ABSTRACT
The aim of the present investigation was to evaluate the impact of Blighia sapida aril level in diet on the growth of albino Wistar rats and their welfare. A quantity of B. sapida arils was cooked and lyophilized. Thirty six young albinos Wistar rats teams up in six with six homogeneous groups were used for a four weeks research. Young rats group were fed ad libitum with six different diets which can be distinguished by the level of B. sapida arils powder. There were control diet (0% B. sapida arils powder), diet Bs1 (6.25% B. sapida arils powder), diet Bs2 (12.5% B. sapida arils powder), diet Bs3 (25% B. sapida arils powder), diet Bs4 (50% B. sapida arils powder) and diet Bs5 (75% B. sapida arils powder). Body weight was measured weekly and the animals were observed for signs of abnormalities throughout the study. At the end of the experimentation, blood sample was collected and analyzed. Results show that the growth of rats fed with diets Bs1, Bs2 and Bs3 were normal as compared to the growth of rats fed with the control diet. On the other hand, growth retardation was noticed on rats fed with diets Bs4 and Bs5. However, rats coat fed with diets Bs4 and Bs5 were also normal. Again, for rats fed with diets Bs4 and Bs5, a level (p<0.05) of total cholesterol and LDL-cholesterol was noticed as compared to values obtained in rats of the other groups.In the case of natural disaster, the consumption of B. sapida aril could be used as nutritional base at a degree neighboring 50% of the nutritional need of the organism. Outside this value, growth retardation and a level of total cholesterol and LDL-cholesterol can be noticed.
Key words: Blighia sapida aril, diet, rats, growth, welfare.
The tree called fisanier in French is scientifically known as Blighia sapida L. (sapindaceae). It originates from west and Central tropical Africa (Hill, 1952; Bressler et al., 1969). It has been spread by cultivation in the tropical region (Arbonnier, 2002). It is available in West Africa, Central Africa and also in Caribbean and Jamaica. B. sapida is grown because it has several uses. In fact, the tree is grown in towns for its shade. His wood, because it is termite hardy, is used as building material and for cooking (Arbonnier, 2002). The leaves are used as herbal medicine to treat amygalite, conjuntivitis, wound, icterus, vertigo and epilepsy (Arbonnier, 2002; Orwa et al., 2009). When the unripe fruits are crushed, they produce moss which is used as soap and also as fishing poison (Arbonnier, 2002).
The main reason why B. sapida is cultivated is for its arils, the freshly cream which colours the pulp of the fruit. These arils can be consumed freshly or when they are made into sauce or fried in oil (Arbonnier, 2002; Ekué et al., 2010). The consumption of B. sapida arils is worldwide. In fact, in Jamaica, they are industrially transformed and put into cans and are sold in America and Great Britain (Orane et al., 2005). Several research results on the beneficial uses of B. sapida arils show that it is an essential element that should not to be neglected in diet in many regions due to it lipids (45%), proteins (11%), ash (4%) and vitamin C (65 mg/kg) content (Morton, 1987; Akintayo et al., 2002; Ouattara et al., 2010). Nevertheless, some molecules such as hypoglycine A, saponin, saccharides and fructoligosaccharides (Jimoh et al., 2007; Benkeblia and Lopez, 2015) have negative effects on the organism function (Moyal, 1985, 1986; Chase et al., 1990; CDC, 1992; Bouree et al., 2002; OPS, 2002; Katibi et al., 2015). These negative effects can end up with death caused by hypoglycemia or hemolytic syndrome (Moyal, 1985, 1986; CDC, 1992; Bouree et al., 2002; OPS, 2002; Katibi et al., 2015). Despite everything, potentialities of B. sapida arils have been exploited in Côte d’Ivoire and in Jamaica when there were natural disasters in these countries. In Côte d’Ivoire, in the year 1984, a drought caused a diminution of cereals production. Then, consumption of B. sapida arils prevented a big famine. Also, the consumption of B. sapida aril prevented a big famine in Jamaica. This famine was due to flood which caused lack of cereals. According to an epidemiological study, mortality was caused either by the action of the toxic molecule call hypoglycin A and the high consumption of arils which do not satisfied the nutritional need of the organism (OPS, 2002). For a better understanding of this situation, some questions may be answered. Can B. sapida aril be a palliative to the lack of food? If not, to what extent can it be safely consumed?
Then, the aim of this study was to evaluate the effect of B. sapida arils consumption in accordance with the level of arils in diet on Albino Wistar rats. In this work, the results of growth, visual observation and blood sample parameters such as lipids parameters, glucose, protein and urea are shown.
Plant material
B. sapida is planted in the North and the Centre of Côte d’Ivoire. In these regions, the arils of the plant are very much consumed. Arils used for the experimentation were brought from Katiola, a town in the North Central of Côte d’Ivoire, in the months of March, April and May. In these months, arils are more available.
At Katiola, arils were spread on polythene paper and exposed to sunlight for two weeks, six hours a day, to get dry. In the night, they were kept on the plastic and put in a house at room temperature (25 - 30°C). After the drying process of the fresh arils, the dried arils were kept in plastic bags and sent to Abidjan in the South of Côte d’Ivoire.
Animals
Thirty six young albino Wistar rats weighing between 49 and 75 g were used. They were bred in the animal house of Ufr Biosciences of the University Félix HOUPHOUËT-BOIGNY of Abidjan (Côte d’Ivoire). During the breeding, rats were fed with food made by a society IVOGRAIN which is specialized in mass production of livestock food. This food is made up of crude protein matter (15%), crude fat matter (3.5%), cellulose matter (12%), mineral matter (9%), calcium (1%), phosphorus (0.9%), sodium (0.3%), vitamin A (15000 UI/kg), vitamin D3 (3000 UI/kg) and vitamin E (10 mg/kg).
Preparation of the arils
In Abidjan, the dry arils were crushed with a laboratory mortar. Each time, 500 g of B. sapida arils powder was cooked, using a gas stone, in one liter of water (500 g/l) for two hours. Approximately, 10 kg of B. sapida arils powder was cooked. Arils pasta obtains were lyophilized. The freeze-dry aril obtained was used to formulate five kinds of experimental diets. These experimental diets were named diets Bs1, Bs2, Bs3, Bs4 and Bs5. Previously, a control diet was prepared. The composition of the control diet was similar to that recommended by scientists of the American Institute of Nutrition for rapid Growth (AIN-93G) on experiment with animals, particularly, rats (Reeves et al., 1993). Table 1 shows the nutritional composition of the control diet.
Difference between the experimental diets
In the experimental diets, a part of the control diet was substituted by the freeze-dry aril. The degree of substitution was 6.5% for diet Bs1, 12.5% for diet Bs2, 25% for diet Bs3, 50% for diet Bs4 and 75% for diet Bs5.
Experimentation
At the beginning of the experimentation, the animals were grouped into six different homogeneous young groups of rats. They were put individually in metabolic cages and maintained under standard laboratory conditions (temperature 25±2°C) with dark and light cycle (12/12 h). In the metabolic cages, rats were acclimatized to this condition and fed with the control diet five days before the beginning of the experiment. After that, the animals were fed ad libitum with the different diets (control diet, diets Bs1, Bs2, Bs3, Bs4 and Bs5) during 28 days according to the different group they belong to.
Treatment of animals during the experimentation
Body weight of each rat was measured weekly and the animals were observed for signs of abnormalities throughout the study. At the end of the experimentation, blood samples were collected at the vena cava level of all the animals and put in individual vacuum valve. The blood samples were taken in the morning after giving ether anesthesia to the albino rats. Samples were collected with a sterile disposable syringe. Also, at the end of the experimentation, the urine (24 h urine) of each animal was taken, measured and analyzed.
Biochemical parameters
The blood samples were centrifuged at 8000 r.p.m for 15 min to harvest the plasma which was used for the various analyses. Glucose was assayed by glucose oxidase-peroxidase method using a kit (Sigma Diagnostics, Sigma kit #315). Total cholesterol and triglycerides were measured as described by Richmond (1973) and Trinder (1969b), respectively. Urea was estimated by the diacetyl method of Wybenga et al. (1971). Total protein was estimated using assay kits (Sigma Diagnostics, St. Louis, MO, USA). Biochemical analysis of serum samples was performed using an automatic chemistry analyzer (Hitachi model 902, Roche).
Statistical analysis
The experimental results were expressed as the mean±S.E.M. Data were assessed by the method of analysis of ANOVA followed by Dunnett test (Ostle, 1966; Woolson, 1987). p value of < 0.05 was considered as statistically significant.
Observation and growth
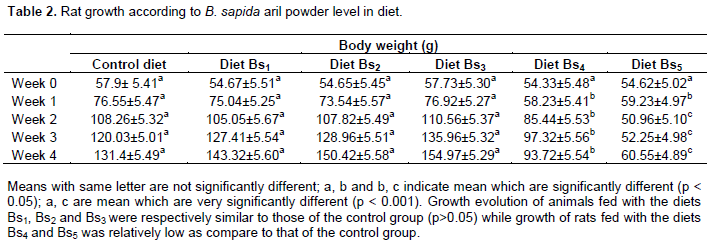
All the rats used in this work had, after the experimentation, generally, either normal coat or normal appearance. At the growth level, some differences were noticed. There were no significant difference (p > 0.05) of growth between the control group and group of rats fed with diets Bs1, Bs2 and Bs3. But, growth of rats fed with diets Bs4 and Bs5 was relatively low (p< 0.05) as compared to that of the control group. The growth of rats fed with diets Bs4 and Bs5 was also low (p< 0.05) when compared with that of rats fed with the diets Bs1, Bs2 and Bs2. Lower growth was observed in the animals fed with diet Bs5 than those which consumed diet Bs4. The percentage of the difference of growth between animals of the control group and those which consumed diet Bs4 was 28.75% (p < 0.05) while this difference between the control group and those which consumed diet Bs5 was 61.53% (p < 0.001). Table 2 shows the curve of rats’ growth according to B. sapida aril powder level in diet.
Effect on the urinary volume
The urinary volumes obtained from rats fed with diets Bs1, Bs2 and Bs3 were not significantly different (p > 0.05) from that obtained from rats fed with the control diet. But, in rats fed with diets Bs4 and Bs5, urinary volumes were respectively high (p < 0.05) and very high (p < 0.001) when compared with urinary volume obtained from rats fed with the control diet.
Urinary volumes obtained was 6.78±1.04 ml/24 h for rats fed with the control diet, 6.57±2.25 ml/24 h for rats fed with diet Bs1, 6.55±1.10 ml/24 h for rats fed with diet Bs2, 6.60±1.34 ml/24 h for rats fed with diet Bs3, 3.11±0.73 ml/24 h for rats fed with diet Bs4 and 1.90±1.11 ml/24 h for rats fed with diet Bs5. These results are shown on Figure 1.
Effect of B. sapida aril powder level in diet on plasmatic triglycerides
Triglycerides level observed were 1.60±0.24 g/l for rats fed with diet Bs1, 1.86±0.17 g/l for rats fed with diet Bs2, 1.42±0.30 g/l for rats fed with diet Bs3, 1.47±0.20 g/l for rats fed with diet Bs4 and 1.37±0.19 g/l for rats fed with diet Bs5. These values were not significantly (p > 0.05) different from that obtained in rats fed with the control diet (1.66±0.11 g/l). These results are shown in Figure 2.
Effect of B. sapida aril powder level in diet on plasmatic total-cholesterol, LDL-cholesterol and HDL-cholesterol
Total-cholesterol and LDL-cholesterol values obtained in rats fed with diet Bs5 were 0.98±0.07 g/l and 0.51±0.07 g/l, respectively. These values were significantly high (p < 0.05) as compared to that obtained in rats of the control group. The total-cholesterol and LDL-cholesterol found in rats of the control group were 0.88±0.07 and 0.40±0.00 g/l, respectively.
The values of total-cholesterol found in rats fed with diets Bs1, Bs2, Bs3, Bs4 and Bs5 were 0.82±0.11, 0.84±0.05, 0.80±0.03, 0.78±0.05 and 0.98±0.07 g/l, respectively. These values were not significantly different (p ˃ 0.05) when compared with that of the control group. The variation of LDL-cholesterol in the experimental group was not significantly different (p ˃ 0.05) from those obtained in the control group. These values were 0.39±0.07 g/l for diet Bs1, 0.41±0.03 g/l for diet Bs2, 0.39±0.04 g/l for diet Bs3, 0.38±0.02 g/l for diet Bs4 and 0.51±0.07 g/l for diet Bs5.
The values of HDL-cholesterol found in rats fed with diets Bs1, Bs2, Bs3, Bs4 and Bs5 were 0.36±0.05, 0.37±0.02, 0.35±0.05, 0.29±0.02 and 0.36±0.05 g/l, respectively. These values were not significantly different (p ˃ 0.05) when compared with that of the control group. The effect of B. sapida aril powder level on plasmatic total-cholesterol, LDL-cholesterol and HDL-cholesterol is shown on Figure 3.
Effect of B. sapida aril powder level diet on plasmatic glucose
Plasmatic glucoses level obtained were 0.62±0.04 g/l for rats fed with diet Bs1, 0.59±0.02 for rats fed with diet Bs2 g/l, 0.65 ± 0.05 g/l for rats fed with diet Bs3, 0.60±0.04 g/l for rats fed with Bs4 and 0.62±0.05 g/l for rats fed with diet Bs5. These values were not significantly different (p > 0.05) from that obtained in rats of the control group which was 0.67±0.02 g/l (Figure 4).
Effect of B. sapida aril powder level in diet on plasmatic protein
Plasmatic protein obtained in rats fed with diets Bs1, Bs2, Bs3, Bs4 and Bs5 were not significantly different (p > 0.05) from that obtained in rats fed with the control diet. These values were 68.50±2.74 g/l for rats fed with control diet, 77.00±5.40 g/l for rats fed with diet Bs1, 72.17±4.26 g/l for rats fed with diet Bs2, 71.00±1.79 g/l for rats fed with diet Bs3, 69.83±1.47 g/l for rats fed with diet Bs4 and 68.50±1.89 g/l for rats fed with diet Bs5 (Figure 5).
Effect of B. sapida aril powder level in diet on plasmatic urea
Plasmatic urea obtained in rats fed with diets Bs1, Bs2, Bs3, Bs4 and Bs5 were not significantly different (p > 0.05) from that obtained in rats fed with the control diet. These values were 0.13±0.01 g/l for rats fed with control diet, 0.12±0.01 g/l for rats fed with diet Bs1, 0.12±0.01 g/l for rats fed with diet Bs2, 0.11±0.01 g/l for rats fed with diet Bs3, 0.13±0.01 g/l for rats fed with Bs4 and 0.11±0.01 g/l for rats fed with Bs5 (Figure 6).
The absence of growth difference in rats fed with experimental diets (diets Bs1, Bs2, Bs3, Bs4 and Bs5) in comparison with growth of rats fed with the control diet could be explained by the fact that these diets are also nutritiously rich. In fact, the different diets may contain satisfactory nutriment elements such as carbohydrate, lipid, vitamin and mineral which are favorable for good growth (Basiotis et al., 2002; American Dietetic Association, 2007; Cindy et al., 2013).
As for rats fed with diets Bs4 and Bs5, growth retardation was noticed when compared with the growth of rats fed with the control diet. This growth retardation was higher among rats fed with diet Bs5 than those fed with diet Bs4. However, the coat of all rats was natural. Growth retardation in rats fed with diets Bs4 and Bs5 may be the result of an inadequate alimentation due to a deficiency of some nutritional elements (IRD, 1996; Katona and Mokhda, 1998; Lefere, 2000). It may be a deficiency of protein (FAO/WHO, 2007). In fact, according to an analysis of the nutritional composition of dry B. sapida aril made by Ouattara et al. (2010) the protein content was 11.99±1.12 g/100 g. This protein content is lower than the one recommended by the Food Agriculture Organization (FAO, 2003) which must be between 12 and 15% in diet. The mixtures of control diet with freeze-dry aril correspond to a diminution of protein in diet. So, when the substitution of the control diet by the freeze-dry aril is important, the diminution of protein content in the diet obtained is higher. This clearly explains the best growth of rats which consumed the diet Bs4 than those which consumed the diet Bs5. Protein restriction in diets Bs4 and Bs5 was very important than protein content of the other diets (control diet, diets Bs1, Bs2 and Bs3) so that it has caused malnutrition due to the deficiency of protein or some amino acids (Drewnowski, 2005). The deficiency of protein or some amino acids justify the growth retardation observed in rats fed with these diets (diets Bs4 and Bs5) than the growth of rats which consumed the other diets (control diet, diets Bs1, Bs2 and Bs3). But, no death was observed in the groups of rats fed with diets Bs4 and Bs5: this suggests that B. sapida aril powder contain adequate quantity of protein and nutritionals elements easy to use by the rats fed with these diets (diets Bs4 and Bs5) even if they do not particularly have sufficient protein.
The absence of lipids level in rats fed with diets Bs1, Bs2, Bs3 and Bs4 as compared to that obtained in rats fed with the control diet suggested that lipids have been used as source of energy in order to satisfy the energy need of the anamal instead of the decrease of carbohydrate in diets. Nevertheless, lipids level obtained in rats fed with diet Bs5 is higher when compare with that obtained in rats of the control diet. This situation is due to the lipids abundance in the diet Bs5 and to the fact that they have not been all utilized as source of energy: they may be accumulated in the rats fed with this diet and then they increased the level of total-cholesterol and LDL-cholesterol. Since no death was noticed, the freeze-dry aril can be used as a palliative to prevent untimely death in the case of lack of food.
Consumption of B. sapida aril powder does not affect the growth of rats until the degree was approximately 50%. At a degree near the value of 50% and above, body weight and growth are affected. At these values of consumption, a rate of total-cholesterol and LDL-cholesterol is noticed. This rate is due to the high content of the diet on fat based on aril. So, B. sapida aril should be consumed with moderation to improve body wellbeing. Therefore, it cannot absolutely be utilized as a palliative to the lack of food but it can contribute to avoiing untimely death in the case of lack of food.
The authors did not declare any conflict of interest.
The author, OUATTARA Howélé, is grateful to the Unité de formation et de Recherche (UFR) Biosciences of University of Félix HOUPHOUËT-BOIGNY for permitting them to work in their laboratories.
REFERENCES
|
Akintayo ET, Adebayo EA, Arogunde (2002). Assessment of dietary exposure to the natural toxin hypoglicin in ackee (Blighia sapida) by Jamaican. Food Res. Int. 37:833-838.
|
|
|
|
American Dietetic Association (2007). Practice paper of the American Dietetic Association: Nutrient Diversity: Meeting nutrient goals within calories needs. J. Am. Diet. Assoc.108: 860-868.
|
|
|
|
|
Arbonnier M (2002). Arbres, arbustes et lianes des zones sèches d'Afrique de l'Ouest. Edition 2, CIRAD-MNHN. pp. 173-179.
|
|
|
|
|
Basiotis P, Carlson A, Gerrior S, Juan W, Lino M (2002). The healthy Eating Index 1999-2000. Washington, DC U.S Department of Agriculture, CNPP. P 12.
|
|
|
|
|
Benkeblia N, Lopez MG (2015). Saccharides and fructooligosaccharides composition of green and ripe Averrhoa carambola, Blighia sapida and Spondias dulcis fruits. Food Chem. 176:314-318.
Crossref
|
|
|
|
|
Bouree P, Frinot JP, Morell-Gil RE, Fernot JP, Nagiroy D (2002). Ackee (Blighia sapida): un arbre aux fruits toxiques mal connu en Afrique et dans l'Ile d'Hispaniola. Med. Afr. Noire 49(7):329-333.
|
|
|
|
|
Bressler R, Corridor C, Brendel K (1969). Hypoglycin and hypoglycin like compounds. Pharmacol. Rev. 21(2):105-130.
|
|
|
|
|
CDC (1992). Toxic Hypoglycemic Syndrome –Jamaica 1989-1991. MMWR 41(4):53-55.
|
|
|
|
|
Chase GWJ, Landen WOJ, Solimon AG (1990). Hypoglicin A content in aril, seeds, and husks of ackee fruit at various stages of repenses. J. Assoc. Official Anal. Chem. 73: 318- 319.
|
|
|
|
|
Cindy WL, Sc D MPH, Elena EH, BA, Ana CLS, DDS, DrPH, Hayley EL, Vanessa AH, MPH, RD, Sophie T, Walter CW, Susan JBE (2013). A Qualitative Study of Diverse Experts Views about Barriers and Strategies to improve the Diets and Health of Supplemental Nutrition Assistance Program (SNAP). J. Acad. Nutr. Diet. 113(1):70-76.
Crossref
|
|
|
|
|
Drewnowski A (2005). Concept of a nutritious food: toward a nutrient density scored 1' 2' 3'. Am. J. Clin. Nutr. 82(4):721-732.
|
|
|
|
|
Ekué MR, Sinsin B, Eyoq-Matiq O, Finkeldey R (2010). Uses, traditional management, perception of variation and preferences in ackee (Blighia sapida K. D Koenig) fruits traits in Benin: implications for domestication and conservation. J. Ethnobiol. Ethnomed. 6(1):1.
Crossref
|
|
|
|
|
FAO (2003). Evolution des graisses alimentaires et évolution de leur consommation. Laboratoire de nutrition tropicale, centre ORSTOM 34032, Montpellier cedex, France.
|
|
|
|
|
FAO/WHO (2007). Protein and amino acid Requirements in Human Nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation, 270 p.
|
|
|
|
|
Hill KR (1952). The vomiting sickness of Jamaica. West Indian Med. J. 1:243.
|
|
|
|
|
IRD (1996). Lepnut, Epidemiology in action, surveillance nutritionnelles, module d'apprentissage, Liverpool/Epidemiology Programm, version Français 1.0 Laboratoire de Nutrition Tropical, IRD, Montpellier.
|
|
|
|
|
Jimoh TO, Bouoro AT, Muriana M (2012). Utilization of Blighia sapida (Akee apple) pod in the removal of lead, cadmium and cobalt ions from aqueous solution. J. Environ. Chem. Ecotoxicol. 4(10):178-187.
Crossref
|
|
|
|
|
Katibi OS, Olaosebikan R, Abdulkar MB, Oqunkunle TO, Ibraheem RM, Murtala R (2015). Ackee Fruits poisoning in Eight Siblings: Implication for Public Health Awareness. Am. J. Trop. Med. Hyg. 93(5):1122-1123.
Crossref
|
|
|
|
|
Katona-apte, Mokhada A (1998). Malnutrition of children in the democratic people's associated in Us children, 1968 through 1991. JMA. 274(14):1143-1148.
|
|
|
|
|
Lefere X (2000). Les conséquences de la dénutrition. Cah. Nutr. Diét. 35:171-175.
|
|
|
|
|
Morton J (1987). Akee. In Fruits of Warm Climates, Julia FM (ed). Morton Publisher: Miami, FL. pp. 269-271.
|
|
|
|
|
Moyal P (1985). Des cas intoxications mortelles par l'arille du fruit de Blghia sapida Koenig (Sapindaceae) en Côte d'Ivoire. Bull. Ethnomed. 33:67-73.
|
|
|
|
|
Moyal P (1986). Les produits naturels peuvent être toxiques: un exemple en Côte d'Ivoire. Phytoma-Défense des cultures. pp.383-386.
|
|
|
|
|
Orane BA, Maurice RB, Jose CJ (2005). Ackee (Blighia sapida) hypoglicin A toxicity:dose response assessment in laboratory rats. Food Chem. Toxicol. 44(2):207-213.
|
|
|
|
|
OPS-Organisation Panamericaine de la Santé (2002). Empoisonnement par consommation d'ackee (Blighia sapida) dans le Département du Nord, Haïti. 22(2):4.
|
|
|
|
|
Orwa C, Mutua A, Kindt R, Jamnadess R, Anthony S (2009). Agroforestree Database: a tree reference and selection guide version 4.0. World Agroforestry Centre, Kenya.
|
|
|
|
|
Ostle B (1966). Statistics in Research. Iowa state university press, Iowa., USA. pp. 310-361.
|
|
|
|
|
Ouattara H, Bobele N F, Dally T, Kati-coulibaly S (2010). Nutritional composition studies of Blighia sapida aril from Côte d'Ivoire. J. Appl. Biosci. 32:1989-1994.
|
|
|
|
|
Reeves PG, Nielsen FH, Fahey GCJ (1993). Purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 123:1939-1951.
|
|
|
|
|
Richmond W (1973). Determination of blood cholesterol. Clin. Chem. 19:1350.
|
|
|
|
|
Trinder P (1969b). Determination of blood triglycerides. Ann. Clin. Biochem. 6:24.
Crossref
|
|
|
|
|
Woolson RF (1987). Statistical methods for the Analysis of Biomedical Data. John Wiley and Sons Inc., New York.
|
|
|
|
|
Wybenga DR, Giorgio D, Pillegi JV (1971). Urea estimation by the diacetyl monoxine method. Clin. Chem. 19:891-895.
|
|