Full Length Research Paper
ABSTRACT
Sorghum is a nutritious and under-utilized cereal whose potential in development of nutritious ready-to-drink beverages remains unexplored. The objective of the study was to optimize malting and fermentation conditions to obtain a nutritionally superior new sorghum product. Four beverage formulations containing sweetened sorghum malt extracts were developed through fermentation using kombucha culture at different temperatures (between 20 and 35°C). The formulations were also malted for three, four and five days and analyzed for nutritional characteristics. The iron, total phenolics and vitamin C contents in the formulations ranged from 0.125 ± 0.292 to 2.956 ± 13.83 mg/100g, 0.1328 ± 0.00594 to 1.6601 ± 0.0640 mg GAE/100 ml and 4.3505 ± 0.2797 to 6.1304c ± 0.2797 mg/100 ml respectively. Iron was used in the process optimization to select the best beverage which had high nutritional and sensory quality. The mean contents of iron in the beverages ranged from 1.25 ± 0.292 mg/kg (formulation 5D1) to 29.56 ± 13.83 mg/kg (formulation 4D). The best formulation was obtained from an optimum of four days malting and fermentation temperature of 25°C (4D). The findings indicate that nutritious beverages can be developed from sorghum by employing different malting days and fermentation conditions to come up with products having varying levels of iron, vitamin c and phenolics depending on your process or consumer needs.
Key words: Sorghum, kombucha, malting, fermentation.
INTRODUCTION
Sorghum is a major source of minerals, calories and proteins for many people in Africa and Asia (Hadebe et al., 2017). It has drought tolerance and is adapted to both tropical and subtropical ecosystems hence it is a very important subsistence crop (Amelework et al., 2016). In terms of cereal production in Kenya, it is ranked third after maize and wheat and it’s a staple crop for many low income households (Kilambya and Witwer, 2013). It is the only indigenous cereal to Kenya, being cultivated even in areas considered poor in terms of potential for agriculture. This cereal has great potential to stimulate regional development and improve food security.
Sorghum in addition to being a protein and minerals source, has potential in functional constituents that promote health, such as fibers, B group of vitamins, waxes that lower cholesterol and antioxidant phenolics (Hassani et al., 2014). The composition and contents of starch in the grain are influenced by the grain’s growth conditions (Hill et al., 2012). It usually ranges between 32.1 and 72.5 g/100 g with amylopectin being (81.0 to 96.5%) and amylose (3.5 to 19.0%) (Shegro et al., 2012). Due to starch granules, proteins and tannins being bound together strongly, it has the lowest starch digestibility among cereals (Mkandawire et al., 2013). Prolamins and non-prolamins are the main proteins present in the sorghum grain. Of the total protein composition, prolamins range between 77 to 82% (7-15 g/100 g) with glutelins, globulins and albumins occupying the other minor proportion (Mokrane et al., 2010).
The cereal’s mineral content varies depending on area of cultivation with the minerals being phosphorus, potassium, iron and zinc. Their bioavailability is still unknown and availability of iron and zinc in the grain ranges from 6.6 to 15.7% and 9.7 to 17.1%, respectively (de Morais Cardoso et al., 2014). The phenolic acid content in some sorghum varieties varies from 135.5 to 479.40 mg/g with ferulic and protocatechuic acids as the major contributors with 120.5 to 173.5 mg/g and 150.3 to 178.2 mg/g, respectively (Afify et al., 2012). Tannins are a group of phenolic compounds and are found in many plants. It plays part in defense against pathogens and predators.
They reduce the availability of minerals, starch and proteins. Despite their anti-nutritional effect, they are better radical scavengers compared to other simple phenolics (Kaufman et al., 2013). Sorghum has a lot of health benefits some of which include alleviation of the negative effects brought about by cardiovascular disease, many chronic diseases and obesity (Salazar-López et al., 2018).
The lag in commercialization of sorghum compared to other cereals in Kenya is mainly due to grain prices that cannot compete with the other cereals levels of production which are low and which also vary, high costs of assembly and high costs of processing (Njagi et al., 2019). Production of sorghum is also conducted majorly by subsistence farmers who produce just enough for their domestic use and seldom excess for sale purposes. Thus, production limitations vary from conventional to commercial scales (Omoro, 2013).
Malting involves germination, under controlled conditions. Its main objective is modification of the chemical composition of the grain through mobilization of the endogenous enzymes, as a result, physical and rapid solubilization are enabled during brewing resulting in a nutritionally rich medium for yeast fermentation which produces carbon dioxide and ethanol (Taylor and Kruger, 2019). In hydrolysis of malt, the most important enzymes are alpha and beta amylases that cause production of fermentable maltose from starch (Taylor and Kruger, 2019).
Fermentation of cereals is usually aimed at preservation, which comes from acids production. The acids include lactic, acetic and propionic or alcohol production which is often combined with a reduction in water activity, safety enhancement of the final products by inhibition of pathogenic microorganisms, enhancement of sensory properties (color, aroma, texture and taste), nutritional value improvement by removal of anti-nutrients such as tannins, phytic acid, enzyme inhibitors and poly-phenols, bio-availability enhancement of some components of carbohydrates, indigestible poly and oligosaccharides reduction (Liptáková et al., 2017).
Epidemiological studies have shown that consumption of fermented foods leads to an improvement in health and a decline in the risk of disease contraction. Probiotics consumption in adequate doses can confer health benefits to the consumer (Rezac et al., 2018). The probiotics use carbohydrates that are available to produce short chain fatty acids, out-compete pathogens for resources, produce antimicrobial agents, they balance the immune system and also produce vitamins (Derrien et al., 2015).
In terms of sorghum beverages, in Kenya there’s locally produced sorghum beer targeted at consumers who want to upgrade to bottled beers from illicit drinks (Orr et al., 2014), coffee substitutes from sorghum for those sensitive to caffeinated beverages (Omer and Abou-zaid, 2022). There is also the malt extract from sorghum which is a sweet wort which is rich in sugar and is also a beverage by itself, but can be made into a flavored syrup through concentration or powder by evaporation of the extract into a product which is dark-colored (Elgorashi et al., 2016). This beverage development will contribute to the sorghum value chain by adding onto the list of existing sorghum products.
MATERIALS AND METHODS
Study design
A completely randomized block design was used with malting and fermentation chosen as the blocks in the experimental design.
Sample collection and preparation
Sorghum (Sorghum bicolor L. Moench) purchased from Busia, finger millet (Eleusine coracana) from the local market in Kangemi, kombucha culture bought from Kombucha Kenya Company (a mushroom-like consortium of yeasts and acetic acid bacteria which are in a symbiotic relationship suspended in previously fermented broth), previously fermented kombucha broth and white sugar purchased from a local supermarket.
Methodology
Product development
Sorghum grains were malted for 3, 4 and 5 days after which malt extraction was conducted, resulting into malt extracts which were further subjected to 7 days fermentation at temperatures between 20 and 35°C by the SCOBY (Table 1). Resulting beverages were analyzed for their nutritional components and the nutrient which had a significant number of reported deficiencies nutritionally and also had significant differences between the formulations was chosen as the determinant factor during the process optimization (Figure 1).
Malting
The method of Aluge et al. (2016) was used, with variations being applied in the germination days per formulation. Foreign matter was sorted out and grain soaking done in buckets with potable water for 12 h in the ratio 1:2. The grains were rid of water by draining then sprouted by spreading on wet blankets on malting trays on wooden benches and finally covered for the sprouting process. Daily sprinkling was done onto the grains and absolute ethanol sprayed onto the grains to prevent growth of mold. Oven drying (memmert oven supplied by GmbH and Co. Stavendamm22 model Schutzart DIN 40050-IP20) for 6 h at 65°C was done to the germinated grains. After drying, meshed trays were used to remove the shoots by rubbing the sprouts on them (Hassani et al., 2014).
Malt extraction and brewing
Slight modifications were applied to the hot water extraction method by Sarkodie et al. (2014). 2 L of portable water at ambient temperature was mixed with ½ a kilo of milled sorghum malt (supplemented with 40% malt from finger millet). The mash was left to sediment for 1 h. 40% (1 L) of the supernatant extract composed of enzymes was decanted and kept aside using calibrated jars. 30 min boiling in stainless steel cooking vessels of the remaining mash which was thick was conducted and the enzyme extract added after cooling.
The cooked mash was then incubated at 72 ± 1°C (memmert oven supplied by GmbH and Co. Stavendamm22 Schutzart DIN 40050-IP20) overnight for saccharification then cooled to room temperature and an adjustment made to the weight to reach 3 kg with distilled water. Filtration was done to the resulting wort using a muslin cloth then finally through filter pads.
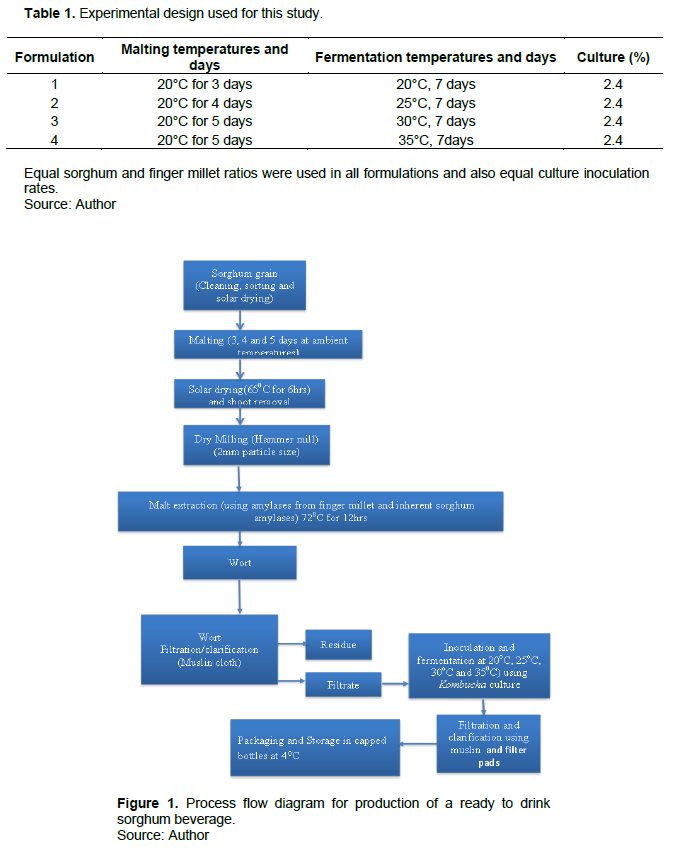
Inoculation and fermentation
The methods according to Jayabalan et al. (2014) and Kumar and Joshi (2016) with slight modifications were used. 1 L of the cool sorghum malt sweetened with 100 g sucrose at ambient temperature was poured Into a wide mouthed clean vessel which had been sterilized with boiling water, 100 ml of previously fermented kombucha was added to prevent the growth of undesirable microbes by lowering the pH and a mat of the culture about 24 g placed on the surface of the infusion and the jar was hygienically covered using clean muslin cloths and properly fastened using rubber bands. The concentrates were placed at different incubation temperatures (between 20 to 30°C) for 7 days.
Analytical methods
Nutritional analysis
Protein: AOAC 2012 method 991.20 was used for determination of crude protein. Into a Kjedahl flask, 0.5 g of the accurately weighed samples was placed while folded in a nitrogen free filter paper. Sulphuric acid and a catalyst tablet were added to digest the sample in a fume chamber. Phenolphthalein indicator was used to indicate the end point before connection of the flask to a distillation unit. For back titration, 40% NaOH solution was used against 0.1N NaOH solution. The standard conversion factor used was 6.25.
Vitamin C: AOAC 967.21 (2006) was used whereby 5 ml of the test solution was titrated against prepared standard solution of ascorbic acid until the end point which was a faint pink color.
Iron and zinc: The method AOAC 999.11 according to 2006 AOAC was used. The beverage samples were subjected to ashing in a muffle furnace overnight and residue collected, acidified with nitric acid to remove acid soluble minerals and heated on a hot plate. The resulting clear solutions were diluted up to 100 ml in volumetric flasks with distilled water then subjected to Buck Scientific Atomic Absorption Spectrophotometer (Model 210VGP) to obtain the mineral content readings directly using the different cathode tubes made from the elements of interest (Fe and Zn).
Total sugars: Done according to Islam et al. (2013) with slight adjustments. 4 ml of anthrone reagent was added into an aliquot of pipetted 1 ml beverage extract in test tubes. This was cooled after boiling for ten minutes. Preparation of a reagent blank was done and it was treated the same way. The resulting solutions’ absorbances were measured at 630 nm in a Perkin Elmer UV-VIS spectrophotometer model 166351. A glucose standard curve was also prepared and used in calculating the concentrations from absorbances obtained. Total sugar content per 100ml sample was calculated using the formula:
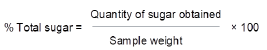
Alcohol content determination
The procedure according to Park et al. (2004) was used. Distillation was performed to a final volume of 50 ml after filtration of 100 ml samples through a strainer. Distilled water was used to readjust the distillate to 100 ml. An alcohol hydrometer was used to determine the strength at room temperature.
Calorific value
This was done according to Mohammed et al. (2011). Calculations of calorific value were done using the Atwater factors: 4 Kcal/g for carbohydrates, 4 Kcal/g for protein, 9 Kcal/g for fat and 7 kcal/g for alcohol.
Total phenolics
Done according to Singleton et al. (1999). The Folin-Ciocalteu method was used with some modifications. 50 ul of the diluted sample was mixed with Folin-Ciocalteu reagent (100 µl) and deionized water was used as the diluent and control. Final dilution was done to a total volume of 1,150 ul with deionized water and mixed thoroughly. 10 min incubation at room temperature was done then 500 µl of 20% Na2CO3 solution added with mixing immediately and this was further incubated for 2 h at room temperature. Absorbance was recorded at 765 nm with all samples being measured in duplicate. Gallic acid (1 mg/ml) was used as the standard and the quantification of total phenolic compounds was done in milligrams per 100 ml gallic acid equivalents (mg GAE/100 ml).
Tannins determination
The method according to Adeyeye et al. (2019)was used. 1 ml sample was weighed and soaked with a solvent mixture 100 ml with acetic acid and acetone in the ratio 1:4 respectively for 5 h so as to extract the tannins. The samples were filtered and absorbance of the filtrate determined using a Perkin Elmer UV-VIS spectrophotometer model 166351 according to AOAC. A calibration curve for the standard (tannic acid) was prepared.
Statistical analysis
Data obtained was analyzed using one-way ANOVA on Genstat statistical software version 15.1. Means obtained were compared using least significant difference at 5% under Tukey test.
RESULTS
Nutritional quality of developed sorghum beverage
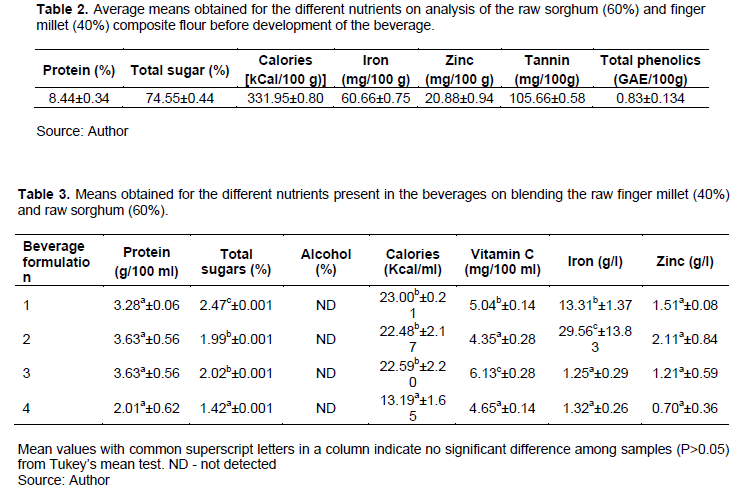
Most nutritional components in the raw materials were significantly increased while the non-nutritional components and sugars were reduced after processing. There was no significant difference between the protein contents of the developed beverages (Tables 2 and 3).
Total sugars varied significantly between the different formulations being highest in beverage formulation 1. This could probably be due to the limited number of malting days such that sugars had not been used up a lot by the germinating seedling compared to the other formulations which have lesser sugars as the days of malting increase, the least being beverage formulation 4 (Tables 2 and 3).
For iron and zinc, there were significant differences between the formulations and the trend was similar for both nutrients. The mineral contents increased with days of malting and fermentation temperatures up to the fourth day then decreased sharply. For both minerals, formulation 2 had the highest concentrations, with 3 and 4 having the least concentrations that were not of significant difference between the two. From the above statistics, it is evident that formulation 2 (Four days malting and one week fermentation at 25°C) is superior in terms of the content of iron, which increases with days of malting and temperature of fermentation up to the four day of malting and 25°C fermentation then subsequently decreases with increase in both factors. Zinc had no significant difference between the different formulations (Table 2).
In terms of vitamin C content, formulation 3 (five days malting and one week fermentation at 35°C) was the most superior while 2 and 4 had the least quantities (Table 2).
Non nutritional composition of developed sorghum beverage
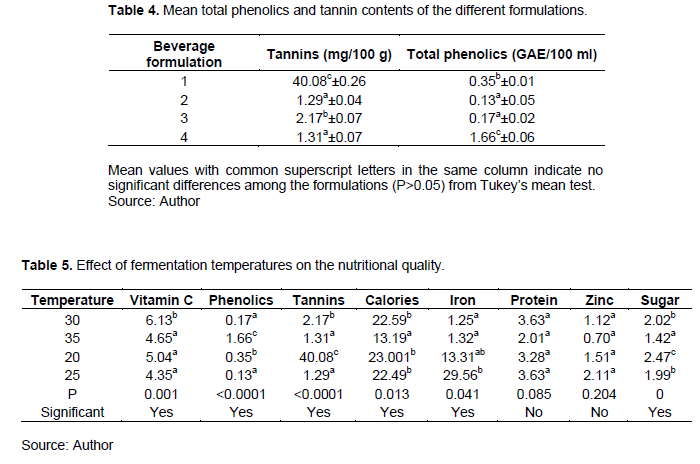
There was a significant difference in the quantities of tannins between the different sorghum beverage formulations with formulation 1 having the highest tannin contents (40 g/100 g) and Formulation 1 had the highest total phenolic content (1.66 mg/100 g). They were highest in formulation 3D and lowest in 4D and 5D2. The malting days for the cereals to have less tannin were 4 days as from the values obtained (Table 4).
For phenolics, despite significant differences between the formulations, the trend was not well defined since 4D and 5D1 had the least phenolics content and 5D2 the highest. Formulation 4 (5 days malting and 1 week fermentation at 35°C) is characterized by the highest total phenolics content (Table 4).
Malting effects on nutritional quality of developed sorghum beverages
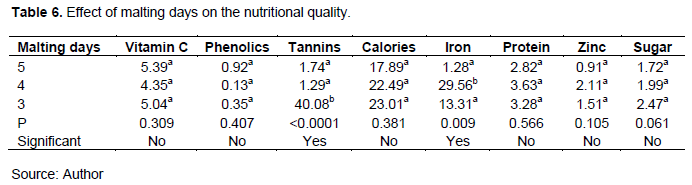
Malting or fermentation as treatments yields nutritionally superior products. Fermentation specifically, has been used to improve the yield of different bioprocesses (Das et al., 2014). The contributions of malting to the different nutrients can be seen especially on tannins and iron content on which there are significant differences between the samples. The fermentation temperatures used also cause significant differences in the quantity of sugar, calories, vitamin c, iron, phenolics and tannins (Table 5). From the data processing on statistical software, there was no interaction between fermentation temperatures and the days of malting owing to the experimental design used.
Effects of fermentation on nutritional quality of developed sorghum beverages
Fermentation temperatures used were found to have a significant effect (p<0.05) on the levels of vitamin c, phenolics, tannins, calories, iron and sugars and no effect (p>0.05) on protein and zinc contents.
The number of malting days used were also found to have a significant effect (p<0.05) on the levels of tannins and iron content. There was no significant effect (p>0.05) on the levels of vitamin C, phenolics, calories, protein, zinc and sugars (Table 6).
DISCUSSION
Compared to most of the beverages currently in the market, the protein content of the developed beverages is higher and can contribute towards meeting the daily protein requirements. Protein content forms an important basis for the quality of a beverage. The interactions among proteins, amino acids and phenols greatly influence the stability and organoleptic characteristics of the beverage. The amino acids also form a great component of the beverage’s aromatic compounds. There was increase in protein content with increase in fermentation period which can be attributed to an increase in microbial mass (Correia et al., 2010). This could be supported by the favorable pH for the growth of lactic acid bacteria with the progress of the fermentation which in turn could cause extensive hydrolysis of the protein molecules to amino acid and other simple peptides (Das et al., 2014). It is also worth noting that increased fermentation time yielded lower protein content. This is because the fermenting microorganisms also uses amino acid which could lower the protein content and quality of some fermented food (Pranoto et al., 2013).
Sugars form an important part for calories provision in an individual. Beverage F1 had the highest sugar content. The initial days of malting facilitated the enzymatic breakdown of carbohydrates into simple sugars through activation of endogenous enzymes such as α-amylase thereby improving digestibility as a result of degradation of starch to provide energy for the seed development (Nkhata et al., 2018). The rest of the beverage formulations had decreased sugars, an occurrence that can be linked to the malting periods. In earlier stage of germination, large portions of soluble sugars are expected to be used up during respiration and not enough α-amylase has been synthesized or activated to hydrolyze starch, leading to less increase in sugars (Okolo et al., 2020). However, after 36 to 48 h of germination, the dormancy is lost as the amylolytic enzymes synthesized in the aleurone layer migrate into the endosperm and initiate the hydrolysis of starch granules. Glucose and fructose levels are generally low in the raw cereals at this moment, however, on germination, the two soluble sugars increase significantly such that their levels supersede that of sucrose activation of invertase which hydrolyzes sucrose into glucose and fructose during germination (Oseguera-Toledo et al., 2020). This explains the gradual increase then decrease to a further increase in sugar content in the formulations depending on malting periods. Calories were not significantly different between formulations 1, 2 and 3 while 4 had the least calories due to also having the least sugars.
Cereals have most of the nutritional elements bound. Malting ensures the bound mineral components are released. This explains the increase in mineral content (iron and zinc) up to day 4. The increase could be due to leaching of the anti-nutritional factors that bind the minerals. It has been hypothesized that the remarkable increase in phytase activity during germination helps reduce phytic acids, which bind minerals subsequently leading to increased mineral availability (Nkhata et al., 2018). After day four, the bound elements have been released hence accounting for the sharp decrease.
The initial increase in tannin content could be attributed to hydrolysis of condensed tannins such as proanthocyanidin. While the eventual decrease may be due to their binding with cotyledon endosperm that are usually undetected by routine method due to their insolubility in solvent or may be due to microbial phenyl oxidase action as explained by (Osman, 2011).
Phenolic content increased with increasing days of fermentation, a factor which may be attributed to an increase in the level of free soluble phenolics, due to hydrolysis of the glycosidic bonds of bound phenolics by hydrolytic enzymes secreted by microorganisms in the culture (Elkhalifa and Bernhardt, 2018). Phenolic compounds provide the antioxidant compounds in a beverage. These phenolic compounds have several functional properties in the beverage and influence its colloidal stability, flavor and color (Adebo and Medina-Meza, 2020). Phenolic compounds are also important antioxidants, and owing to this antioxidant capacity and low alcoholic content, consumption of beer helps to improve the plasma antioxidant activity and reduce the risk of cardiovascular diseases (Das et al., 2014). For phenolics, they affect the taste of products especially when they are very high in concentration. They were highest in this formulation due to increased number of malting days as suggested by Carciochi et al. (2016)and also fermentation temperature (Aguilar et al., 2019).
For vitamin C, consumption of 300 ml of either of the four beverages is enough to meet the daily requirements of the nutrient. Osman (2011)confirmed that malting and fermentation increase the quantity of vitamin C. Vitamin C can be synthesized during malting by the hydrolysis of starch using amylases and diastases that avail glucose for this process. This enhanced content of glucose is the one that acts as a precursor to formation of vitamin c. This study confirmed that C-6 of glucose could be oxidized to form the carboxyl carbon of the ascorbic acid concluded that the same could happen in plants during fermentation or malting.
For process optimization, iron content was chosen as the standard due to the fact that previous studies have shown that phenolics can contribute to bitter taste especially when in exceeding amounts, for example in olive oil as suggested by Shahidi and Ambigaipalan (2015)despite them having health benefits against cancer and cardiovascular diseases. Based on the recommended dietary allowances issued by WHO (8 mg/day), this beverage will easily meet the requirements of all individuals. Formulation 4D is well balanced in terms of phenolics which are lower in amounts compared to the rest hence the issue of bitterness may not be present and also in terms of the content of vitamin C it contains an amount which is almost similar to the other formulations when the means are compared.
CONCLUSION
It is quite interesting to note that varying the number of malting days and use of different fermentation temperatures can lead to considerable differences nutritionally in the final cereal products despite similarities in certain nutrient compositions as in the above scenario. The best formulation for the beverage development for good nutritional output is four days malting at 25°C fermentation temperature for one week. Sensory analysis output can also be used for optimization purposes then the products during commercialization can be improved nutritionally via fortification as is done by most food industries.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENT
The authors acknowledge the National Research Fund (NRF) Multidisciplinary Research Grant, 2016-2017 financial and material support under the “Harnessing the genetic resources of Sorghum (Sorghum bicolor L) in Kenya to improve drought resistance and striga resistance of farmer preferred cultivars for climate change adaptation by utilizing innovative genomic, value chain and participatory approaches” project.
REFERENCES
|
Adebo OA, Medina-Meza IG (2020). Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules 25(4):1-19. |
|
|
Adeyeye SAO, Adebayo OAO, Olalekan SA, Fyemi OA, Tiamiyu HK, Oke EK, Soretire AA (2019). Effect of co-fermentation on nutritional composition, anti-nutritional factors and acceptability of cookies from fermented sorghum (Sorghum bicolor) and soybeans (Glycine max) flour blends. Journal of Culinary Science and Technology 17(1):59-74. |
|
|
Afify AEMMR, El-Beltagi HS, El-Salam SMA, Omran AA (2012). Protein solubility, digestibility and fractionation after germination of sorghum varieties. PLoS ONE 7(2):e31154. |
|
|
Aguilar J, Miano AC, Obregon J, Colchado JS, Jauregui GB (2019). Malting process as an alternative to obtain high nutritional quality quinoa flour. Journal of Cereal Science 90:102858. |
|
|
Aluge OO, Akinola S, Osundahunsi OF (2016). Effect of malted sorghum on quality characteristics of wheat-sorghum-soybean flour for potential use in confectionaries. Food and Nutrition Sciences 7(13):1241-1252. |
|
|
Amelework BA, Shimelis HA, Laing MD, Ayele DG, Tongoona O, Mengistu F (2016). Sorghum production systems and constraints, and coping strategies under drought-prone agro-ecologies of Ethiopia. South African Journal of Plant and Soil 33(3):207-217. |
|
|
Carciochi RA, Dimitrov K, D'Alessandro LG (2016). Effect of malting conditions on phenolic content, Maillard reaction products formation, and antioxidant activity of quinoa seeds. Journal of Food Science and Technology 53(11):3978-3985. |
|
|
Correia I, Nunes A, Guedes S, Barros AS, Delgadillo I (2010). Screening of lactic acid bacteria potentially useful for sorghum fermentation. Journal of Cereal Science 52(1):9-15. |
|
|
Das AJ, Seth D, Miyaji T, Deka SC (2014). Fermentation optimization for a probiotic local northeastern Indian rice beer and application to local cassava and plantain beer production. Journal of the Institute of Brewing 121(2):273-282. |
|
|
de Morais Cardoso L, Montini TA, Pinheiro SS, Pinheiro-Sant'Ana HM, Martino HS, Moreira AV (2014). Effects of processing with dry heat and wet heat on the antioxidant profile of sorghum. Food Chemistry 152:210-217. |
|
|
Derrien M, Johan ET, Vlieg VH (2015). Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends in Microbiology 23(6):354-366. |
|
|
Elgorashi AGM, Elkhalifa EA, Sulieman AE (2016). The effect of malting conditions on the production of non-alcoholic sorghum malt beverage. International Journal of Food Science and Nutrition Engineering 6(4):81-86. |
|
|
Elkhalifa AEO, Bernhardt R. (2018). Combination effect of germination and fermentation on functional properties of sorghum flour. Current Journal of Applied Science and Technology 30(1):1-12. |
|
|
Hadebe ST, Modi AT, Mabhaudhi T (2017). Drought Tolerance and Water Use of Cereal Crops: A Focus on Sorghum as a Food Security Crop in Sub-Saharan Africa. Journal of Agronomy and Crop Science 203(3):177-191. |
|
|
Hassani A, Zarnkow M, Becker T (2014). Influence of malting conditions on sorghum (Sorghum bicolor (L.) Moench) as a raw material for fermented beverages. Food Science and Technology International 20(6):453-463. |
|
|
Hill H, Slade L, Henry R (2012). Variation in sorghum starch synthesis genes associated with differences in starch phenotype. Food Chemistry 131:175-183. |
|
|
Islam MK, Khan MZH, Sarkar MAR, Absar N, Sarkar SK (2013). Changes in acidity, TSS, and sugar content at different storage periods of the postharvest mango (Mangifera indica L.) influenced by Bavistin DF. International Journal of Food Science 2013. |
|
|
Jayabalan R, Malbasa RV, Loncar ES, Vitas JS, Sathishkumar M (2014). A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Comprehensive Reviews in Food Science and Food Safety 13(4):538-550. |
|
|
Kaufman RC, Wilson JD, Bean SR, Presley H, Canqui BH, Mikha M (2013). Effect of nitrogen fertilization and cover cropping systems on sorghum grain characteristics. Journal of Agricultural and Food Chemistry 61(24):5715-5719. |
|
|
Kilambya D, Witwer M (2013). Analysis of incentives and disincentives for sorghum in Nigeria. Technical notes series. MAFAP, FAO. Rome. Available at: www.fao.org/publications (Accessed: 23 February 2022) |
|
|
Kumar V, Joshi VK (2016). Kombucha: Technology, microbiology, production, composition and therapeutic value. International Journal of Food and Fermentation Technology 6(1):13-24. |
|
|
Liptáková D, Matej?eková Z, Valík L (2017). Lactic acid bacteria and fermentation of cereals and pseudocereals. Fermentation Processes 10:65459. |
|
|
Mkandawire NL, Kaufman RC, Bean SR, Weller CL. Jackson DS, Rose DJ (2013). Effects of sorghum (Sorghum bicolor (L.) Moench) tannins on α-amylase activity and in vitro digestibility of starch in raw and processed flours. Journal of Agricultural and Food Chemistry 61(18):4448-4454. |
|
|
Mohammed NA, Ahmed IAM, Babiker EE (2011). Nutritional evaluation of sorghum flour (Sorghum bicolor L. Moench) during processing of injera. International Journal of Nutrition and Food Engineering 51:72-76. |
|
|
Mokrane H, Amoura H, Bensemra NB, Courtin CM, Delcour JA, Nadjemi B (2010). Assessment of Algerian sorghum protein quality [Sorghum bicolor (L.) Moench] using amino acid analysis and in vitro pepsin digestibility. Food Chemistry 121(3):719-723. |
|
|
Njagi T, Onyango K, Kirimi L, Makau J (2019). Sorghum Production in Kenya: Farm-level Characteristics, Constraints and Opportunities. Tegemeo Institute of Agricultural Policy and Development, Egerton University. Nairobi, Kenya. |
|
|
Nkhata SG, Ayua E, Kamau EH, Shingiro JB (2018). Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Science and Nutrition 6(8):2446-2458. |
|
|
Okolo BN, Moneke AN, Nwagu TN, Nnamchi CI (2020). Influence of malted barley and exogenous enzymes on the glucose/maltose balance of worts with sorghum or barley as an adjunct. Journal of the Institute of Brewing 126(1):46-52. |
|
|
Omer F, Abou-zaid F (2022). Evaluation of coffee substitute produced from quinoa. Asian Research Journal of Current Science 4(1):125-133. |
|
|
Omoro W (2013). Factors for low sorghum production: A case study of small-scale farmers in East Kano sub location, Nyando District, Kenya. |
|
|
Orr A, Mwema C, Mulinge W (2014). The value chain for sorghum beer in Kenya. Socioeconomics Discussion Paper Series (16). |
|
|
Oseguera-Toledo ME, Contreras-Jimenez B, Hernandez-Becerra E, Rodriguez-Garcia ME (2020). Physicochemical changes of starch during malting process of sorghum grain. Journal of Cereal Science 95:103069. |
|
|
Osman MA (2011). Effect of traditional fermentation process on the nutrient and antinutrient contents of pearl millet during preparation of Lohoh. Journal of the Saudi Society of Agricultural Sciences 10(1):1-6. |
|
|
Pranoto Y, Anggrahini S, Efendi Z (2013). Effect of natural and Lactobacillus plantarum fermentation on in-vitro protein and starch digestibilities of sorghum flour. Food Bioscience 2:46-52. |
|
|
Rezac S, Kok CR, Heermann M, Hutkins R (2018). Fermented foods as a dietary source of live organisms. Frontiers in Microbiology 9:1785. |
|
|
Salazar-López NJ, Julieta N, Gonzalez-Aguilar G, Rouzaud-Sandez O, Robles-Sanchez M (2018). Technologies applied to sorghum (Sorghum bicolor L. moench): Changes in phenolic compounds and antioxidant capacity. Food Science and Technology 38(3):369-382. |
|
|
Sarkodie PA, Agyapong D, Dawuona S, Dwomoh AJ, Owusu-Ansah (2014). Improving the saccharification of sorghum mash with supplementary enzymes from local crops. Indian Journal of Scientific Research and Technology 2(3):71-78. |
|
|
Shahidi F, Ambigaipalan P (2015). Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects - A review. Journal of Functional Foods 18:820-897. |
|
|
Shegro A, Shargie NM, Biljon AV, Labuschagne MT (2012). Diversity in starch, protein and mineral composition of sorghum landrace accessions from Ethiopia. Journal of Crop Science and Biotechnology 15(4):275-280. |
|
|
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999). Analysis of Total Phenols and other oxidation substrates and Antioxidants by means of Folin-Ciocalteu Reagent. Methods in Enzymology 299(1974):152-178. |
|
|
Taylor JRN, Kruger J (2019). Sorghum and Millets: Food and Beverage Nutritional Attributes. Sorghum and Millets pp. 171-224. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0