ABSTRACT
In this study, jambolan and grape juices were used to produce polysaccharide-based edible films by the solvent-casting technique. The polysaccharides used were carboxymethyl cellulose, hydroxypropyl methyl cellulose, high-methoxyl pectin, low-methoxyl pectin, sodium alginate, and locust bean gum. The films exhibited good mechanical resistance and flexibility, with tensile strength (8 to 28 Mpa), elongation at break (6 to 36%), adhesion force (0.4 to 1.4 N), swelling index (1.0 to 2.3), and disintegration time (0.5 to 60 min) that varied as a function of the polysaccharide and the fruit juice used. The surface pH was, respectively, ~5.5 and ~4.6 for the films produced with grape and jambolan juices, regardless of the polysaccharide used. All films presented the typical color of the fruit juices, which was characterized by the L*, a* and b* parameters. The films produced with jambolan juice had the higher anthocyanin content (3.4 mg/g, d.b.) and antioxidant capacity (198 mMol Trolox equivalent/g, d.b.) when compared to those produced with grape juice (0.28 mg/g and 85 mMol Trolox equivalent/g, d.b.). The results are interesting for the food industry, specifically in edible or biodegradable films production, since alternative fruit juices can be used in food formulations and their natural compounds can replace synthetic additives.
Key words: Alternatives fruits, Syzygium cumini L., Vitis vinifera L., antioxidant, anthocyanin, natural colorants.
Edible films can be produced from polysaccharides as cellulose or starch derivatives, pectin, pullulan and gelatin (Borges et al., 2015; Prajapati et al., 2018; Tedesco et al., 2017). Many synthetic additives can be used for the formulation of edible films, including plasticizers, colorants, stabilizers, salivary secretion stimulants, buffer systems, sweeteners, taste masking agents, and palatability enhancers (Borges et al., 2015; Silva et al., 2015). Fruit juices contain mainly sugars, organic acids, phenolic compounds, vitamins and mineral elements, among others (Coelho et al., 2016; Gurak et al., 2010)and can be used in the formulation of edible films. Thus, glucose and fructose can contribute to the film sweetness and act as a matrix plasticizer, due to its low molecular weight (Olivas and Barbosa-Cánovas, 2008; Santacruz et al., 2015). Organic acids, besides contributing to the organoleptic characteristics of the films, can also act as salivary secretory stimulants. Phenolic compounds and anthocyanins can confer an attractive color and astringency, and provide antioxidant properties to the films.
Jambolan (Syzygium cumini L.) is an Asian native fruit, found in Brazil as an ornamental tree (Tavares et al., 2016). Its high anthocyanins content imparts an intense and attractive purple color to the bark (Silva et al., 2018) , as well as potential biological activities, including antioxidant capacity, anti-inflammatory properties, antibacterial, anti-ulcerogenic, cardioprotective, anticancer, anti-allergic properties, anti-diabetes effect, among others (Singh et al., 2018; Tavares et al., 2016). Grape juice is a product with high antioxidant potential, capable of combating the oxidative processes in the body (Mendes Lopes et al., 2016; Cosme et al., 2018). Studies have shown that its consumption can positively affect the risk factors associated with cardiovascular health, cancer, neurodegenerative diseases, and age-related cognitive decline (Blumberg et al., 2015; Vislocky and Fernandez, 2010; Wightman and Heuberger, 2015). Thus, the aim of this study was to use fruit juices (jambolan and grape) in the development of polysaccharides edible films.
Polysaccharides
Sodium carboxymethylcellulose (CEKOL 150, CPKelco, Brazil); hydroxypropyl methylcellulose (Benecel K4M PHARM, Ashland, Brazil); high-methoxyl pectin (GENU Pectin D Slow Set Z, CPKelco, Brazil); low-methoxyl pectin (GENU Pectin LM-102-AS-Z, CPKelco, Brazil); sodium Alginate (CAS 9005-38-3, Dinâmica Química, Brazil); and locust bean gum (GENU-GUM RL 200 Z, CPKelco, Brazil) were used.
Fruit juice extraction
Jambolan juice in natura was extracted from ripened jambolan fruits collected at State University of Campinas, Campinas - SP, Brazil, 22° 81’ 95” S, 47° 06’ 49. The fruits were sanitized with sodium hypochlorite solution (50 mg/L; 15 min) with a subsequent water rinse. The fruits were pulped in a brush-type pulping machine (Sterling Power System Inc. Lionel Corporation), packed in polyethylene bags (500 g) and stored at -20°C. The thawed pulp was filtrated in a 270 mesh sieve and centrifuged (Dammom IEC Model HN–S, USA) at 1100 x g for 15 min to obtain a clear juice
(~10 °Brix).
Grape juice concentrate (68 °Brix) was purchased from Golden Sucos, Brazil. The fruit juices were characterized for pH, acidity, and reducing sugars according to the AOAC methods (AOAC, 1997a, b, 2000)in triplicate, and the results were expressed on a dry basis (d.b.) (Table 1). The anthocyanins content and antioxidant capacity of the juices were also determined (Brand-Williams et al., 1995; Lee et al., 2005)and the determinations are described in subsequently.
Film production
The films were produced using the solvent-casting technique that consists in the dispersion of a film-forming solution (solution casting) on a plate surface followed by the evaporation of the solvent. The film-forming solutions (FFS) were prepared to contain 2% (w/w) and 1.5% (w/w) soluble solids from jambolan and grape juices, respectively. These concentrations were established to form a structural matrix sufficiently cohesive so that the film can be easily removed from the support without breaking. Jambolan or grape juices were mixed with the polysaccharides solution was prepared according to Table 2, using a magnetic stirrer until complete homogenization (10 min). To improve the grape juice dispersion into the polysaccharide solution, the concentrate grape juice was diluted to 20 °Brix, while jambolan juice was kept at 10°Brix. The FFS were dispersed in polyester plates (13.7 cm in diameter) and dried in an oven with air circulation at 30°C/16 h. The films were conditioned at 23±0.5°C and 33% relative humidity for 5 days before characterization. After conditioning, the moisture contents were 9.1±0.8 and 8.1±0.5% for the films produced with grape and jambolan juices, respectively. This film production process was repeated three times for each formulation.
Film characterization
Mechanical properties
The mechanical properties were determined using a TA.xT2i (TA Instruments, New Castle, USA) texture analyzer, 25 kg cell loading, according to the ASTM (1995)method D882-95, and carried out at 23°C and 40 to 50% relative humidity (RH). A grip separation and crosshead speed of 50 mm and 1 mm/s, respectively, were applied to the films (25 mm wide and 10 cm long). Film thickness was determined from the mean of five measurements across the films using a digital micrometer (Mitutoyo, Japan) with a range of 0 to 12.7 mm and an accuracy of 0.001 mm. The mean values were used for calculation of tensile strength, Young's modulus, and elongation at rupture. The typical film thickness was 0.044±0.004 and 0.055±0.007 mm for the films prepared with grape and jambolan juices, respectively. Mechanical measurements were done in triplicate.
Adhesion strength in vitro
The adhesion strength of the films was determined in a TA.xT2i texture analyzer (25 kg cell loading, TA Instruments, New Castle, USA) using gelatin gel as a buccal model (Okeke and Boateng, 2016)upon which the films were allowed to adhere. Gelatin (GELITA, 150 BLOM/30 MESH) was solubilized in water (10%, 90°C), placed in the Petri plate (5.2 cm in diameter and 1 cm height), cooled down, and stored under refrigerated storage overnight for gelling (5°C). The film (20 mm in diameter) was attached with double-sided adhesive tape to the Perspex support (20 mm), connected to the mobile arm of the texture analyzer. Artificial saliva (40 uL) was added onto the gelatin surface, and the film and the gelatin gel were allowed to adhere. The adhesion strength was measured as the maximum applied force (N) to detach the film from the gelatin gel. The contact force, contact time, and the speed of probe withdrawal during the adhesion experiment was fixed at 1 N, 10 s, and 0.5 mm/s, respectively.
Swelling index
Films with 20 mm in diameter were placed on a Petri plate (9.5 cm in diameter) containing 30 mL of artificial saliva, and the changes were measured at different time intervals up to constant diameter. The swelling index was measured in triplicate and calculated using Equation 1 (Nair et al., 2013), where At is the area of the film at time t, and A0 is the area of the film at time zero.
Swelling index = At/A0 (1)
In vitro disintegration time
The film was fixed on an acrylic cell (Figure 1) and 200 μL of artificial saliva was added onto the center of the support. The time the drop takes to disintegrate the film and reach the interior base of the cell was defined as the in vitro disintegration time (measured in triplicate).
Surface pH
The surface pH of the films was determined using artificial saliva, according to Prabhu et al. (2011)with modifications. The artificial saliva was prepared with phosphate buffer solution (pH = 7.1-7.2) and mucin from porcine stomach (SIGMA-ALDRICH, TYPE II) at 2 mg/mL (Sánchez et al., 2011). The film was placed inside a 5 mL flask (at the bottom and at the sides). The artificial saliva (~ 0.5 mL) was spread on the film surface. The electrode was placed in contact with the wetted film for 10 s for stabilization, and the pH was measured (in triplicate).
Color and opacity
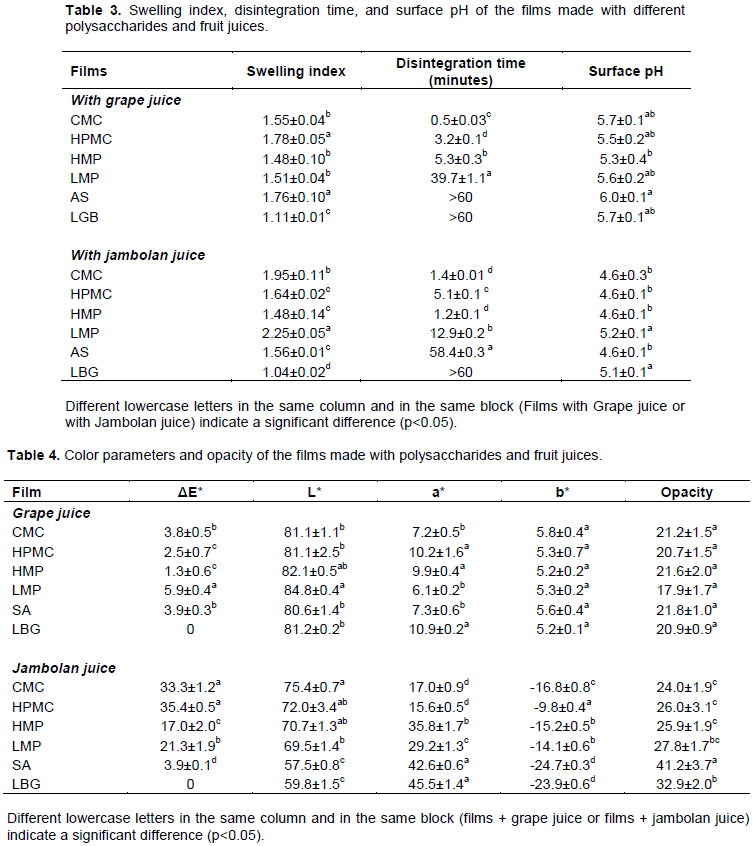
Film color was evaluated by L*, a*, and b* parameters, measured by transmittance using CIELab color scale. Film opacity was determined by reflectance, and calculated from the relationship between the opacity of the film over the black (Yb) and white (Yw) reflectance color standard. Determinations were carried out in triplicate, using a spectrophotometer UltralScan PRO D65 Hunterlab (Reston, USA) and the software EasyMatch QC. All determinations were made by placing the film surface in contact with the air in the direction of light. The color difference (ΔE) was calculated using Equation 2, where Ls*, as*, bs* are the color parameters of the locust bean gum (LBG) film (Table 3) made with grape juice or jambolan juice. The LBG film containing grape and jambolan juices showed high a* and low b* values, which allowed determining the differences (∆E) between the samples.
∆E = [(L* - Ls*)2 + (a*- as*)2 + (b*- bs*)2]1/2 (2)
Anthocyanins content
Anthocyanins content was determined by the differential method (Lee et al., 2005), in triplicate, in a dark environment at 20°C. The fruit juices were adjusted to 20 and 10 °Brix for grape and jambolan, respectively. While the films (0.3 g) made with or without fruit juices were dispersed in 30 g distilled water. When necessary, the samples were diluted with distilled water to obtain absorbance readings lower than 0.9. The samples were mixed with two buffer solutions separately: 0.025 M potassium chloride buffer (pH 1.0) and 0.4 M sodium acetate buffer (pH 4.5). The absorbance of the buffer solutions was measured at 520 and 700 nm. Anthocyanin content was expressed as cyanindin-3-glucoside equivalents according to Equation (3), and then converted to a dry basis:
Anthocyanin content (mg/L) = [A x MW x DF x 103]/[Æ x PL] (3)
Where A = [(A520nm–A700nm)pH 1.0 – (A520nm–A700nm)pH 4.5]; MW (molecular weight) = 449.2 gmol-1, for cyaniding-3-glucoside (cyd-3-glu); DF = dilution factor; PL = path length, 1 cm; e = 26,900 molar extinction coefficient (Lmol-1cm-1), for cyd-3-glu; and 103 = conversion factor from g to mg.
Antioxidant capacity
The antioxidant capacity was determined by DPPH assay (Brand-Williams et al., 1995), in triplicate, in a dark environment at 20°C. Films (0.3 g) were dispersed in 30 g distilled water, and the fruit juices were adjusted to 20 and 10 °Brix for grape and jambolan, respectively. When necessary, the samples were diluted with distilled water to obtain absorbance readings lower than 0.9. Sample or Trolox standard (0 – 2000 mM in ethanol) aliquots of 50 mL were mixed to 4.95 mL of 0.06 mM DPPH solution (in ethanol) in dark and kept for 16 h to achieve a constant concentration of remaining DPPH for grape juice concentrate. After the reaction, the absorbance was measured at 517 nm, and the results were expressed as Trolox equivalent antioxidant capacity.
Statistical analysis
The results were submitted to analysis of variance (ANOVA) and Tukey's comparison test to identify the differences at a 5% level of significance, using the software SAS 9.4.
Mechanical properties
The edible films made with different polysaccharides with the addition of the grape and jambolan juices were visually homogeneous and with no insoluble particles. In general, the mechanical properties values of the films varied from 7 to 27 Mpa (tension at rupture - TR), 20 to 570 MPa (Young's Modulus - MY), and from 6 to 36% (elongation at rupture - ER) (Figure 2). It is recommended that these films have sufficient tension to be handled without breaking during packaging or handling, but not so flexible to easily extend and deform during cutting in the production line (Borges et al., 2015). The present results were close to those reported for films made with hydroxypropylmethylcellulose or hydroxypropylcellulose with different medicinal plants extracts (TR = 0.2 - 2.5 Mpa; ER = 6 - 70%; MY = 23 - 321 Mpa, thickness = 69 - 192 mm) (Visser et al., 2016), and those reported for films made with high methoxyl pectin, glucomannan, methylcellulose and their blends (TR = 37 - 73 Mpa; ER = 2 - 10%; thickness = 19 - 28 mm) (Chambi and Grosso, 2011a).
Regardless of the type of juice used in the formulation, the films produced with SA, HMP, LMP, and HPMC were resistant to handling, without damages during the characterization process carried out at 23°C and 40 - 50% RH. Above 50% RH, the films were sticky, due to the presence of sugar from the fruits that are hygroscopic in high RH, which should be considered for future applications. Therefore, all characterizations were carried out at 40 to 50% RH (Figure 2).
Adhesion strength
The results of maximum adhesive strength of the films varied between 0.4 and 1.4 N (Figure 3), depending on the polysaccharide and the fruit juice used. The films made with jambolan juice had higher adhesion strength than those made with grape juice (Figure 4). The films made with jambolan juice had lower soluble solids when compared to the films made with grape juice. Moreover, the jambolan juice presented the lowest reducing sugar content (Table 1). The results are interesting from the technological point of view, once a smaller amount of jambolan juice was required to produce films with good resistance and flexibility, and high adhesive strength. Studies have shown that the higher the amount of additives the higher the adhesive strength (Perumal et al., 2008).
The films made with CMC and SA exhibited a greater adhesion to the buccal surface model, while a lower adhesion was observed for the films made with HPMC and LBG (Figure 4). During the contact process, the film adsorbs the artificial saliva on the buccal mucosa model and hydrates, initiating the interpenetration of polymer chains within the model buccal layer and vice versa. The adhesion effect is probably due to the high number of hydroxyl and carboxyl groups of polysaccharides, which improves the hydrogen bonding to the mucosa. Electrostatic interactions may also be formed with polysaccharides, loaded as CMC and SA. These polysaccharides facilitate strong interactions with the mucosa, resulting in high adhesive strength. Studied have shown that charged polymers increase the adhesion by leading to stronger bonds between the film and the buccal surface (Morales and McConville, 2011; Okeke and Boateng, 2016).
Swelling index
The swelling index of the films was dependent on the type of juice and the polysaccharide used in the formulation (Table 3). Films made from LMP and jambolan juice had the highest swelling index (2.3), while the LBG films exhibited the lowest index (1.1) for both juices. These results ​​were similar to those reported for films made with methylcellulose and hydroxypropylmethylcellulose blends, which presented a swelling index from 1 to 1.5 (Attama et al., 2008). A higher swelling index indicates a greater hydration capacity of the polymer film. The hydration capacity of the film is an important characteristic in the manufacture of films, once it is related to the adhesive strength and the ease of release of compounds naturally present in juices (Mahcene et al., 2020; Piñeros-Hernandez et al., 2017).
In general, the higher the swelling index, the greater the adhesive strength (Mortazavian et al., 2014). For the films produced with grape juice, the adhesive strength (Figure 3) was directly related to the swelling index (Table 4), with an exception for the film made with HPMC. This relationship was not observed for the films made with jambolan juice. Although sugars and organic acids from fruit juices act as additives in the film production, the minor components as anthocyanins (present in a higher proportion in the jambolan juice) also contribute with the different interactions in the polymeric matrix, resulting in films with different values of swelling index. Each polysaccharide has a particular structure with hydroxyl groups that in its turn could form hydrogen bonding with the anthocyanin hydroxyl groups. Starch-BBE (anthocyanin-rich bayberry extract) films had their properties modified (water vapor permeability, tensile strength, UV-vis light barrier, among others) due to the presence of anthocyanins in BBE (Yun et al., 2019).
Disintegration time
The disintegration time of the films changed according to the type of polysaccharide used (Table 3). Films made with CMC, HPMC and HMP had the lower disintegration times (0.5 to 5.3 min), while those made with SA and LBG took longer to disintegrate (60 min). Both fast and slow disintegrating films can have pharmaceutical applications. Due to the rapid disintegration time (30 s) of the CMC films made with grape juice, they can be easily administered in people with dysphagia, nausea, vomiting and mental disorders (Sudhakar et al., 2006). HPMC and HMP films can be used when the continuous release of the active ingredient is required within a few minutes. On the other hand, the SA and LBG films can be used as patches for applications requiring long periods of time, which should be removed at the end of the application. The use of SA and LBG in the production of adhesive films has the advantage of preventing the use of organic solvents generally used in the solubilization of polymers for the manufacture of insoluble films.
Surface pH
The polysaccharide films containing both grape and jambolan juice presented a surface pH (Table 4) near the pH of saliva (5-7) (Sudhakar et al., 2006). Thus, the consumption of these films should not cause irritation to the oral mucosa. Oral films produced from NaCMC (matrix), glycerol (plasticizer) and nystatin (an antifungal agent) had similar results, pH = 5 to 5.4 (Gajdošová et al., 2016).
Color and opacity
All films presented the typical color of the fruit juice used in their formulations. Figure 4 illustrates the jambolan fruit, and the film resulting from the addition of juice to the film formulation. The films with jambolan juice exhibited a bright purple color, while those made with the addition of grape juice were purplish blue.
The color of the films was determined by the parameters L*, a*, b* and ΔE* (Table 4), which varied according to the polysaccharide and the fruit juice used in the formulation. The natural pigments present in the juices and the polysaccharides used in the formulation can provide great differences in color between the films, resulting in differences in consumer perception. The films made with grape juice presented no significant variations in L* and b*, as a function of the polysaccharide, with small variations for a* and ΔE*. In contrast, the films made with jambolan juice presented the most significant variations in L*, a*, b* and ΔE* depending on the polysaccharide used.
Anthocyanins are a group of water-soluble flavonoids that are responsible for the bright red, blue and purple colors in fruits like jambolan (Jampani et al., 2014). The anthocyanin concentration in jambolan juices was higher when compared to the grape juice (Table 1), thus significant differences were observed for the films produced with jambolan juices, probably due to the anthocyanins stability in the film matrix. The anthocyanin stability is affected by several factors such as pH, temperature, light, presence of copigments, metal ions, oxygen, enzymes, ascorbic acid, sugars, and their degradation products (Fang et al., 2020). The surface pH values were similar for the films containing jambolan juices (Table 4), which affected the color to a lesser extent. All films were prepared under the same conditions, thus the parameters temperature and light did not affect the color of the films produced.
In nature, anthocyanin molecules are normally associated with colorless molecules (copigments) that affects the plant color (Falcão et al., 2003; Fan et al., 2019). The different interactions between anthocyanins (present in a higher proportion in the jambolan juice) and the polysaccharides (copigments) resulted in different color parameters and intensity of bright purple color. This type of copigmentation is known as being intermolecular (Lopes et al., 2007).
The film opacity was dependent on both the type of polysaccharide and the fruit juice. In general, the films were translucent, presenting low opacity, with values around 20 and 25 for the films containing grape juice and jambolan juice, respectively. Only the LBG and SA films had high opacity values (33 and 41, respectively), which resulted in an unattractive color for the films made with jambolan juice. Different values of film transparency are related to their internal structure that is defined by a component rearrangement in the film matrix during the drying process (Chambi and Grosso, 2011b).
Anthocyanins content and antioxidant capacity
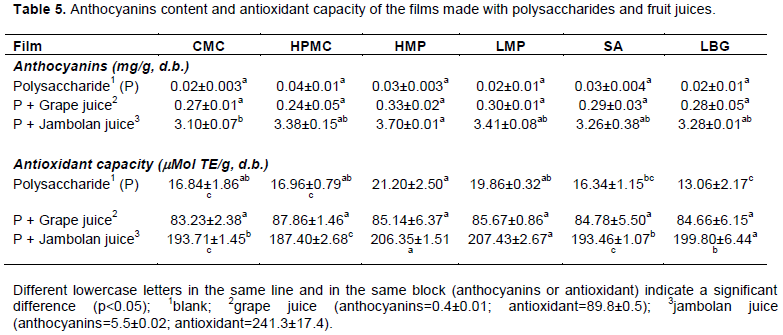
The total monomeric anthocyanins content and the antioxidant capacity of the films were similar to the values found for the fruit juices used in the formulations (Table 5). These results indicate that the manufacturing process did not lead to significant losses of the functional properties. The anthocyanins content and the antioxidant capacity of the films made with jambolan juice (Table 5) were higher than those observed for the films made with grape juice (Table 1), due to the higher anthocyanins levels of jambolan juice. The antioxidant capacity of the films produced only with polysaccharides was related to their ability to remove free radicals such as DPPH (Wang et al., 2018) acting as antioxidants to protect living organism from oxidative damage (Wang et al., 2016a). This antioxidant ability will vary depending on the type of polysaccharide (Wang et al., 2018). Polysaccharides such as pectins from grapefruit peel, apple pomace and citrus peel presented antioxidant capacity that would be related to the hydroxyl groups presents in the pectin structure (Wang et al., 2016b, 2014). The anthocyanin concentration was similar for all films prepared with the same fruit juice, with a mean anthocyanin content of 3.4±0.2 for the films containing jambolan juice (Table 5). Therefore, it was possible to produce films with different color intensities with similar anthocyanin concentrations (Table 4). The films showed antioxidant capacity, which together with the attractive color make the fruit juices potential ingredients for the production of edible films.
In the polysaccharide based edible films, glucose, fructose, organic acids, phenolics, and anthocyanins from fruit juices acted as synthetic additives replacer as well as active ingredients due to their antioxidant potential. The properties of the films were mainly modulated by the different polysaccharides used, and allow several applications, including those that require a rapid disintegration (CMC and HPMC, 0.5 and 3.2 min, respectively) and long application (SA and LGB, 58.4 and > 60 min, respectively) of the active ingredient. The films presented an attractive color, and those made with jambolan juice stood out among the others. The results are useful for the food and pharmaceutical industry since alternative fruit juices can be used in food formulations or as drug delivery matrices in the oral cavity.
The authors have not declared any conflict of interests.
The authors extends their appreciation to coordination for the Improvement of Higher Education Personnel (CAPES) for the Postdoctoral Fellowship of the first author and to the State University of Campinas for the Undergraduate Research Fellowship of the third and fourth authors as well as for the installation and equipment used.
REFERENCES
|
AOAC (1997a). Method 981.12: pH of Acidified Foods. Washington, USA: Association of Official Analytical Chemist.
|
|
|
|
AOAC (1997b). Method 942.15: Acidity (Titratable) of Fruit Products. Washington, USA: Association of Official Analytical Chemist.
|
|
|
|
|
AOAC (2000). Method 923.09: Invert Sugar in Sugars and Syrups. Lane-Eynon General Volumetric Method, Washington, USA: Association of Official Analytical Chemist.
|
|
|
|
|
ASTM (1995). D882-95a. Standard Test Method for Tensile Properties of Thin Plastic Sheeting. New York, United States.
|
|
|
|
|
Attama AA, Akpa PA, Onugwu LE, Igwilo G (2008). Novel buccoadhesive delivery system of hydrochlorothiazide formulated with ethyl cellulose- hydroxypropyl methylcellulose interpolymer complex. Scientific Research and Essay 3(6):343-347.
|
|
|
|
|
Blumberg JB, Vita JA, Oliver Chen CY (2015). Concord grape juice polyphenols and cardiovascular risk factors: Dose-response relationships. Nutrients 7(12):10032-10052.
Crossref
|
|
|
|
|
Borges AF, Siva C, Coelho JFJ, Simões S (2015). Oral films : Current status and future perspectives I - Galenical development and quality attributes. Journal of Controlled Release 206:1-19.
Crossref
|
|
|
|
|
Brand-Williams W, Cuvelier ME, Berset C (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology 28(1):25-30.
Crossref
|
|
|
|
|
Chambi HNM, Grosso CRF (2011a). Mechanical and water vapor permeability properties of biodegradables films based on methylcellulose, glucomannan, pectin and gelatin. Food Science and Technology 31(3):739-746.
Crossref
|
|
|
|
|
Chambi HNM, Grosso CRF (2011b). Effect of surfactants on the functional properties of gelatin-polysaccharide-based films. European Food Research and Technology 232:63-69.
Crossref
|
|
|
|
|
Coelho EM, de Azevêdo LC, Corrêa LC, Bordignon-Luiz MT, Lima MDS (2016). Phenolic Profile, Organic Acids and Antioxidant Activity of Frozen Pulp and Juice of the Jambolan (Syzygium Cumini). Journal of Food Biochemistry 40(2):211-219.
Crossref
|
|
|
|
|
Cosme F, Pinto T, Vilela A (2018). Phenolic compounds and antioxidant activity in grape juices: A chemical and sensory view (Review). Beverages 4:1-14
Crossref
|
|
|
|
|
Falcão LD, Barros DM, Gauche C, Luiz MTB (2003). Copigmentação intre e intermolecular de antocianinas: Uma revisão. Boletim Centro de Pesquisa de Processamento de Alimentos 21:351-366.
Crossref
|
|
|
|
|
Fan L, Wang Y, Xie P, Zhang L, Li Y, Zhou J (2019). Copigmentation effects of phenolics on color enhancement and stability of blackberry wine residue anthocyanins: Chromaticity, kinetics and structural simulation. Food Chemistry 275:299-308.
Crossref
|
|
|
|
|
Fang J, Luo Y, Yuan K, Guo Y, Jin S (2020). Preparation and evaluation of an encapsulated anthocyanin complex for enhancing the stability of anthocyanin. LWT - Food Science and Technology 117:108543.
Crossref
|
|
|
|
|
Gajdošová M, Vetchý D, Doležel P, Gajdziok J, Landová H, Muselík J, Jekl V (2016). Evaluation of mucoadhesive oral films containing nystatin. Journal of Applied Biomedicine 14(4):247-256.
Crossref
|
|
|
|
|
Gurak PD, Cabral LMC, Rocha-Leão MHM, Matta VM, Freitas SP (2010). Quality evaluation of grape juice concentrated by reverse osmosis. Journal of Food Engineering 96(3):421-426.
Crossref
|
|
|
|
|
Jampani C, Naik A, Raghavarao KSMS (2014). Purification of anthocyanins from jamun (Syzygium cumini L.) employing adsorption. Separation and Purification Technology 125:170-178.
Crossref
|
|
|
|
|
Lee J, Durst RW, Wrolstad RE (2005). Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. Journal of AOAC International 88(5):1269-1278.
Crossref
|
|
|
|
|
Lopes TJ, Xavier MF, Gabriela M, Quadri N, Quadri MB (2007). Antocianinas: Una breve revisão das características estruturais e da estabilidade. Revista Brasileira de Agrociência 13:291-297.
|
|
|
|
|
Mahcene Z, Khelil A, Hasni S, Kubra P, Bozkurt F, Birech K, Tornuk F (2020). Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants. International Journal of Biological Macromolecules 145:124-132.
Crossref
|
|
|
|
|
Mendes Lopes ML, Miguel MAL, Fialho E, Valente-Mesquita VL (2016). Grape juice obtained using steam extraction and other small-scale extraction methods: phenolic content, antioxidant capacity and stability during storage. International Journal of Food Science and Technology 51(7):1696-1702.
Crossref
|
|
|
|
|
Morales JO, McConville JT (2011). Manufacture and characterization of mucoadhesive buccal films. European Journal of Pharmaceutics and Biopharmaceutics 77(2):187-199.
Crossref
|
|
|
|
|
Mortazavian E, Dorkoosh FA, Rafiee-Tehrani M (2014). Design, characterization and ex vivo evaluation of chitosan film integrating of insulin nanoparticles composed of thiolated chitosan derivative for buccal delivery of insulin. Drug Development and Industrial Pharmacy 40(5):691-8.
Crossref
|
|
|
|
|
Nair AB, Kumria R, Harsha S, Attimarad M, Al-Dhubiab BE, Alhaider IA (2013). In vitro techniques to evaluate buccal films. Journal of Controlled Release 166(1):10-21.
Crossref
|
|
|
|
|
Okeke OC, Boateng JS (2016). Composite HPMC and sodium alginate based buccal formulations for nicotine replacement therapy. International Journal of Biological Macromolecules 91:31-44.
Crossref
|
|
|
|
|
Olivas GI, Barbosa-Cánovas GV (2008). Alginate-calcium films: Water vapor permeability and mechanical properties as affected by plasticizer and relative humidity. LWT - Food Science and Technology 41(2):359-366.
Crossref
|
|
|
|
|
Perumal VA, Lutchman D, Mackraj I, Govender T (2008). Formulation of monolayered films with drug and polymers of opposing solubilities. International Journal of Pharmaceutics 358(1-2):184-191.
Crossref
|
|
|
|
|
Piñeros-Hernandez D, Medina-Jaramillo C, López-Cordoba A, Goyanes S (2017). Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocolloids 63:488-495.
Crossref
|
|
|
|
|
Prabhu P, Malli R, Koland M, Vijaynarayana K, D′Souza U, Harish N, Charyulu R (2011). Formulation and evaluation of fast dissolving films of levocitirizine di hydrochloride. International Journal of Pharmaceutical Investigation 1(2):99-104.
Crossref
|
|
|
|
|
Prajapati VD, Chaudhari AM, Gandhi AK, Maheriya P (2018). Pullulan based oral thin film formulation of zolmitriptan: Development and optimization using factorial design. International Journal of Biological Macromolecules 107:2075-2085.
Crossref
|
|
|
|
|
Sánchez GA, Miozza V, Delgado A, Busch L (2011). Determination of salivary levels of mucin and amylase in chronic periodontitis patients. Journal of Periodontal Research 46(2):221-227.
Crossref
|
|
|
|
|
Santacruz S, Rivadeneira C, Castro M (2015). Edible films based on starch and chitosan. Effect of starch source and concentration, plasticizer, surfactant's hydrophobic tail and mechanical treatment. Food Hydrocolloids 49:89-94.
Crossref
|
|
|
|
|
Silva BMA, Borges AF, Silva C, Coelho JFJ, Simões S (2015). Mucoadhesive oral films: The potential for unmet needs. International Journal of Pharmaceutics 494(1):537-551.
Crossref
|
|
|
|
|
Silva WP, Nunes JS, Gomes JP, Silva CMDPS (2018). Obtaining anthocyanin from jambolan fruit: kinetics, extraction rate, and prediction of process time for different agitation frequencies. Food Science and Nutrition 6:1664-1669.
Crossref
|
|
|
|
|
Singh B, Singh JP, Kaur A, Singh N (2018). Insights into the phenolic compounds present in jambolan (Syzygium Cumini) along with their health-promoting effects. International Journal of Food Science and Technology 53:2431-2447.
Crossref
|
|
|
|
|
Sudhakar Y, Kuotsu K, Bandyopadhyay AK (2006). Buccal bioadhesive drug delivery - A promising option for orally less efficient drugs. Journal of Controlled Release 114(1):15-40.
Crossref
|
|
|
|
|
Tavares IMDC, Lago-Vanzela ES, Rebello LPG, Ramos AM, Gómez-Alonso S, García-Romero E, Hermosín-Gutiérrez I (2016). Comprehensive study of the phenolic composition of the edible parts of jambolan fruit (Syzygium cumini L. Skeels). Food Research International 82:1-13.
Crossref
|
|
|
|
|
Tedesco MP, Monaco-lourenço CA, Carvalho RA (2017). Characterization of oral disintegrating film of peanut skin extract - Potential route for buccal delivery of phenolic compounds. International Journal of Biological Macromolecules 97:418-425.
Crossref
|
|
|
|
|
Vislocky LM, Fernandez ML (2010). Biomedical effects of grape products. Nutrition Reviews 68(11):656-670.
Crossref
|
|
|
|
|
Visser JC, Eugresya G, Hinrichs WLJ, Tjandrawinata RR, Avanti C, Frijlink HW, Woerdenbag HJ (2016). Development of orodispersible fi lms with selected Indonesian medicinal plant extracts. Journal of Herbal Medicine 7:37-46.
Crossref
|
|
|
|
|
Wang X, Chen Q, Lü X (2014). Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocolloids 38:129-137.
Crossref
|
|
|
|
|
Wang J, Hu S, Nie S, Yu Q, Xie M (2016a). Reviews on mechanism of in vitro antioxidant activity of polysaccharides. Oxidative Medicine and Cellular Longevity 2016:1-13.
Crossref
|
|
|
|
|
Wang W, Ma X, Jiang P, Hu L, Zhi Z, Chen J, Liu D (2016b). Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocolloids 61:730-739.
Crossref
|
|
|
|
|
Wang JM, Sun XY, Ouyang JM (2018). Structural characterization, antioxidant activity, and biomedical application of Astragalus polysaccharide degradation products. International. Journal of Polymer Science 2018:1-13.
Crossref
|
|
|
|
|
Wightman JD, Heuberger RA (2015). Effect of grape and other berries on cardiovascular health. Journal of the Science of Food and Agriculture 95(8):1584-1597.
Crossref
|
|
|
|
|
Yun D, Cai H, Liu Y, Xiao L, Song J, Liu J (2019). Development of active and intelligent films based on cassava starch and chinese bayberry (Myrica rubra Sieb. Et Zucc.) anthocyanins. Royal Society of Chemistry 9:30905-30916.
Crossref
|
|