ABSTRACT
A study was conducted to assess retention of moisture and ascorbic acid in tender pumpkin (Cucurbita moschata) leaves subjected to different blanching times and dehydration conditions. Equal portions of the leaves were blanched at 5, 10 and 15 min. A half of each portion was dehydrated under a shade and the other under direct sunlight. All the samples were then analysed for moisture and ascorbic acid contents in comparison with those of the raw-leaf sample. The fresh samples (wet basis) had 79.26±5.08 mg/100 g ascobic acid compared to the processed samples, which ranged from 41.10±2.94 to 73.39±5.87 mg/100 g in the blanched shade-dehydrated sample and 17.61±0.00 to 35.23±5.08 in the sun-dehydrated blanched sample. The results further showed that the samples that were blanched for a shorter time and dehydrated under a shade retained a significantly higher (p<0.05) amount of ascorbic acid compared to those that were blanched longer and dehydrated under direct sunlight. There was no significant difference in moisture content between the shade-dehydrated and sun-dehydrated samples, which were found to be in the ranges of 13.22±0.09 to 14.23±0.27% and 12.45±0.035 to 13.42±0.52%, respectively. It was, therefore, concluded that shorter blanching time and shade-dehydration can retain ascorbic acid in tender C. moschata leaves without compromising moisture content of the product.
Key words: Cucurbita moschata, sun-dehydration, shade-dehydration, ascorbic acid, pumpkin leaves.
Pumpkin plant (Cucurbita moschata), also known as ‘tropical pumpkin’, is one of the well-known and highly utilised plants cultivated throughout the world, particularly in lowland areas of Asia, Africa and America. Pumpkin plant is unique in a way that almost every part of it (except the roots) is edible. Flowers, fruit, and long tendril shoots and leaves are relished as vegetable. The leaves and tender young shoots are cooked as vegetables and used as potherb or added to soups and stews (Lim, 2012a). Pumpkin blossoms are edible raw or cooked but when mature, the fruit is cooked as a main course or side dish, and used as an ingredient, in pies, soups, stews, and bakery preparations (Lim, 2012a; Durante et al., 2014). Seeds are eaten raw, dried or roasted and can be served as a snack but can also be ground into a powder and used with cereals and in bread making (Lim, 2012a).
This has made pumpkin a plant of interest for researchers. Although a lot of studies have been conducted on the pumpkin plant, most of them, however, have targeted the fruit and seeds only (Stevenson et al., 2007; Mala et al., 2016). Pumpkin leaf is one of the most consumed parts of the plant in some parts of the world, including the Sub-Saharan Africa (Lymo et al., 1991; Lim, 2012a), but limited information on the same is available.
Vegetables play crucial roles in alleviating hunger and food security by contributing bulk of the nutritional components in the diets of people where animal products are scarce (Mepba et al., 2007). Just like many other green leafy vegetables, pumpkin leaf is tasty and nutritious and is popular in countries such as Kenya, Malawi, Zambia, Zimbabwe, among others. The leaves are a valuable source of nutrients (which are usually in short supply in daily diets) especially in rural areas (Lymo et al., 1991; Mepba et al., 2007) where they contribute substantially to minerals, fiber, protein and vitamins, especially β–carotene and ascorbic acid (FAO,1988; Mwaniki et al., 1999; Adegunwa et al., 2011; Kakade and Neeha, 2014). Pharmacologically, leaves in the family of Cucurbitaceae are believed to have a number of health benefits. In ethno medicine users, it has been reported that the leaves are used for reduction of fever, treatment of nausea and boosting haemoglobin content. It is also believed that the leaves help in the prevention of convulsion, in which young leaves are sliced and mixed with coconut water and salt, then stored and used for the treatment. Moreover, the leaves have been reported to have the ability of boosting fertility as a result of zinc and essential fatty acids present, protect the liver and cure anaemia (due to the presence of iron). Furthermore, the sufficient amount of vitamin is reported to help in boosting of vision as well as supplementing effect on the daily protein requirement of the body (Dhiman et al., 2012; Lim, 2012a, b). A study by Kwak and Ju (2013), in-vitro, has shown the anti-cancer propertied of extracts from C. moschata leaves.
In most cases, pumpkin plant is mainly grown for the fruit, as such, availability of the leaf as vegetable depends on the time of the year when the fruit is considered to do well. Although pumpkins are not necessarily seasonal in nature, in countries like Malawi, they are mostly grown during the rainy season; as a result, the leaves are in abundance during this period and become scarce thereafter. To ensure their constant availability, in most developing countries, pumpkin leaves are traditionally processed into their dehydrated form, which mostly involves blanching and sun-dehydration (Lymo et al., 1991; Mepba et al., 2007). Although this is done traditionally, the scientific reasoning is that the blanching deactivates enzymes, while dehydration reduces water activity, which prevents growth of moulds and other microorganisms; thereby preventing spoilage of the preserved vegetable (Fellow, 2009).
In fact, there are several methods of processing vegetables for preservation, which include sun-dehydration, canning, vacuum packing, minimal processing, refrigeration, freezing and irradiation (Fellow, 2009). Of all these processing methods, sun-dehydration has been regarded as the most effective, cheap and popular method of processing pumpkin leaves for preservation by the local people in Malawi compared to the other methods. Under this method, people prefer blanching then sun-dehydration compared to the one which only involves sun-dehydration without blanching with the same reasoning that blanching will help to deactivate spoilage enzymes and kill spoilage microorganisms hence help the vegetable to be of higher quality. While fresh pumpkin leaves are perishable due to high water activity, dehydrated pumpkin leaves stay longer as compared to the fresh ones. Dehydrated vegetables stored in good containers and kept in dry conditions can have a shelf life of more than a year (Musarirambi et al., 2010). Dehydration also reduces weight of the vegetable thereby making it easy for transportation (Fellow, 2009).
Although this kind of processing has been regarded as significant in preserving pumpkin leaves, the steps of blanching and sun-dehydration have great potential of reducing some nutrients in the vegetable, more especially, ascorbic acid (vitamin C), which is soluble in water and prone to oxidation upon exposure to light (Lawal et al., 2015; Okpalamma et al., 2013; Adegunwa et al., 2011). Despite the fact that, there is a possibility that the blanching and dehydration can lead to ascorbic acid loss, there is no clear information on the amount that is lost as a result of this processing method. Lack of knowledge on the retained amount might interfere with the formulation of balanced diets hence a need for a study of this nature that focuses on the effect of cooking time and/or dehydration condition on ascorbic acid loss and suggest how best the vegetable can be processed to ensure its possible maximum retention.
Sample collection
Fresh and tender pumpkin leaves of the C. moschata species (over 2 kg) were purchased in morning hours (around 8:00 am) from a single seller at a local market. The leaves were immediately brought in airtight plastic carrier bags to a laboratory, which was about 3 km from the market, for processing. Thus the vegetables arrived the laboratory still very fresh. While analysing initial contents of ascorbic acid and moisture in the fresh unprocessed portion of the sample, the rest of the vegetables were stored in the same carrier bag in a refrigerator and were processed within 4 h. This was done to minimise action of spoilage microorganisms and enzymes that could alter characteristics of the vegetables. All chemicals used were of analytical grade.
Sample preparation
The pumpkin leaves were first cut into slices of about 1 cm in width using a stainless steel knife then divided into 4 portions of 400 g each. One portion was analysed immediately for moisture and ascorbic acid contents. The remaining 3 portions were immersed into separate beakers of 500 mL pre-boiling distilled water and boiled further for 5, 10 and 15 min, respectively. At the end of the boiling time, each sample was drained in a polypropylene colander until the liquid stopped dripping. Each sample was then divided further into two equal portions. Each portion of these blanched samples was spread on a separate clean traditional bamboo winnower and one portion dehydrated under shade at ambient temperature of about 22 to 26°C, while the other one under direct sunlight (32±4°C). The dehydration process was observed for 5 days and the leaves were removed and put into air-tight polythene bags and kept at dry, well ventilated place, ambient temperature (22 to 26°C) until analysis. The samples were named according to the blanching time and method of dehydrations for example; ‘5 min SD’ was a sample which was blanched for 5 min and sun-dehydrated, while the ‘5 min SHD’ represents one that was blanched for the same 5 min but was shade-dehydrated.
Moisture retention determination
Moisture content was determined using the AOAC (2000) method. Crushed sample (2 g) was put into beaker, which was previously cleaned, dehydrated for 1 h in an oven and cooled in a desiccator for 30 min. The initial weight of the beaker with sample was recorded. The sample in the beaker was then dehydrated for 6 h in an air circulating oven set at 100°C, cooled in a desiccator for 1 h, then reweighed. Moisture content was calculated as a percentage using the following formula:
Moisture content (%) = (A - B) / C × 100
Where: A = initial weight of beaker with sample; B = final weight of the beaker plus sample after oven-drying; C = initial weight of the sample before oven-drying.
Moisture retention was calculated as a percentage of the dehydrated sample moisture content to that of original fresh leaves.
Ascorbic acid retention determination
Ascorbic acid (AA) was analysed by AOAC (2000) titrimetric method using 2,6-dichlorophenolindophenol (DCPIP) as a redox dye. To begin with, 30 g of dehydrated sample was ground finely using a motor and pestle to pass through a 100 mesh sieve. Then, 90 mL of water was added to make a ratio of 1:3, and the mixture transferred into a 200 mL beaker. Two spatulas of activated charcoal were added and the mixture was boiled for 10 min to remove the green colour that would interfere with the observation of colour change during titration. After cooling in a water bath, the sample was filtered through a Whatman No. 1 filter paper. The filtrate, in triplicate, was then used for the analysis of ascorbic acid content in the sample using the above AOAC standard method. Ascorbic acid retention was calculated as a percentage of dehydrated sample ascorbic acid content to that of original fresh leaves.
Statistical analyses
One way analysis of variance (ANOVA), with Duncan's multiple range test, using a SAS program (version 8.1, SAS Institute Inc., Cary, NC, USA) was conducted to assess significance of differences (p<0.05) among the obtained mean values.
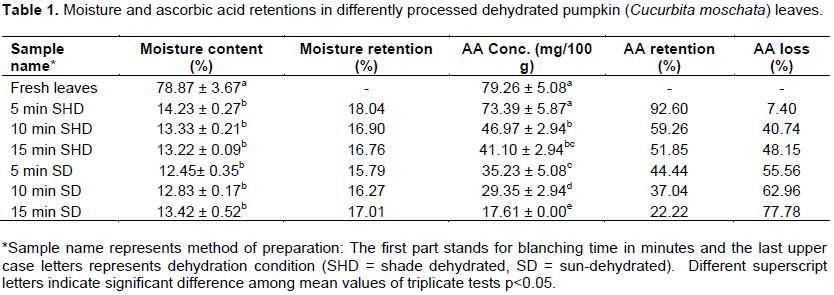
The results for both moisture content and ascorbic acid determinations and retentions of the differently processed dehydrated pumpkin leaf samples are presented in Table 1.
Moisture retention
In the preservation of vegetables by dehydration technique, moisture content of the final product is of great importance as it determines its longevity on the shelf.
Usually, dehydration under direct sunlight is preferred as it is believed to reduce the moisture to the minimum level. However, from Table 1, it can be observed that there were no significant differences (p>0.05) among samples dehydrated under direct sunlight and those dehydrated under a shade. Much as the direct sunlight might be efficient in terms of the dehydration time, it has great potential to affect retention of some nutrients such as vitamin C and being a free provision, the sun’s efficiency has no any economic value. As such, dehydration techniques that can retain nutrients would be of great importance. Compared to the findings of similar studies, the moisture contents in the fresh and processed dehydrated leaves were substantially lower than those reported by Onoja (2014) in fluted pumpkin (Telfairia occidentalis) leaves. This difference in the moisture content of the fresh leaves may be attributed to differences in plant species and water composition of the area where the plants for these two studies were grown, while those of dehydrated leaves may be due to the length of dehydration time, ambient temperature and air circulation of the dehydration environment.
A thorough scrutiny of the results in this study further revealed that samples dehydrated under the direct sunlight retained less moisture compared to the shade dehydrated ones, but the contents increased as blanching time increased. Thus, the sample, which was blanched for 15 min retained more moisture followed by the ones blanched for 10 then 5 min, in that order. This scenario may not necessarily reflect that the blanching process led to absorption of more water by the sample. This is so because, observation has shown that as leafy vegetables get boiled, they tend to shrink and liquid gets released from them resulting in an increase in the amount of liquid in the boiling vessel. However, there is a possibility that as the liquid got released from the leaf, external cells of the leaf got compacted together to form a semi-permeable membrane that prevented some water from getting out of the leaf. The exposure to the direct sun radiation possibly assisted in the faster formation of this membrane compared to shade-dehydration, where the scenario was different. The shade-dehydrated samples had their moisture retention decreasing with increasing blanching time. These different trends are clearly presented in Figure 1. No study, however, was found to compare these findings with.
Ascorbic acid retention
The results of the ascorbic acid retention in the processed pumpkin leaves are also presented in Table 1. There was a significant difference (p<0.05) between the fresh and processed samples, except the 5 min SHD, with the fresh samples having the highest amount. Among the processed samples, all of them differed significantly (p<0.05) in the order of 5 min SHD > 10 min SHD > 15 min SHD > 5 min SD > 10 min SD > 15 min SD. This showed that the samples, which were dehydrated under a shade, had generally higher values than the sun-dehydrated ones. At the same time, ascorbic acid kept reducing as blanching time increased. The ascorbic acid in the 5 min shade-dehydrated sample did not differ significantly with the fresh one. However, with the scope of this study, it was not certain as to whether the 5 min of blanching were enough to achieve green colour retention, deactivation of microorganisms and enzymes, and improvement of flavour, which are the main reasons for blanching vegetables (Kakade and Neeha, 2014; Ahmed et al., 2001). The study conducted by Vyankatrao (2014) in mint, coriander, curry leaves and bitter gourd revealed that highest retention of ascorbic acid alternated between sun-dehydrated and shade-dehydrated among different vegetables, indicating that the findings of this study were specific to leaves of C. moschata and cannot be easily generalised to all leaves that would be dehydrated under the same conditions. Type of the leaf is also an important factor. However, a number of studies in drumstick (Molinga) leaves (Joshi and Mehta, 2010) are in concord with the findings of this study.
The findings of this study have shown that in the preservation of C. moschata leaf vegetables by dehydration method, duration of branching and light intensity during dehydration have an effect on the retention of vitamin C. Reduced blanching duration accompanied by shade dehydration can retain more of the vitamin. Proper shade dehydration of the vegetable cannot compromise the shelf-life of the vegetable as the retained moisture may not be different from that of the vegetables dehydrated under direct sunlight.
The authors have not declared any conflict of interests.
REFERENCES
|
Adegunwa MO, Alamu EO, Bakale HA, Oyeniyi CO (2011). Proximate and bioactive contents of some selected vegetables in Nigeria: Processing and varietal effects. Am. J. Food Nutr. 1: 171-177.
Crossref
|
|
|
|
Ahmed J, Shivhare US, Singh G (2001). Drying characteristics and product quality of coriander Leaves. Food Bioprod. Proc. 79(2):103-106.
Crossref
|
|
|
|
|
Dhiman K, Gupta A, Sharma DK, Gill NS, Goyal A (2012). A review on the medicinally important plants of the family Cucurbitaceae. Asian J. Clin. Nutr. 4:16-26.
Crossref
|
|
|
|
|
Durante M, Lenucci MS Mita G (2014). Supercritical carbon dioxide extraction of carotenoids from pumpkin (Cucurbita spp.): A Review. Int. J. Mol Sci. 15:6725-6740.
Crossref
|
|
|
|
|
FAO (1988). Traditional Food Plants. Rome: FAO.
|
|
|
|
|
Fellows PJ (2009). Food processing technology. Principles and practice. 3rd Edition. Oxford, Cambridge, New Delhi: Woodhead Publishing Limited.
Crossref
|
|
|
|
|
Joshi P, Mehta D (2010). Effect of dehydration on the nutritive value of drumstick leaves. J. Metabolomics. Syst. Biol. 1:5-9.
|
|
|
|
|
Kakade SB, Neeha VS (2014). Dehydration of green leafy vegetable: Review. Int. J. Innov. Res. Technol. 1:58-64.
|
|
|
|
|
Kwak Y, Ju J (2013). Antioxidant and anti-cancer activities of squash (Cucurbita moschata Duch.) leaf extract in vitro. Korean J. Food Sci. Technol. 45:770-776.
Crossref
|
|
|
|
|
Lawal OO, Essien NC, Essien NM, Ochalla F (2015). Vitamin C content of some processed green leafy vegetables. Eur. J. Exp. Biol. 5:110-112.
|
|
|
|
|
Lim TK (2012a). Cucurbita moschata. The encyclopedia of earth. Edible medicinal and non-medicinal plants. Volume 2. Fruits. Netherlands: Springer. pp. 266-280.
|
|
|
|
|
Lim TK (2012b). Cucurbita pepo L. The encyclopedia of earth. Edible medicinal and non-medicinal plants. Volume 2. Fruits. Netherlands: Springer. Pp. 281-294.
|
|
|
|
|
Lymo MH, Nyagwegwe S, Mnkeni AP (1991). Investigation on the effect of traditional food processing, preservation and storage methods on vegetable nutrients: a case study in Tanzania. Plant Foods Hum. Nutr. 41: 53-57.
Crossref
|
|
|
|
|
Mala KS, Kurian AE, Srinivasulu K (2016). Effect of pre-treatments on the proximate composition of pumpkin flour. Int. J. Inn. Stud. Sci. Eng. Technol. 2:17-24.
|
|
|
|
|
Mepba HD, Eboh L, Banogo DE (2007). Effect of processing treatments on nutritive composition and consumer acceptance of some Nigerian edible leafy vegetables. Afr. J. Food Agri. Nutri. Dev. 7:1-18.
|
|
|
|
|
Musarirambi MT, Mavuto V, Songwe VD, Nkambule TP, Mhazo N (2010). Indigenous post-harvest handling and processing of traditional vegetables in Swaziland: A review. Afr. J. Agric. Res. 5:3333-3341.
|
|
|
|
|
Mwaniki DL, Omwega AM, Muniu EM, Mutunga JN, Akelola R, Shako BR, Gotink MH, Pertet AM (1999). Anaemia and status of iron, vitamin A and zinc in Kenya. The National Survey. Nairobi: Ministry of Health.
|
|
|
|
|
Okpalamma F, Ojimelukwe PC, Mazi EA (2013). Post-harvest storage and processing changes in carotenoids and micronutrients in Fluted Pumpkin (Telferia occidentalis Hook F.). J. Agric. Vet. Sci. 6:34-39.
|
|
|
|
|
Onoja IU (2014). The effect of different processing methods on the Proximate, β-Carotene and Ascorbate Composition of Fluted Pumpkin (Telfairia Occidentalis) Leaves and its Product, the Leaf Curd. Int. J. Nutr. Food Sci. 3:404-410.
Crossref
|
|
|
|
|
Stevenson DG, Eller FJ, Wang L, Jane JL, Wang T, Inglett GE (2007). Oil and tocopherol content and composition of pumpkin seed in 12 cultivars. J. Agric. Food Chem. 5: 4005-4013.
Crossref
|
|
|
|
|
Vyankatrao NP (2014). Effect of drying methods on nutritional value of some vegetables. Proceeding of the National Conference on Conservation of Natural Resources & Biodiversity for Sustainable Development. Biosci. Discov. 6:72-79.
|
|