Full Length Research Paper
ABSTRACT
INTRODUCTION
MATERIALS AND METHODS
Materials
Seven brands of IFM powders for the age bracket 0-6 months were purposively sampled from stores in Nairobi, Kenya and coded T, U, V, W, X, Y and Z.
Chemicals and reagents
All chemicals and reagents were of analytical grade. Concentrated nitric acid, hydrogen peroxide and aluminum sulphate, were sourced from Thomas Backers Chemicals Ltd Mumbai India. Standards for nickel, cadmium and lead were purchased from Fluka Chemie GmbH Aldrich chemical company, INC, USA.
Laboratory procedures
Digestion of the powders followed the procedure by Picciano (2001). Briefly, 2.5 g of powder was accurately weighed into a Kjeldahl flask, 15 ml of concentrated nitric acid and 5 ml of 10% hydrogen peroxide were added and the resulting solution heated until there were no more brown fumes. The resulting mixture was filtered through Whatman paper No.1 into 50 ml volumetric flask and its volume topped up with deionized water to the mark. The measurements for Al, Cd, Pb and Ni were done in triplicates using computerized Varian Atomic absorption Spectrometer (Model: AA-10, Varian, USA). The instrumental parameters are presented in Table 1.
Method validation procedures
Freshly prepared standard stock solutions were serially diluted andused to obtain calibration curves with linearity values as presented in Table 2. The correlation coefficient values were a good indicator of the linearity for AAS instrument for precision and accuracy of results (Duan et al., 2003). Further, using recovery tests where samples were repeatedly spiked with known amounts of standards prior to measurements were performed to confirm the accuracy of the instruments (Duan et al., 2003). The percentage recoveries obtained were good, falling between 98.1-99.9%.
Statistical analysis
Data were analyzed with SPSS 17.0 for windows. The mean and standard deviation of means were calculated. The Estimated Weekly Intake (EWI) per body weight of the infant for the metal ions in each brand of formula milk were then calculated based on the feeding table provided in each brand of formula milk (0-6 months). The data were analyzed by one-way analysis of variance (ANOVA) and Duncan’s multiple range tests was used to separate means (P < 0.05).
RESULTS AND DISCUSSION
RESULTS AND DISCUSSION
The levels of Al, Cd, Pb and Ni in seven brands of IFM for infants aged 0-6 months sold in Nairobi County, Kenya are presented in Table 3 and the EWI are presented in Table 4.
Except for Cd, all brands of IFM (0-6 months) contained Al, Pb and Ni. These ranged as follows: Al (1.054±0.085 µg/g in brand T to 2.156±0.423 µg/g in brand X); Pb(0.018±0.001 µg/g in brand V to 0.059±0.002 µg/g in brand W), Ni (0.022±0.001 µg/g in brand X to 0.032±0.001 µg/g in brand T). Except for brands T and V, all other brands had levels of Pb above the KEBS maximum limit of 0.02 ppm. Infants are at a critical point of their brain development and exposure to elements pose severe health risks since the effect is compounded by the fact that even at low levels of exposure, metals bio-accumulate in vital organs and persists in adulthood (Nevin, 2000; Needleman et al., 2002; Lanphear et al., 2008). The findings therefore raise a health concern toinfants who consume the brands. A further concern is the labelling of the packages. In this study, Pb was identified in the samples, but not reported on the labels of the containers. These study findings indicate an increased health risk with the consumption of IFM resulting in not only the exposure, but the bioaccumulation of the elements Al, Pb and Ni.
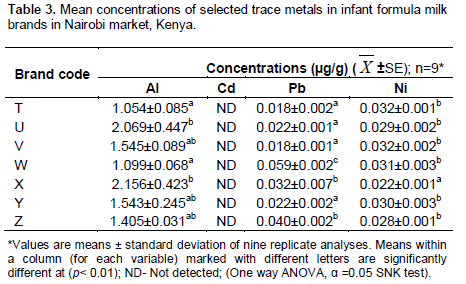
There were statistically significant differences of the elements between brands (p<0.001). This would be expected and these differences can be attributed to the different manufacturing practices, quality of raw materials, and packaging containers used (Khalifa and Ahmad, 2010). Comparing the metal ion levels and the maximum possible weekly intake (Table 4), the EWI of Al and Pb were below the Provisional Tolerable Weekly Intake (PTWI) set by the Joint Committee on Food Additives of the FAO and the EU Scientific Committee for infant feeding, an indication that the IFM are safe for consumption. These findings may generate a debate on whether the campaigns on consumption of IFM should be encouraged although it is understandable that under some unavoidable circumstances exclusive breast feeding is an impossible undertaking. The labels on the IFM packages should include even the minimal safe amounts of all elements as this not only informs the consumers, but have implications on their choice in promoting their consumption.
CONCLUSION
In this study, we affirm the presence of trace elements (Al, Cd, Pb and Ni) in IFM brands in the Kenyan market. Although below the WHO set limits, the findings are an indication of caution regarding consumption of IFM unless under unavoidable circumstances as the risks of their bioaccumulation are inevitable.
CONFLICT OF INTERESTS
The authors did not declare any conflict of interest.
ACKNOWLEDGEMENTS
REFERENCES
| contaminated milk. The Guardian (London). Archived from the original on 5 December 2008. Retrieved 2 April 2010. | ||||
|
Burrell S, Exley C (2010). There is too much aluminium in infant formula. BMC Pediatrics 10:63. Crossref |
||||
| CDC (Center for Disease Control) (2006). Promotion and support of breastfeeding and obesity control. Int. Med. J. 1:21-24. | ||||
|
Duan Q, Gupta H, Sorooshian S, Rousseau A, Turcotte R (Eds.). (2003). Advances in Calibration of Watershed Models, Water Science and Application, Series 6. American Geophysi-cal Union, Washington, DC, p. 345. Crossref |
||||
| Gian C, Zaheer D, Christian D, Angela M, Eva M, Hanna M, Margot E, Jennifer W (2009). Analysis of toxic heavy metals in selected infant formula milk commercially available in Philippines by AAS. E-Int. Sci. Res. J. 1:40-51 | ||||
| KEBS (Kenya Bureau of Standards). (2014). Approved list of standards by 103rd standards approval committee meeting on 19th june 2014 KS EAS 4:2013 kenya standard-infant formula-specification, First edition. | ||||
| Kenya National Bureau of Statistics (2010). Kenya Demographic and Health Survey | ||||
| Khalifa A, Ahmad D (2010). Determination of key elements by ICP-OES in commercially available infant formulae and baby foods in Saudi Arabia. Afr. J. Food Sci. 4:464-468. | ||||
| Komen L (2009). Overview of Infant and young child feeding in Kenya. Infant Young Child Feed. J. 2: 1-14. | ||||
| Lanphear B, Dietrich K, Auinger P, Christopher C (2008). Cognitive deficits associated with blood lead concentrations less than ten micrograms per deciliter in US children and adolescents. Public Health Rep. 4:87-96. | ||||
|
Lawrence RM (2004). Breast milk and infection. Clin. Perinatol. J. 31:501-528. Crossref |
||||
|
Ljung K, Palma B, Grander M, Vahter M (2011). High concentrations of essential and toxic elements in infant formula foods- a matter of concern. Food Chem. J. 40:1-9. Crossref |
||||
|
Mamiro S, Kolsteren P, Robertfoid D, Opsumer A (2005). Feeding practices and factors contributing to wasting, stunting and iron deficiency anemia in rural Tanzania. J. Health Popul. Nutr. 23:222-230. |
||||
| Nakashima T, Matsuno K, Matsushita T (2009). Lifestyle determined gender and hierarchical differences in the lead contamination of bones from a feudal town of Edo period. J. Occup. Health 49 (2):139-157. | ||||
| National Defence Resource Council (NDRC) (2005). Breastfeeding around the world. Food Chem. J. 61:213-215. | ||||
|
Needleman J, Gleavas D, Ness R, Fernberg S, Tobin M (2002). Bone lead levels in adjudicated delinquents, a case control study. Neurotoxicol. Teratol. 24:711-717. Crossref |
||||
|
Nevin R (2000). How lead exposure relates to temporal changes in IQ, crime and owed pregnancy. Environ. Res. 83:1-22. Crossref |
||||
|
Picciano MF (2001). Nutrient composition of human milk. Pediatr. Clin. J. North Am. 48:53-67. Crossref |
||||
| Salah FA (2012). Assessment of toxic heavy metals in some dairy products and the effect of storage on their distribution. J. Am. Sci. 8 (8):665-670. | ||||
| WHA (2001). Infant and young nutrition. | ||||
| WHO (World Health Organization) (2004). HIV transmission through breastfeeding. A review of available evidence. | ||||
| WHO (World Health Organization) (2015). Breastfeeding advocacy initiative, For the best start in life. | ||||
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0