ABSTRACT
The effects of microwave (MW) dry blanching of mango in comparison with conventional water blanching and blanching in closed plastic bags prior to hot air drying (70°C) were evaluated on the retention of vitamin C and β-carotene and on the activity of polyphenol oxidase (PPO) and ascorbic acid oxidase (AAO) enzymes. Blanching conditions for MW and water blanching were 2 min at 90°C high temperature and short time (HTST) or for 10 min at 70°C low temperature and low time (LTLT). PPO was completely inactivated by the blanching treatments, while low AAO activity remained. High retentions (~100%) of vitamin C were found in dried mango after blanching treatments HTST with MW and blanching in closed plastic bags, while lower retention was observed after LTLT with MW (81.8 ± 4.5%), and conventional water blanching 86.7 ± 2.6% (HTST) and 78.6 ± 2.5% (LTLT). Blanching resulted in partial oxidation of L-AA into dehydroascorbic acid (DHAA). Lower retention of all-trans-β-carotene was obtained in MW and HTST water blanched dried mango (82 to 90%) compared with LTLT water blanched dried mango (~100%). In all dried blanched mango samples the levels of 13-cis-β-carotene isomer increased. A slight darkening of colour was observed only in conventional blanched mango samples.
Key words: Microwave heating, mango, β-carotene, vitamin C, ascorbic acid oxidase.
Mango (Mangifera indica L.) is one of the most nutritious tropical fruits, widely consumed in fresh and processed forms. It is seasonal, with relatively short postharvest shelf life due to its perishability (Zhao et al. 2014). Mango can be considered to be a good source of bioactive compounds such as vitamin C, provitamin A carotenoids and phenolic compounds (Ribeiro et al., 2007; Shieber et al., 2000).
Processing of seasonal fruits and vegetables is desired to prolong their shelf-life and control the activity of enzymes such as polyphenol oxidase (PPO) and ascorbic acid oxidase (AAO) naturally present in fruits and vegetables and responsible for causing quality modifications in texture, flavour, colour and nutritive value (Leong and Oey, 2012; Yamaguchi et al., 2003). The appearance and organoleptic properties of fruits and vegetables are affected by enzymatic browning as the result of polyphenol oxidase (PPO) catalyzed oxidation of mono and diphenols to o-quinones, which are highly reactive compounds subject to further reactions, enzymatically catalyzed or not, leading to the formation of brown pigments (McEvily et al., 1992). Ascorbic acid oxidase (AAO) catalyzes the oxidation of vitamin C in the L-AA form to dehydroascorbic acid (DHAA), which is irreversibly hydrolyzed to 2,3-diketogulonic acid that has no vitamin C activity (Lima et al., 2010). Vieira et al. (2000) reported the degradation of L-AA to DHAA and subsequent degradation during conventional blanching of cupuaçu nectar in the temperature range of 60 to 99°C.
Blanching is the most common method used to stabilize foods via the destruction of microorganisms and inactivation of spoilage enzymes; however, it may lead to a loss of nutritional quality (Puligundla et al., 2013; Ramesh et al., 2002), due to the destruction of vegetable cell membranes during blanching. This causes leaching of water-soluble compounds such as vitamin C during conventional water blanching as well as thermal degradation (Ponne et al., 1994).
Novel heating techniques are gaining more interest in optimizing the quality of processed foods. These involve the use of dry conservation techniques such as microwave and infrared radiation (Vishwanathan et al., 2013; Jeevitha et al., 2013; Lin and Brewer, 2005). Microwave have been applied in a broad range of food processing including blanching, showing a high nutrient retention capacity and minimal loss of heat-labile nutrients such as B and C vitamins, dietary antioxidant phenols and carotenoids (Jeevitha et al., 2015, 2013; Puligundla et al., 2013; Ruiz-Ojeda and Peñas, 2013; Ramesh et al., 2002; Muftugil, 1986). It has advantages compared with conventional heat blanching such as in-depth heating (volumetric) in the absence of a temperature gradient and avoidance of the leaching of water-soluble components (Lin and Brewer, 2005). However, MW lack uniformity in heating and have a limited penetration range (Ramesh et al., 2002; Ramaswamyi and Pillet-Will, 1992).
Microwave (MW) blanching was shown to inactivate oxidative enzymes in papaya, strawberry and kiwi purees with minor losses of carotenoids were detected (De Ancos et al., 1999). Ruiz-Ojeda and Peñas (2013) reported about 50% higher retention of vitamin C in microwaved blanched green bean pods in comparison with conventional hot water treatment. Similar results were reported by Muftugil (1986) and Brewer and Begum (2003) for microwaved green beans and by Ramesh et al. (2002) for spinach, carrot and bell peppers. However, studies on the effect of MW blanching with subsequent conventional drying on the retention of bioactive compounds in fruits and vegetables including mango are lacking.
Thus, the objective of this work was to evaluate the effect of MW dry blanching prior to hot air drying on the retention of vitamin C and carotenoids in dried mango in comparison with conventional water blanching at high temperature and short time (HTST) or at low temperature and long time (LTLT). The remaining activity of PPO and AAO enzymes after blanching was also evaluated.
The experimental work was performed at the SP Technical Research Institute of Sweden, and at the Division of Food and Nutrition Science, Chalmers University of Technology, in Sweden.
Fruit preparation
Fresh, medium ripe mango (Mangifera indica L. cv. ‘Osteen’), purchased in a local market and stored at 8 to 10°C, was sliced in a dimmed room into 2 cm diameter and 0.5 cm thick pieces. The average moisture content and water activity of the samples was 0.84 ± 0.02 g/g mango and 0.983 ± 0.002, respectively.
Blanching processes and drying processes
Preliminary tests were performed with 200 g of mango slices to obtain the experimental set-up, given the target combination of time-temperature: 2 min at 90°C (High Temperature Short Time - HTST) or for 10 min at 70°C (Low Temperature Long Time - LTLT) and similar temperature histories for water bath (conventional) or in closed plastic bags and microwave blanching. The temperature of three mango cylinders central point was recorded every second during the heating processes using optic fiber (microwave) or thin-wire copper-constantan thermocouples (water) previously calibrated and connected to a data log. Water blanching was carried out by immersion of a plastic strainer or closed air evacuated plastic bags containing 200 g of sliced mango into thermostatic water bath Julabo Shake Temp SW23, with a 10,000 ml volume of water at constant temperature (70 or 90°C) for a corresponding blanching time (10 or 2 min, respectively).
Microwave blanching was performed using a domestic MW oven (Panasonic NE–C1453, 2450 MHz), with turntable plate. Preliminary studies were carried out to identify the MW conditions (power and time) required for the treatments. Thus, HTST at 90°C required 1350 W/120 s, while LTLT at 70°C demanded a set of power and time sequence of 1350 W/60 s + 420 W/540 s. After blanching, the mango was cooled in iced water for 5 min to lower the residual enzymatic activity, drained and weighed before hot air drying at 70°C using an air circulation oven (Elektro Helios Garomat) with an air velocity of 1 m/s up to a water activity of approximately 0.6. The time required was determined in screening tests and validated by determination of water content and water activity of mango after drying. Samples of fresh, blanched and dried mangoes were collected, filled with nitrogen and stored at -80°C until determinations of vitamin C, carotenoids and enzymatic activity were carried out. The blanching experiments were performed in duplicate.
Chemicals and standards
All chemicals for extraction and reagents were of analytical or high performance liquid chromatography (HPLC) grade obtained from Sigma–Aldrich (Stockholm, Sweden) or Fischer Scientific GTF (Göteborg, Sweden). The water was generated by Millipore Milli-Q plus an ultra-pure water system (Millipore, Solna, Sweden). All-trans-β-carotene standard (synthetic, crystalline, Type II, product C-4582), ACS grade L-Ascorbic acid (≥ 99%), ethylenediaminetetraacetate disodium salt (EDTA) and Tris(2-carboxyethyl) phosphine (TCEP) was obtained from Sigma–Aldrich (Stockholm, Sweden).
Enzyme activity measurements
Enzymatic activities of fresh and blanched mango were determined and the average of three measurements of the duplicates was recorded for both polyphenol oxidase (PPO) and for ascorbic acid oxidase (AAO) enzymes.
Polyphenol oxidase activity
The procedure to measure the PPO enzyme activity in the mango samples has earlier been reported in detail (Guiamba and Svanberg, 2016) and was based on the method described by Palou et al. (1999) and Ndiaye et al. (2009). After extraction of the PPO enzyme using a McIlvaine citric-phosphate buffer (pH 6.5) and addition of a 4-methylcatecol solution, the colour reaction was recorded by measuring the absorbance at 420 nm using a Cary 50Bio UV-visible spectrophotometer.
Ascorbic acid oxidase activity
The activity of AAO in the mango samples was measured according to the procedure earlier described in detail by Guiamba and Svanberg (2016) and based on the method according to Oberbacher and Vines (1963) as modified by Munyaka et al. (2010). The AAO enzyme was extracted from the mango samples using a phosphate buffer (0.1 M, pH 5.6, 0.5 mM EDTA) and the AAO activity was determined by measuring the decrease in substrate concentration (0.5 mM L-AA) using a Cary 50Bio UV-visible spectrophotometer at 265 nm.
Determination of vitamin C (L-AA and total ascorbic acid)
Vitamin C and total ascorbic acid was determined using an HPLC method that was equipped with an electrochemical detector and the procedure was described in detail by Guiamba and Svanberg (2016) with minor modifications. Approximately 1.5 g of fresh or blanched mango and 0.5 g of dried mango was homogenized in an extraction buffer (20 mM NaH2PO4, pH 2.1, 1 mM EDTA) and aliquots of the supernatant were diluted both in McIlvaine citric-phosphate buffer (pH 4.5) alone and McIlvaine citric-phosphate buffer containing 0.312 mM TCEP (Tris[2-carboxyethyl]phosphine hydrochloride). TCEP was used to reduce DHAA into L-AA, thereby enabling the determination of both L-ascorbic acid and total ascorbic acid. Results of L-AA and total vitamin C were expressed as milligrams per 100 g dry weight (DW). The percentage retention of vitamin C was calculated as the ratio of vitamin C in the treated sample to the fresh sample × 100.
Determination of β-carotene (trans/cis isomers)
Carotenoids analysis was performed using the method described by Bengtsson et al. (2008) with minor modifications as described by Guiamba and Svanberg (2016). Approximately 0.2 g of finely ground flour of freeze dried fresh or processed mango (in duplicate) was added to a test tube and extracted using hexane. After extraction, the carotenoids were analysed by reversed phase HPLC equipped with a C30 polymeric column and a UV–visible photodiode array detector operating at 450 nm. The concentration of each carotenoid was expressed as micrograms per g dry matter, given as the mean of four extractions. The percentage retention of each carotenoid was calculated as the ratio of carotenoid in processed samples to fresh sample × 100.
Analytical determinations
All analytical determinations were performed in triplicate. Water activity was measured using an Aqua Lab Series 3 – Decagon. The moisture content was measured by drying samples in a vacuum oven at 80°C at 900 mmHg until a constant weight was reached. Colour measurements were carried out using a Digieye verivide (Serie DE00367, Colour Edge CG211, UK) and were based on the CIELAB parameters of L* (lightness), a* (redness) and b* (yellowness). The total colour change (ΔE*) was the analysed parameter calculated using the following equation:
Statistical analysis
All the tests were performed in triplicate of duplicate samples and the results are presented as mean ± standard deviation. Differences between variables were tested for significance by one-way analysis of variance (ANOVA) and Tukey´s HSD post hoc multiple range test. Differences were considered to be significant at P < 0.05 (or at a level of α = 0.05).
Temperature profile
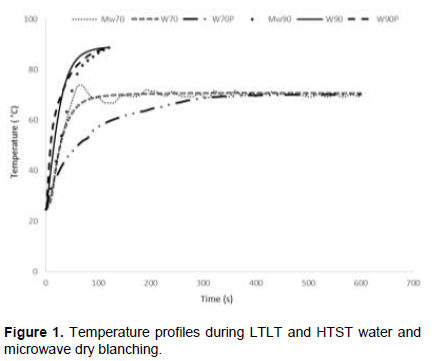
Mango cylinders were blanched under similar temperature-time conditions either by microwave (MW) heating or by immersion in a water bath in closed plastic bags or directly in a plastic strainer (conventional) for 10 min at 70°C (Low Temperature Long Time - LTLT) or 2 min at 90°C (High Temperature Short Time - HTST). Samples will be referred as MW70, W70P, W70 and MW90, W90P, W90, respectively. Although blanching in plastic bags is not a current practice in the industry, it was implemented in the present work to evaluate its possible effect on the retention of nutrients. However, the possibility of its application requires further studies and the identification of suitable material that will not affect the quality of the product. Figure 1 shows the centre temperature profile of mango cylinders obtained during the blanching processes. The increase of mango temperature during water blanching at LTLT was smoother compared to MW blanching due to the relatively low thermal conductivity of mango. A slower temperature increase was observed in mango water blanched in closed plastic bags due to agglomeration of mango cylinders within the plastic bag. On the other hand, the faster temperature increases at the initial stage during MW LTLT heating resulted in a temperature peak at ~75°C, slightly overshooting the set temperature for some few seconds. However, the temperature attained at the end of all blanching processes was 89 and 70°C for HTST and LTLT, respectively.
Enzyme inactivation
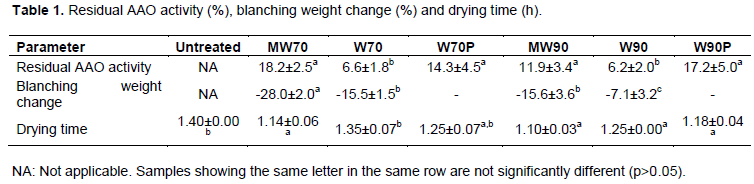
The remaining enzymatic activity of PPO and AAO as affected by the blanching processes was evaluated. The initial PPO activity in the fresh mango (n=8) was 68.0 ± 24.4 units, and it was observed that a complete inactivation of polyphenol oxidase (PPO) enzyme was achieved with all blanching processes (data not shown). On the other hand, some residual enzymatic activity of AAO was detected in mango samples after the treatments (Table 1). Minor AAO activity (~6%) remained in the conventional blanched samples (W70) and (W90). Higher activity (12%) was found in microwave blanched samples at high temperature (MW90), 18% at low temperature (MW70) and about 16% in water blanched samples in plastic bags at both temperatures (W70P) and (W90P). The initial AAO activity in the fresh mango was 1.7 ± 0.2 units. Blanching mango of the variety “Tommy Atkins” using conventional water and infrared dry blanching under similar experimental conditions as in the present work resulted in complete inactivation of PPO, and some residual activity of AAO (Guiamba et al., 2015). The results of these studies indicate that AAO in mango seems to be more resistant than PPO. In a comparative study of several blanching methods, Jeevitha et al. (2015) reported that water and steam blanching required less time than microwave, and in turn less than the infrared blanching to inactivate PPO and POD in green bell pepper. This was attributed to a rapid increase in slice temperature during convective heating with water and steam, which led to faster inactivation of the enzymes.
Weight change during blanching and drying time
The results of weight changes that occurred during the blanching treatments are shown in Table 1. It was observed that there was weight loss in all the treatments except for the blanched samples in closed plastic bags since the plastic was a barrier that limited mass change between internal mango samples with the external environment. The weight loss during microwave blanching at low and high temperatures (MW70, MW90) was significantly higher than with similar water blanching conditions (W70, W90). This was mainly due to the drying action of MW heat, which caused moisture vaporization (Ramesh et al., 2002; Muftugil, 1986). The loss of tissue samples and soluble solids by leaching was thought to be the probable cause of the weight reduction in water blanching. The longer blanching times at 70°C also resulted in higher weight loss when each blanching method was compared separately.
Hot air drying at 70°C was performed for both untreated fresh and blanched mango cylinders in order to reduce the water activity to a level that ensures microbiological stability of the fruit (~0.6). The time required for each process is presented in Table 1. It was observed that a comparatively higher drying time was needed to dry untreated mango samples (1.40 ± 0.00 h) and LTLT water blanched samples (W70 and W70P). MW heated samples showed the lowest drying time. This was due to the relatively higher weight loss during microwave blanching.
Colour
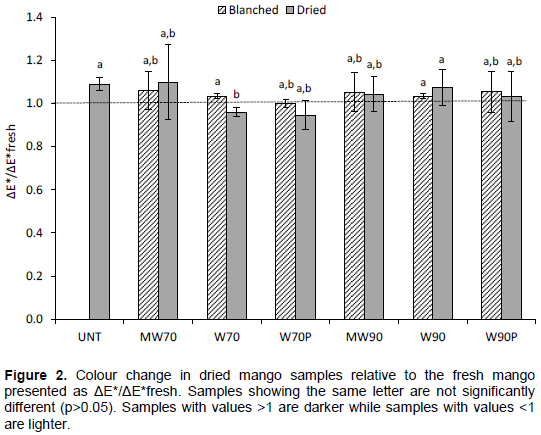
Colour is usually the most influential factor in evaluation of food by consumers, and often the off-colour products are non-acceptable in spite of good taste and flavour (Piližota and Šubarić, 1998). The effect of water and MW thermal treatments, and also combined with hot air drying, as well as drying untreated samples in the colour variation is presented in Figure 2. The total colour difference parameter, ΔE*, was selected to represent the
colour variation of mango, and the results were normalized with the colour of a fresh sample (ΔE*/ΔE*fresh) to compensate for variations in colour between mango from different batches. In comparison with the dried fresh mango, the blanched and dried blanched colour samples was, in general, not affected (ΔE*/ΔE*fresh=1). This could be due to inactivation of PPO during the blanching processes, the enzyme that is responsible for browning (Ioannou and Ghoul, 2013; Palma-Orozco et al., 2012). However, dried untreated (UNT) and conventionally blanched samples (W70, W90) showed some increase in ΔE* ratio values, as an indication of a slight darkening of these samples, while dried W70 samples resulted in a lower ΔE* ratio, indicating a lighter colour than in fresh ones. These changes could be related to the thermal treatments, which induce several reactions such as pigment degradation, especially carotenoids, Maillard reactions, and oxidation of ascorbic acid (Barreiro et al., 1997). The Maillard reaction and ascorbic acid oxidation has been shown to produce a yellow brown colour in mango dried at high temperature (70°C) (Chong et al., 2013).
Vitamin C
The total amount of vitamin C (L-AA + DHAA) in fresh mango samples of cv. ‘Osteen’ was 82.2 ± 27.3 mg/100 g DW. Figure 3 shows the retention of vitamin C in mango after blanching and drying. MW blanching at either high or low temperatures (MW70, MW90) and water blanching in closed plastic bags (W70P, W90P) had no effect on the vitamin C content. The retention after conventional water blanching was however significantly lower, 86.2% (W90) and 81.5% (W70). On the other hand, no further degradation of vitamin C was caused by the drying process, except for MW70 and untreated samples where the retention of 81.8 and 82.9%, respectively was achieved. The degradation of vitamin C in the mango during the drying stage could be associated with a thermal breakdown of the matrix structure (Ponne et al., 1994), which may have caused oxidation of vitamin C in the presence of oxygen.
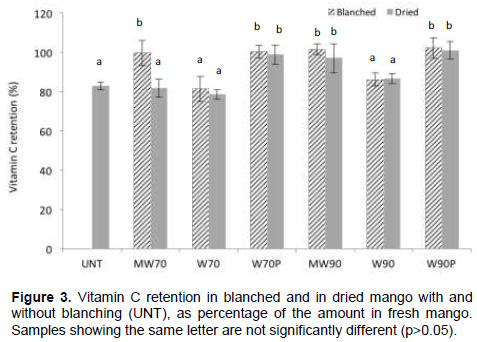
Compared with conventional treatment, microwave blanching resulted in a better retention of vitamin C, which may be explained by less leaching losses during processing and internal heat penetration by microwaves (Lin and Brewer, 2005; Ramesh et al., 2002). Similar findings of high retention of vitamin C in microwave blanching over conventional treatment have been reported by several authors for green beans (Ruiz-Ojeda and Peñas, 2013; Muftugil, 1986), spinach, bell peppers and carrot (Jeevitha et al., 2015, 2013; Ramesh et al., 2002). The comparable results obtained in this work for MW and water blanching in closed plastic bags may be associated with the limited leaching of water-soluble nutrients in both processes. The analysis of AAO residual activity after blanching versus vitamin C retention in the final dried products did not show a direct relation between these variables.
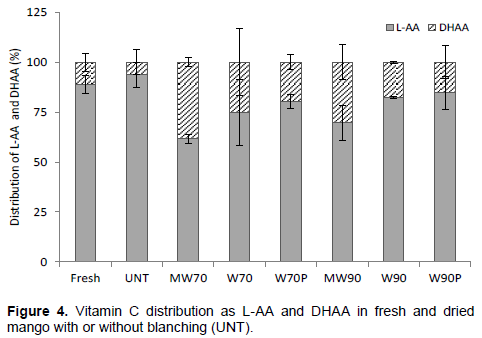
The total vitamin C is the sum of L-AA and DHAA, both having vitamin C activity (Santos and Silva, 2008). The distribution of vitamin C as L-AA and DHAA in the fresh and dried mango is shown in Figure 4. The retention of vitamin C was mainly as L-AA and the levels were significantly higher in the fresh and dried untreated samples, 89 and 93%, respectively. Higher levels of DHAA were found in the blanched samples. Obviously, the blanching treatments activate the AAO enzyme, resulting in a conversion of L-AA to DHAA. However, there was a trend toward higher levels of DHAA in the LTLT treated samples compared with the HTST treated samples. A possible explanation could be that the lower blanching temperature for the longer time resulted in a slower inactivation of the AAO enzyme and thus a higher oxidation of LAA to DHAA in these samples. Thus, the high level of DHAA in MW70 mango may be associated to the effect of both long blanching time and comparatively higher remaining AAO residual activity (Table 1). Transformation of L-AA into DHAA is influenced by factors such as temperature, light, pH, concentration and metal ions and is facilitated by a naturally occurring enzyme, ascorbic acid oxidase (AAO) in the presence of oxygen (Brewer and Begum, 2003; Davey et al., 2000; Brinkman et al., 1942).
β-carotene
The average amount of total β-carotene in fresh samples, as the sum of all-trans-β-carotene and 13-cis-β-carotene isomers, was found to be 1759.9 ± 589.6 µg/100 g DW, containing 95.2% (1675.9 µg/100 g DW) of all-trans-β-carotene and 4.8% (84.0 µg/100 g DW) of 13-cis-β-carotene. These values were in the same range as reported for mango by other authors (Vásquez-Caicedo et al., 2005; Ndawula et al., 2004).
The retention of all-trans-β-carotene in the dried mango samples, calculated in relation to the original amount in fresh mango (DW), is shown in Figure 5. The retention of all-trans-β-carotene in LTLT water blanched dried samples (W70 and W70P) was not affected by the pre-treatment. However, in dried mango that was water blanched (W90 and W90P), the retention was higher (~90%) compared with the microwave blanched mango (MW70 and MW90) where the retention decreased to values of about 82%. Severe degradation of all-trans-β-carotene was noted in dried untreated (UNT) mango, with a retention of around 57%. The explanation might be the PPO activity causing an indirect co-oxidation of the all-trans-β-carotene when exposed to high temperature in the presence of oxygen (Xianquan et al., 2005; Dorantes-Alvarez and Chiralt, 2000).
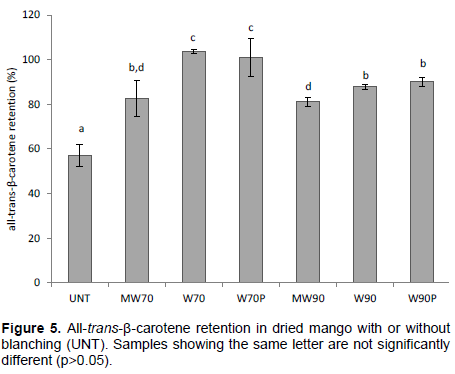
Contrary to the results on vitamin C, the retention of all-trans-β-carotene in mango blanched using plastic bags did not differ from the mango conventionally water blanched. This may be associated to the fact of carotenoids being water-insoluble pigments but lipid soluble (Mao et al., 2009). In addition, carotenoids are highly unsaturated and prone to isomerization and oxidation during processing and storage due to the effects of chemical, mechanical and thermal stresses (Qian et al., 2012; Mao et al., 2009; Boon et al., 2009). Thermal processing of carotenoid containing foods with cooking oil has been shown to significantly reduce the retention of all-trans-β-carotene and to increase the level of 13-cis-β-carotene (Bengtsson et al., 2010). In mango, the β-carotene is embedded in lipid droplets (Brackmann et al., 2011; Bartley and Scolnik, 1995), which then may explain the lower retention in mango subjected to a high blanching temperature (90°C).
Microwave blanching of other plant foods than mango has been reported in the literature. According to De Ancos et al. (1999), microwave heating induced a loss of total carotenoid content in papaya purée as high as 57%. Opposite results were reported by Jeevitha et al. (2015, 2013), i.e., a retention of more than 100% of β-carotene in green and red bell peppers after microwave blanching, which was attributed to an enhanced extractability of nutrient from blanched samples.
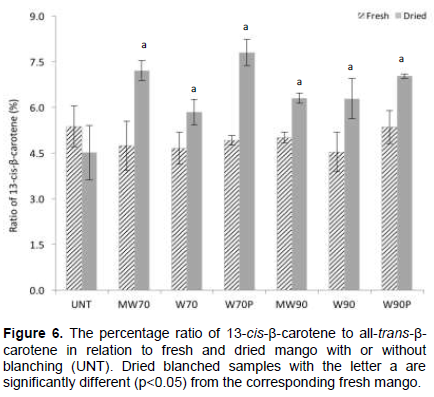
The presence of cis-β-carotene isomers in fresh mango has been reported by several authors (Chen et al., 2007; Pott et al., 2003; Godoy and Rodriguez-Amaya, 1994). The ratio of 13-cis-β-carotene (%) to all-trans-β-carotene in the fresh and dried mango samples as affected by the treatments is presented in Figure 6. The ratio of 13-cis-β-carotene remained unchanged in untreated (UNT) mango after drying. However, a significant increase in the ratio of this isomer was observed in all dried blanched samples. These results suggest that the thermal treatment (blanching) affected the stability of all-trans-β-carotene, leading to isomerization. Reports in the literature show that heat treatment promotes isomerization of the carotenoids in foods, from trans to cis isomeric forms, and that the degree of isomerization is directly correlated with the intensity and duration of heat processing (Lemmens et al., 2013; Achir et al., 2011; Vásquez-Caicedo et al., 2007; Rodriguez-Amaya et al., 2006; Chen and Huang, 1998) and with the presence of oil in the process (Bengtsson et al., 2010).
Selection of suitable technology and conditions for blanching can improve the nutritional value of dried mango and inactivate relevant enzymes. Complete inactivation of PPO in mango was achieved with heat treatments at 90°C for 2 min (HTST) and at 70°C for 10 min (LTLT), while AAO showed a low remaining activity for the conditions tested. A higher retention of vitamin C (water-soluble, heat and oxygen sensitive) in dried mango was achieved using MW dry blanching or water blanching in plastic bags in comparison to conventionally water blanching, due to the limited leaching of water-soluble compounds in these processes. The distribution of vitamin C as L-AA and DHAA in fresh and dried mango was mainly as L-AA, but with relatively higher amounts of DHAA in blanched samples, thus showing an influence of blanching on the oxidation of L-AA to DHAA.
LTLT or HTST blanching before drying is an effective way to improve the retention of carotenoids (water insoluble, prone to isomerization and oxidation) in dried mango. The retention of all-trans-β-carotene was higher in LTLT water blanched dried samples compared with HTST, while the corresponding MW blanched dried mango had lower retention. Moreover, blanching in plastic bags did not differ from water conventional blanching. MW blanching showed no significant difference between LTLT and HTST treatments. In all pre–blanched mango, the levels of 13-cis-β-carotene isomer were higher than in the fresh samples.
The authors have not declared any conflict of interests.
The financial support from the Swedish International Development Cooperation Agency (Sida) under the project “Technology processing of natural resources for sustainable development” is gratefully acknowledged.
REFERENCES
|
Achir N, Pénicaud C, Avallone S, Bohuon P (2011). Insight into β-carotene thermal degradation in oils with multiresponse modeling. Journal of the American Oil Chemists' Society, 88(12):2035-2045.
Crossref
|
|
|
|
Barreiro JA, Milano M, Sandoval AJ (1997). Kinetics of colour change of double concentrated tomato paste during thermal treatment. Journal of Food Engineering, 33:359-371.
Crossref
|
|
|
|
|
Bartley GE, Scolnik PA (1995). Plant carotenoids: Pigments for photoprotection, visual attraction, and human health. The Plant Cell, 7(7):1027.
Crossref
|
|
|
|
|
Bengtsson A, Namutebi M, Alminger L, Svanberg U (2008). Effects of various traditional processing methods on the all-trans-β-carotene content of orange-fleshed sweet potato. Journal of Food Composition and Analysis, 21(2):134-143.
Crossref
|
|
|
|
|
Bengtsson A, Brackmann C, Enejder A, Larsson Alminger M, Svanberg U (2010). Effects of thermal processing on the in vitro bioaccessibility of β-carotene in orange-fleshed sweet potato. Journal of Agricultural and Food Chemistry, 58(20):11090-11096.
Crossref
|
|
|
|
|
Boon CS, McClements DJ, Weiss J, Decker EA (2009). Role of iron and hydroperoxides in the degradation of lycopene in oil-in-water emulsions. Journal of Agricultural and Food Chemistry, 57(7):2993-2998.
Crossref
|
|
|
|
|
Brackmann C, Bengtsson A, Larsson Alminger M, Svanberg U, Enejder A (2011). Visualization of β-carotene and starch granules in plant cells using CARS and SHG microscopy. Journal of Raman Spectroscopy, 42:586-592.
Crossref
|
|
|
|
|
Brewer MS, Begum S (2003). Effect of microwave power level and time on ascorbic acid content, peroxidase activity and colour of selected vegetables. Journal of Food Processing and Preservation 27(6):411-426.
Crossref
|
|
|
|
|
Brinkman EVS, Halliday EG, Hinman WF, Hamner RJ (1942). Effect of various cooking methods upon subjective qualities and nutritive values of vegetables. Food of Food Science, 7(4):300-309.
Crossref
|
|
|
|
|
Chen BH, Huang JH (1998). Degradation and isomerization of chlorophyll a and β-carotene as affected by various heating and illumination treatments. Food Chemistry, 62:299-307.
Crossref
|
|
|
|
|
Chen JP, Tai CY, Chen BH (2007). Effects of different drying treatments on the stability of carotenoids in Taiwanese mango (Mangifera indica L.). Food Chemistry, 100:1005-1010.
Crossref
|
|
|
|
|
Chong CH, Law CL, Figiel A, Wojdyło A, Oziembłowski M (2013). Colour, phenolic content and antioxidant capacity of some fruits dehydrated by a combination of different methods. Food Chemistry, 141:3889-3896.
Crossref
|
|
|
|
|
Davey MW, Van Montagu M, Inzé D, Sanmartin M, Kannellis A, Smirnoff N, Benzie IJJ, Strain JJ, Favell F, Fletcher J (2000). Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. Journal of the Science of Food and Agriculture, 80(7):825-860.
Crossref
|
|
|
|
|
De Ancos B, Cano MP, Hernandez A, Monreal M (1999). Effects of microwave heating on pigment composition and colour of fruit purees. Journal of the Science of Food and Agriculture, 79:663-670.
Crossref
|
|
|
|
|
Dorantes-Alvarez L, Chiralt A (2000). Color of Minimally processed fruits and vegetables as affected by some chemical and biochemical changes. In: Minimally processed fruits and vegetables. Alzamora SM, Tapia MS, Lopez-Malo A (Eds.), Aspen Publishers, Inc., USA. pp. 111-116.
|
|
|
|
|
Godoy HT, Rodriguez-Amaya DB (1994). Occurrence of cis-isomers of provitamin A in Brazilian fruits. Journal of Agricultural Food Chemistry, 42:1306-1313.
Crossref
|
|
|
|
|
Guiamba IRF, Svanberg U, Ahrné L (2015). Effect of infrared blanching on enzyme activity and retention of β-carotene and vitamin C in dried mango. Journal of Food Science, 80:E1235-E1242.
Crossref
|
|
|
|
|
Guiamba IRF, Svanberg U (2016). Effects of blanching, acidification, or addition of EDTA on vitamin C and β-carotene stability during mango purée preparation. Food Science and Nutrition, 4(5):706-715.
Crossref
|
|
|
|
|
Ioannou I, Ghoul M (2013). Prevention of enzymatic browning in fruit and vegetables. European Science Journal, 9(30):1857-7881.
|
|
|
|
|
Jeevitha GC, Hebbar HU, Raghavarao KSMS (2013). Electromagnetic Radiationâ€Based Dry Blanching of Red Bell Peppers: A Comparative Study. Journal of Food Process Engineering, 36(5):663-674.
Crossref
|
|
|
|
|
Jeevitha GC, Anto A, Chakkaravarthi A, Hebbar HU (2015). Application of electromagnetic radiations and superheated steam for enzyme inactivation in green bell pepper. Journal of Food Processing and Preservation, 39(6):784-792.
Crossref
|
|
|
|
|
Lemmens L, Tchuenche ES, Loey AMV, Hendrickx ME (2013). Beta-carotene isomerization in mango puree as influenced by thermal processing and high-pressure homogenization. European Food Research and Technology, 236(1):155-163.
Crossref
|
|
|
|
|
Leong SY, Oey I (2012). Effects of processing on anthocyanins, carotenoids and vitamin C in summer fruits and vegetables. Food Chemistry, 133:1577-1587.
Crossref
|
|
|
|
|
Lima JR, Elizondo NJ, Bohuon P (2010). Kinetics of ascorbic acid degradation and colour change in ground cashew apples treated at high temperatures (100-180 °C). International Journal of Food Science and Technology, 45(8):1724-1731.
Crossref
|
|
|
|
|
Lin S, Brewer MS (2005). Effects of blanching method on the quality characteristics of frozen peas. Journal of Food Quality, 28(4):350-360.
Crossref
|
|
|
|
|
Mao LK, Xu DX, Yang J, Yuan F, Gao YX, Zhao J (2009). Effects of small and large molecule emulsifiers on the characteristics of β-carotene nanoemulsions prepared by high pressure homogenization. Food Technology and Biotechnology, 47(3):336-342.
|
|
|
|
|
McEvily AJ, Iyengar R, Otwell WS (1992). Inhibition of enzymatic browning in foods and beverages. Critical Reviews in Food Science and Nutrition, 32(3):253-273.
Crossref
|
|
|
|
|
Muftugil N (1986). Effect of different types of blanching on the colour and the ascorbic acid and chlorophyll contents of green beans. Journal of Food Processing and Preservation, 10(1):69-76.
Crossref
|
|
|
|
|
Munyaka AW, Makule EE, Oey I, Loey AV, Hendrickx M (2010). Thermal stability of L-ascorbic acid and ascorbic acid oxidase in broccoli (Brassica oleracea var. italica). Journal of Food Science, 75(4):C333-C340.
Crossref
|
|
|
|
|
Ndawula J, Kabasa JD, Byaruhanga YB (2004). Alterations in fruit and vegetable β-carotene and vitamin C content caused by open-sun drying, visqueen-covered and polyethylene-covered solar-dryers. African Health Sciences, 4(2):125-130.
|
|
|
|
|
Ndiaye C, Xu SY, Wang Z (2009). Steam blanching effect on polyphenoloxidase and colour of mango (Mangifera indica L.) slices. Food Chemistry, 113:92-95.
Crossref
|
|
|
|
|
Oberbacher MF, Vines HM (1963). Spectrophotometric assay of ascorbic acid oxidase. Nature, 197:1203-1204.
Crossref
|
|
|
|
|
Palma-Orozco G, Sampedro JG, Ortiz-Moreno A, Nájera H (2012). In situ inactivation of polyphenol oxidase in mamey fruit (Pouteria sapota) by microwave treatment. Journal of Food Science, 77(4):C359-C365.
Crossref
|
|
|
|
|
Palou E, López-Malo A, Barbosa-Cánovas GV, Welti-Chanes J, Swanson BG (1999). Polyphenoloxidase activity and colour of blanched and high hydrostatic pressure treated banana puree. Journal of Food Science, 64(1):42-45.
Crossref
|
|
|
|
|
Piližota V, Šubarić D (1998). Control of enzymatic browning of foods. Food Technology and Biotechnology, 36(3):219-227.
|
|
|
|
|
Ponne CT, Baysal T, Yuksel D (1994). Blanching leafy vegetables with electromagnetic energy. Journal of Food Science, 59(5):1037-1041.
Crossref
|
|
|
|
|
Pott I, Marx M, Neidhart S, Muhlbauer W, Carle R (2003). Quantitative determination of β-carotene stereoisomers in fresh, dried and solar-dried mangoes (Mangifera indica L.). Journal of Agricultural and Food Chemistry, 51(16):4527-4531.
Crossref
|
|
|
|
|
Puligundla P, Abdullah SA, Choi W, Jun S, Oh SE, Ko S (2013). Potentials of microwave heating technology for select food processing applications - a brief overview and update. Journal of Food Processing and Technology, 4(11):2-9.
Crossref
|
|
|
|
|
Qian C, Decker EA, Xiao H, McClements DJ (2012). Physical and chemical stability of β-carotene-enriched nanoemulsions: influence of pH, ionic strength, temperature, and emulsifier type. Food Chemistry, 132:1221-1229.
Crossref
|
|
|
|
|
Ramaswamyi HS, Pillet-Will T (1992). Temperature distribution in microwave-heated food models. Journal of Food Quality, 15:435-448.
Crossref
|
|
|
|
|
Ramesh MN, Wolf W, Tevini D, Bognár A (2002). Microwave blanching of vegetables. Journal of Food Science, 67(1):390-398.
Crossref
|
|
|
|
|
Ribeiro SMR, De Queiroz JH, De Queiroz MELR, Campos FM, Sant'Ana HMP (2007). Antioxidant in mango (Mangifera indica L.) Pulp. Plant Foods for Human Nutrition, 62(1):13-17.
Crossref
|
|
|
|
|
Rodriguez-Amaya DB, Rodriguez EB, Amaya-Farfan J (2006). Advances in food carotenoid research: chemical and technological aspects, implications in human health. Malaysian Journal of Nutrition, 12(1):101-121.
|
|
|
|
|
Ruiz-Ojeda LM, Pe-as FJ (2013). Comparison study of conventional hot-water and microwave blanching on quality of green beans. Innovative Food Science and Emerging Technologies, 20:191-197.
Crossref
|
|
|
|
|
Santos PHS, Silva MA (2008). Retention of vitamin C in drying processes of fruits and vegetables - A review. Drying Technology, 26(12):1421-1437.
Crossref
|
|
|
|
|
Shieber A, Ulrich W, Carle R (2000). Characterization of polyphenols in mango puree concentrate by HPLC with diode array and mass spectrometric detection. Innovative Food Science and Emerging Technologies, 1:161-166.
Crossref
|
|
|
|
|
Vásquez-Caicedo AL, Sruamsiri P, Carle R, Neidhart S (2005). Accumulation of all-trans-β-carotene and its 9-cis and 13-cis stereoisomers during postharvest ripening of nine Thai mango cultivars. Journal of Agricultural and Food Chemistry, 53:4827-4835.
Crossref
|
|
|
|
|
Vásquez-Caicedo AL, Schilling S, Carle R, Neidhart S (2007). Effects of thermal processing and fruit matrix on β-carotene stability and enzyme inactivation during transformation of mangoes into purée and nectar. Food Chemistry, 102:1172-1186.
Crossref
|
|
|
|
|
Vieira MC, Teixeira AA, Silva CLM (2000). Mathematical modeling of the thermal degradation kinetics of vitamin C in cupuaçu (Theobroma grandiflorum) nectar. Journal of Food Engineering, 43:1-7.
Crossref
|
|
|
|
|
Vishwanathan KH, Giwari GK, Hebbar HU (2013). Infrared assisted dry-blanching and hybrid drying of carrot. Food and Bioproducts Processing, 91(2):89-94.
Crossref
|
|
|
|
|
Xianquan S, Shi J, Kakuda Y, Yueming J (2005). Stability of lycopene during food processing and storage. Journal of Medicinal Food, 8(4):413-422.
Crossref
|
|
|
|
|
Yamaguchi T, Katsuda M, Oda Y, Terao J, Kanazawa K, Oshima S, Inakuma T, Ishiguro Y, Takamura H, Matoba T (2003). Influence of polyphenol and ascorbate oxidases during cooking process on the radical-scavenging activity of vegetables. Food Science and Technology Research, 9(1)79-83.
Crossref
|
|
|
|
|
Zhao J, Hu R, Xiao H, Yang Y, Liu F, Gan Z, Ni Y (2014). Osmotic dehydration pretreatment for improving the quality attributes of frozen mango: effects of different osmotic solutes and concentrations on the samples. International Journal of Food Science and Technology, 49(4):960-968.
Crossref
|
|