilization at 121°C for 15 min. Tolerance to bile salts allows bacterial strains to live in the intestines of hosts and to fulfil their metabolic activity while tolerating such harsh conditions. Thus, the ability of L. lactis to grow in the presence of different concentrations of bile salts (0.1, 0.15, 0.20, 0.25 and 0.30%) was studied individually and in conjunction with varying pH values. Figure 4 shows that the growth rate of L. lactis reached its maximum at a bile salt concentration of 0.3% after 9 h of incubation at 37°C (OD of approximately 2.5). The degree of tolerance of L. lactis against bile salts was quite apparent. Resistance to bile salt of the isolates could be attributed to their ability to produce bile hydrolase(Savage 1992).Bile salt hydrolase (BSH) protects the cells that produce it from the toxicity of conjugated bile salts by deconjugating the bile acids (Walker and Gilliland, 1993).
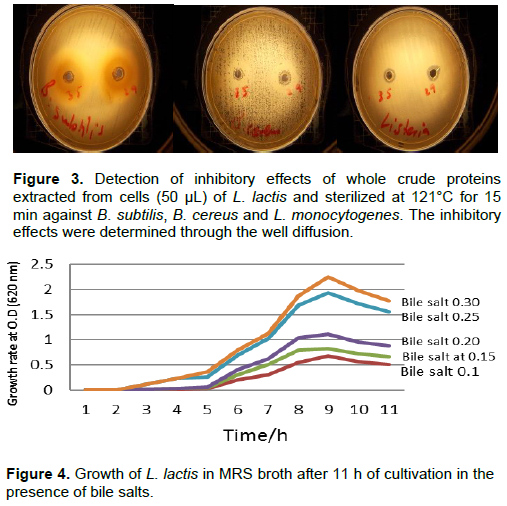
Figure 5 shows the effect of different concentrations of bile salts (0.1, 0.2, and 0.3%) and different pH values (3 and 6) on growth rates. The maximum growth of L. lactis was obtained at the bile salt concentration of 0.3% and a pH value of 6, with an OD value greater than 0.45 after 24 and 48 h and an OD value greater than 0.35 after 72 h. The results presented in Table 2 show that the growth of L. lactis in the presence of bile salt concentrations of 0.1 and 0.2% was better than that observed at a bile salt concentration of 0.3% and allowed the bacteria to reach their maximum growth (OD: 6.5) after 48 h. Increasing the bile salt concentration to 0.3% reduced the growth rate to an OD of 2.0 within the same incubation period. Although, L. lactis grew well in the presence of bile salts in the absence of Tween 80, the growth in the presence of 1% Tween 80 was enhanced. At 72 h, a high decrease in the growth of bacterial strain was noted probably due to the depletion of most nutrients of the media, and the stress that the bacterial strain was exposed to, during growth in the presence of bile salt and Tween 80.
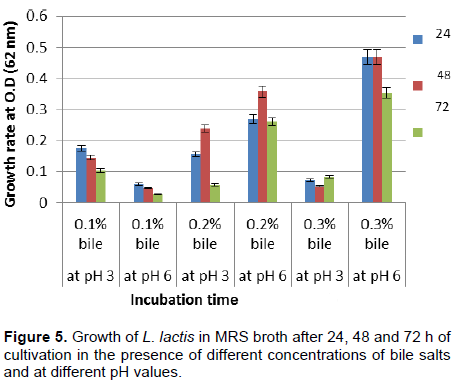
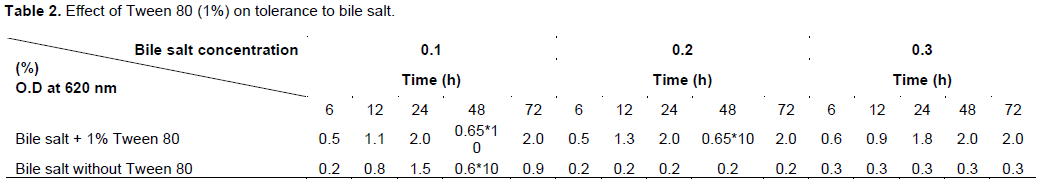
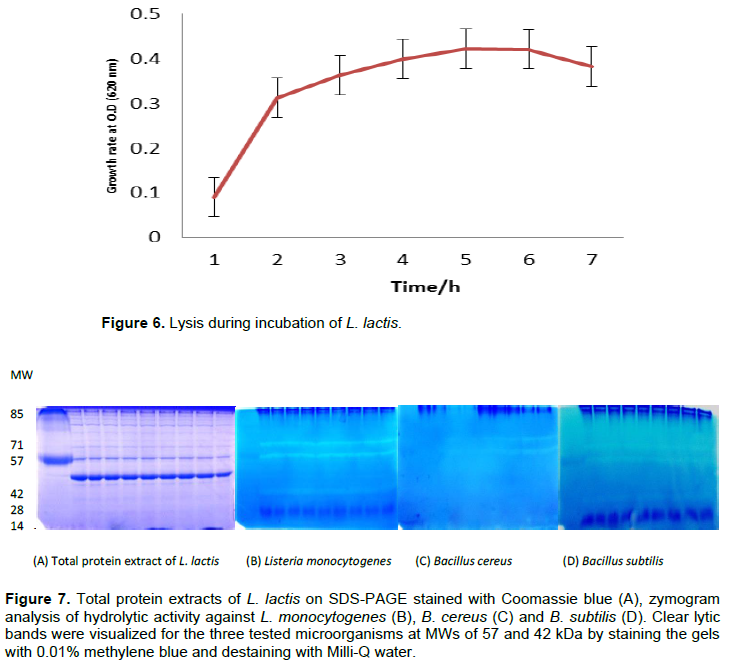
Figure 6 shows a reduction in the growth phase and autolysis of the bacterial strain L. lactis at the end of the exponential phase. Lysis at 18% was obtained after 7 h of incubation, suggesting that the strain of L. lactis was partially autolysed. Figure 7A shows the Coomassie blue staining of the total protein extracted from L. lactis. A zymogram analysis of the lytic activities of whole-cell extracts of L. lactis using B. cereus, B. subtilis and L. monocytogenes as substrates, was performed using renaturing SDS-PAGE (Figures 7B to D). Two bacteriolytic activity bands were revealed after 2 h of incubation in renaturation buffer at 37°C. The two major bands had apparent molecular masses of approximately 57 and 42 kDa. The expression of genes encoding the autolysin of the two bands that resulted in the lytic zone was observed on the gels. No bands were detected for M. luteus or S. aureus. A band at 14 kDa revealed another lytic band referred to as the lysozyme, which was used for the degradation of bacterial cells.
The results, regarding Lactobacillus species isolated from chicken carcasses in Saudi Arabia, are similar to those reported in other countries. For example, Ibourahema et al. (2008) isolated L. casei, Lactococcus lactis, L. plantarum and L. paraplantarum from poultry farms in Senegal. In Nigeria, Adesokan et al. (2008) isolated three different LAB species from poultry meat and they reported that L. plantarum represented the highest percentage of total species at 90%, whereas L. mesenteroides and L. brevis each represented 5% of the total bacterial populations. In Indonesia, Lengkey et al. (2009), isolated L. lactis ssp. lactis 1 and lactis 2, L. fermentum 1, Lactobacillus paracasei 1 and Lactobacillus rhamnosus from raw poultry meat. Lin et al. (2007) showed that L. fermentum is the major LAB species found in the gastrointestinal tracts of swine and poultry. All the above-mentioned authors used API CHL 50 kits for the identification of LAB species in poultry meat. Zhang et al. (2013) stated that L. lactis isolated from the intestine of black porgy fish is most effective towards E. coli O157, S. typhimurium, B. subtilis, P. vulgaris, V. parahaemolyticus, V. alginolyticus, V. harveyi and Shigella, whereas L. lactis displayed a lower inhibitory activity towards S. aureus and did not show any inhibition on L. monocytogenes, L. lactis subsp. cremoris and A. niger. The protein produced by L. lactis was found to be thermostable at high temperatures, which may be of great importance for its action against microorganisms in food treated at high temperatures during the preservation process.
Due to the presence of bile salts and low pH in the intestine, the evaluation of LAB as probiotics depends on their ability to tolerate these conditions (Fontana et al., 2013). It is also important for probiotic bacteria to be able to grow in 0.15±0.30% oxgall (Goldin and Gorbach 1992).
Kimoto et al. (2002) showed that the addition of Tween 80 (a non-ionic detergent) to the microbial medium containing bile salt enhances the microbe’s tolerance to bile, although the effect of Tween 80 was influenced by the type of strain and bile constituents. Kimoto et al. (2002) also investigated the effect of adding Tween 80 on cellular permeability, and the results revealed that the addition of Tween 80 in the presence of bile reduced the cellular leakage (caused by 0.3% oxgall). Cellular leakage was caused by oxgall, whereas cell lysis was not enhanced by oxgall. Therefore, the leakage of UV-absorbing materials is due to an increase in cellular permeability and not cell lysis. These results indicate that exogenously added Tween 80 reduces the cellular permeability caused by bile. Starvation conditions are obtained by transferring bacterial cells from the culture medium to a buffer system, and this technique is widely used to estimate the potential autolytic properties of bacteria (Cibik and Chapot-Chartier, 2004; Lortal et al., 1989; Lemee et al., 1994). Similar results have been reported for L. lactis lactis (Mou et al., 1976; Ostlie et al., 1995a). Buist et al. (1995) reported that inactivation of the L. lactis gene encoding the major autolysin AcmA results in the detection of several bands by renaturing gel electrophoresis that correspond to degradation products or to a precursor form of AcmA. A similar situation occurs in S. aureus, in which most of the multiple bands detected by renaturing SDS-PAGE result from the processing of the major autolysin Atl (Foster, 1995). In agreement with previous reports (Ostlie et al., 1995b; Chapot-Chartier 1996; Lortal et al., 1997), this study revealed that the peptidoglycan hydrolase profile of strains within a species is conserved.
However, in contrast to previous observations for the Lactobacillus genus (Lortal et al., 1997), the peptidoglycan hydrolase profile does not appear to be specific to one species in the Leuconostoc genus but may rather be an indication of species relatedness. Ostlie et al. (1995a, b) and mentioned that cells express the highest level of autolysis in Propionibacterium and Lactobacillus acidophilus during the exponential phase of growth, which supports the hypothesis of the involvement of autolysins in cell growth (Crow et al., 1995). In contrast, Buist et al. (1995) postulated that a major autolytic enzyme from lactococcal strains is expressed during the stationary phase. Alternatively, maximal autolysis for Streptococcus thermophiles has been observed in the transitional phase between the exponential and stationary phases (Sandholm, and Sarimo, 1981). Additionally, Valence and Lortal (1995) reported two optimal harvesting points for L. helveticus: the first occurs during the transitional phase, and the second occurs during the early part of the exponential phase.
In this study, the lactic acid bacterium L. lactis isolated from chickens was found to display a tolerance to 0.3% bile salts at pH 6, suggesting that this bacterium is well-adapted to colonize and survive in the harsh environment of the intestinal tract. Its inhibitory activity on the growth of certain Gram-positive foodborne pathogenic bacteria such as L. monocytogenes, B. cereus and B. subtilis, enhances its importance and contributes to its potentially probiotic properties, which may be of benefit in food preservation technologies.
The authors have not declared any conflict of interests.
REFERENCES
|
Adesokan A, Odetoyinbo BB, Olubamiwa AO (2008). Biopreservative activity of lactic acid bacteria on suya produced from poultry meat. Afr. J. Biotechnol. 7:3799-3803.
|
|
|
|
Buist G, Kok J, Leenhouts KJ, Dabrowska M, Venema G, Haandrikman AJ (1995). Molecular cloning and nucleotid sequence of the gene encoding major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J. Bacteriol. 177:1554-1563.
Crossref
|
|
|
|
|
Chapot-Chartier MP (1996). Les autolysines des bactéries lactiques. Le Lait. 76:91-109.
Crossref
|
|
|
|
|
Cibik R (2010).Biochemical Factors Influencing Autolysis of Leuconostocs in Buffer. Uludag Univ. J. Fac. Vet. Med. 29:37-41.
|
|
|
|
|
Cibik R, Chapot-Chartier MP (2004). Characterization of autolytic enzymes in Lactobacillus pentosus. Lett. Appl. Microbiol. 38:459-463.
Crossref
|
|
|
|
|
Coovadia YM, Solwa Z, J.van den Ende (1987). Meningitis caused by vancomycin-resistant Leuconostoc sp. J. Clin. Microbiol. 25:1784-1785.
|
|
|
|
|
Coovadia YM, Solwa Z, van den Ende J (1988). Potential pathogenicity of Leuconostoc. Lancet i:306.
Crossref
|
|
|
|
|
Crow VL, Coolbear T, Gopal PK, Martley FG, McKay LL, Riepe H (1995). The role of autolysis of lactic acid bacteria in the ripening of cheese. Int. Dairy J. 5:855-875.
Crossref
|
|
|
|
|
FAO/WHO (2002). Guidelines for the evaluation of probiotics in food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; Ontario, Canada. April 30, May 1.
|
|
|
|
|
Fontana L, Brito MB, Diaz JP, Quezada SM, Gil A (2013). Sources, isolation, characterisation and evaluation of probiotics. Br. J. Nutr. 109:35-50.
Crossref
|
|
|
|
|
Franz CMAP, Van Belkum MJ, Holzapfel WH, Abriouel H, Ga’lvez A (2007). Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol. Rev. 31(3) 293-310.
Crossref
|
|
|
|
|
Foster S (1995). Molecular characterization and functional analysis of the major autolysin of Staphylococcus aureus 8325/4. J. Bacteriol. 177(9):5723-5725.
Crossref
|
|
|
|
|
Fuller R (1991). Probiotics in human medicine. Gut 32(4):430-442. Goldin BR, Gorbach SL (1992). Probiotics for humans. In: Fuller, R. (Ed.), Probiotics, the Scientific Basis. Chapman & Hall, London. pp. 355-376.
Crossref
|
|
|
|
|
Holzapfel WH, Geisen R, Schillinger U (1995). Biological preservation of foods with reference to pro¬tective cultures, bacteriocins and food-grade enzymes. Int. J. Food Microbiol. 24:343-362.
Crossref
|
|
|
|
|
Ibourahema C, Dauphin RD, Jacqueline D, Thonart P (2008). Characterization of lactic acid bacteria isolated from poultry farms in Senegal. Afr. J. Biotechnol. 7(12).
|
|
|
|
|
Jay JM (1996). Microorganisms in fresh ground meats: The relative safety of products with low versus high numbers. Meat Sci. 43:59-66.
Crossref
|
|
|
|
|
Kimoto H, Ohmomo S, Okamoto T (2002). Enhancement of bile tolerance in lactococci by Tween 80. J. Appl. Microbiol. 92:41-46.
Crossref
|
|
|
|
|
Lemee R, Rouault A, Guezenec S, Lortal S. (1994) Autolysis of 57 strains of dairy propionibacteria. Le Lait 74.4:241-251.
Crossref
|
|
|
|
|
Lengkey HAW, Adriani L (2009). Uticaj mleka fermentisanog sa Lactobacillus acidophilus I Bifidobacterium spp. na sadržaj mlecne i sircetne kiseline i Staphylococcus aureus i Pseudomonas aeruginosa. Biotechnol. Anim. Husb. 25(7):19-724.
|
|
|
|
|
Lepeuple AS, Van Gemert E, Chapot-Chartier MP (1998b). Analysis of the bacteriolytic enzymes of the autolytic Lactococcus lactis subsp. cremoris strain AM2 by renaturing polyacrylamide gel electrophoresis: identification of a prophage encoded enzyme. Appl. Environ. Microb. 65(41):42-4148.
|
|
|
|
|
Lin WH, Yu B, Jang SH, Tsen HY (2007) . Different probiotic properties for Lactobacillus fermentum strains isolated from swine and poultry. Anaerobe 13:107-113.
Crossref
|
|
|
|
|
Lortal FS. Valence C, Bizet JL (1997). Maubois. Electrophoretic pattern of peptidoglycan hydrolases, a new tool for bacterial species identification: application to 10 Lactobacillus species. Res. Microbiol. 148:461-474.
Crossref
|
|
|
|
|
Lortal S, Boyaval P, Van Heijenoort J (1989). Influence de plusieurs facteurs sur l'autolyse de Lactobacillus helveticus CNRZ 414. Le Lait 69.3:223-231.
Crossref
|
|
|
|
|
Mou L, Sullivan JJ, Jago GR (1976). Autolysis of Streptococcus cremoris. J. Dairy Res. 43(2):275-282.
Crossref
|
|
|
|
|
NCCLS (National Committee for Clinical Laboratory Standards) (2002). Performance standards for antimicrobial susceptibility testing: Twelfth informational supplemen NCCLS document M100- S12. PA, USA.
|
|
|
|
|
Ogunshe AAO (2008). Bioinhbition of diarrhogenic Gram-positive bacterial pathogens by potential indigenous probiotics in industrial infant weaning food. Asian Pac. J. Trop. Med. 1(2):7-11.
|
|
|
|
|
Ostlie H, Vegarud G, Langsrud T (1995a). Autolysis of lactococci: detection of lytic enzymes by polyacrylamide gel electrophoresis and characterization in buffer systems. Appl. Environ. Microbiol. 61(10):3598-3603.
|
|
|
|
|
Ostlie H, Vegarud G,Langsrud T(1995b). Autolysis of dairy propionibacteria in buffer systems. J. Dairy Sci. 78(11):2315-2325.
Crossref
|
|
|
|
|
Potvin C, Leclerc D, Tremblay G, Asselin A, Bellemare G, Cloning (1988.) Sequencing and expression of a Bacillus bacteriolytic enzyme in Escherichia coli. Mol. Gen. Genet. 214(2):241-248.
Crossref
|
|
|
|
|
Sandholm E, Sarimo SS (1981) Autolysis of Streptococcus thermophilus. FEMS Microbiol. Lett. 11:125-129.
Crossref
|
|
|
|
|
Savage DC (1992). Gastrointestinal microbial ecology: possible modes of action of direct-fed microbials in animal production - a review of the literature. In: Direct-Fed Microbials in Animal Production. National Feed Ingredients Association, Iowa. pp. 11-81.
|
|
|
|
|
Stiles MEN (1996). Biopreservation by lactic acid bacteria. Anton Leeuw. Int. J. G 70:331-345.
Crossref
|
|
|
|
|
Valence F,Lortal S (1995). Zymogram and preliminary characterization of Lactobacillus helveticus autolysins. Appl. Environ. Microbiol. 61(9):3391-3399.
|
|
|
|
|
Walker DK, Gilliland SE (1993).Relationships among bile tolerance, bile salt deconjugation and assimilation of cholesterol by Lactobacillus acidophilus. J. Dairy Sci. 76(4):956-961.
Crossref
|
|
|
|
|
Yehia HM, AL-Dagal MM (2014). Prevalence of Campylobacter jejuni in chicken produced by major poultry companies in Saudi Arabia. Int. J Food Contam 1:2.
Crossref
|
|
|
|
|
Zhang W, Liu M, Dai X (2013). Biological characteristics and probiotic effect of Leuconostoc lactis strain isolated from the intestine of black porgy fish. Braz. J. Microbiol. 44(3):3685-691.
Crossref
|
|





