Full Length Research Paper
ABSTRACT
Wine, a fruit-based fermented and non-distilled product, containing most of the nutrients in the raw fruit. Wine production, particularly grape wine, is one of the oldest technologies in the history of man. Other fruits such as pineapple, have been used for wine production. The research was conducted to determine the phytochemical characteristics and consumer acceptability of watermelon wine produced using four commercial Saccharomyces cerevisiae strains (Lalvin EC 1118, Lalvin QA 23, Redstar star rouge and Redstar premier classique). The data obtained on the phytochemical and free radical scavenging activities were analyzed using Tukey Pair Wise comparison at 95% confidence level for statistical difference. Sensory data was translated into means and ranked. The average mean of total phenols ranged in the young wine samples from 93.36 to 105.33 GAEmg/g) and that of the matured samples wine from 156.08 to 275.99 GAEmg/g). Flavonoids was 35.29 to 52.48 and 34.72 to 58.38 in the young and mature wines, respectively. The mean antioxidant activity and antioxidant capacity ranged between 1.28 to 13.59; 79.14 to 246.72 in the young wine samples and that of the matured wine samples 1279.93 to 1914.02 and 808.62 to 2219.37, respectively. Any of the four commercial yeast strains used in this study was suitable for watermelon winemaking. However, it evinced that the Redstar premier classique produced the most desirable sensory attributes, followed by the Lalvin EC 1118 yeast strains
Key words: Sacchromyces cerevisiae, wine, antioxidant, phytochemicals, scavenging activity, watermelon.
INTRODUCTION
Wine, a fruit-based fermented and a non-distilled product contains most of the nutrients present in the raw fruit. Therefore, fruits are important sources of compounds demonstrating beneficial effects on human health, of which one of the most attention-grabbing compounds for researchers today is dietary polyphenol (Mojzer et al., 2016). These dietary polyphenols are termed antioxidants because they can scavenge radicals in the biological system (Zhou et al., 2016). However, in the raw form of fruits, polyphenols are strongly bonded together, making it less soluble and accessible when eaten raw. In contrast, in wine, polyphenols are present in the soluble form rendering them biologically accessible (Mojzer et al., 2016).
Wine production involves the metabolism of soluble sugars (glucose and fructose) into ethanol and carbon dioxide. Wine is a rich source of most polyphenols. Antioxidant characteristics of red wine have been linked to the materials phytochemical profile (Lingua et al., 2016). Wine production is one of the oldest technologies in the human era (Morrison and Rabellotti, 2017). Nevertheless, wine gained popularity in the 17th century due to the effective use of sulphur in the sterilization of storage barrels which paved the way for the production of highly recommended wines and their associated increased shelf-life capacities (Raposo et al., 2018). Furthermore, the clinical benefits of wine, such as the prevention of cardiovascular diseases and cancer, influenced consumers to shift from consumption of other alcoholic beverages such as beer to wine (Pavlidou et al., 2018). Hence, it is now one of the most famous drinks in the world (Morrison and Rabellotti, 2017).
According to the European Union, wine is legally classified as the fermented juice of grapes; however, several plant fruits can be fermented to produce the alcoholic beverage (Saranraj et al., 2017), by using yeast strains (Saranraj and Ramesh, 2019). Different fruits can be used for wine production due to their sugar content, which is the main substrate for alcoholic fermentation. For instance, one of the widely known non-grape fruit wine is apple fruit wine (cider) (Saranraj et al., 2017). However, non-grape fruit must with little natural sugar could be seeded with table sugar to spur the fermentation process (Saranraj et al., 2017).
In the development of novel foods, fruits are mostly seen as significant rich sources as they contribute to the sensory characteristics in foods and serve as bioactive compounds such as vitamins, dietary fibre and polyphenols (Saranraj and Ramesh, 2019). Unfortunately, most fruits have a shorter shelf-life at the farm gate and on the market shelves. The postharvest loss of fruits even worsens in exportation; fruits that do not meet export regulations are left to waste, resulting in financial loss to the farmers (Yusufu et al., 2018). Saranraj et al. (2017)opined that oenology and other alcoholic beverage drinks industries are the recommended ‘bail out’ measures to salvage the menace in fruit production farms. Fruits such as apple, pear and strawberry, cherries, plum, banana, pineapple, oranges, cucumber, watermelon and guava have been used for home-making wine (Velic et al., 2018).
Watermelon (C. lanatus) from the Cucurbitaceae family harbours quite several bioactive compounds besides vitamins A and C, which are available in most fruits (Romdhane et al., 2017). Watermelon is well known for its ability of “thirst quenching”, it is also a rich source of lycopene (Romdhane et al., 2017). Earlier clinical studies reported that lycopene has antioxidant activity and free scavenging property on biological systems (Przybylska, 2020). The nutritional profile of watermelon reported by Wong et al. (2016), revealed that carbohydrates, sodium, vitamins, minerals, fatty acids, and amino acids constitute the important nutrient content of watermelon. Wang et al. (2018) compared fermented and unfermented watermelon juice and reported that fermented juice showed strong antioxidant activity. Similarly, blended watermelon juice and ginger extract fermented into wine revealed that watermelon wine demonstrates improved scavenging activity, hence a promising raw material for fruit winemaking (Yusufu et al., 2018). Despite the numerous promising fruits for wine-making, watermelon has not attracted much attention for wine making from non-grape juice.
The roles of fruits in human nutrition have led most nutritionists to recommend their daily consumption; in line with that, fruit production within the tropics has substantially increased, resulting in postharvest losses in a chunk of the produce. Moreover, the short shelf-life of fruits continues to be the major challenge (Mundaragi and Thangadurai, 2017) that bedevils fruit production worldwide, particularly in developing countries such as Ghana. Postharvest losses in developing countries like Ghana is projected at 20 to 50% of fruits, vegetables, and root and tubers annually (Yakubu et al., 2018), and that of watermelon is not different. Considering the percentages of postharvest loss in Ghana, it shows that it is not only a wastage of food products but also a waste of investment and a burden on the economy as the country spends more to import the same fruits from neighbouring countries such as Burkina Faso, especially at their off-season (Addo et al., 2015).
To stem challenges confronting the fruit production sector, efforts have been made to use fruits for alcoholic beverage production (Saranraj and Ramesh, 2019). Therefore, using watermelon juice for wine production could serve as one of the additional attractive measures to help curb the postharvest loss of the fruit. Considering the nutrient composition of watermelon, its wine is hypothesized to exhibit clinical benefits as other wines (Umeh et al., 2021). Furthermore, food or beverage is worth producing, distributing or marketing when its organoleptic characteristics are accepted (Raposo et al., 2018). Therefore, this study aimed to evaluate the polyphenols, antioxidant activities and sensory attributes of watermelon wine before and after storage by exploring different commercial yeast strains.
MATERIALS AND METHODS
Experimental design and sample preparation
Fresh whole watermelon (Charleston grey) varieties were purchased from the Tamale central market in the Northern Region of Ghana. A 3x4 factorial experimental design was used for the research. The factors include the maturation period (three months) and yeast type (four strains). The watermelon juice was fermented at 25°C with 3 grams of the four differently sourced yeast strains (Lalvin EC 1118, Lalvin QA 23, Redstar premier classique and Redstar premier rouge). The finished wines were then matured with oak chips (French oak chips) in glass bottles for three months (90 days). All four (4) treatments (the yeast strains) were in triplicates. The production and sensory evaluation of the wine samples were done at the Family and Consumer Science Food laboratory, University for Development Studies. The phytochemical quantification was carried out at the Cocoa Research Institute of Ghana (CRIG), Tafo.
Juice preparation
The sample preparation was done following modification by Darman et al. (2010). First, all containers and equipment were thoroughly sterilized (with 1% sodium metabisulphite), and fruits were processed under aseptic conditions. Next, the watermelons were washed using potable water and rinsed with 1% sodium metabisulphite. Instead of grating and mixing with water before filtration, as stated in the above protocol, the watermelon fruits were cut into pieces separating the red flesh from the rind and seeds. The red flesh (without the seeds) was extracted by manually squeezing out the juice, and clear juice was obtained by filtration with a sterilized filter cloth. Approximately 20 l of the juice was extracted for the experiment. Thus five (5) liters of juice was used for each treatment. The clear juice was then transferred to the fermenting drum (bioreactor). Approximately 1 g of tartaric and citric acids each was added to the clear juice per litre to adjust the pH from 5.15 to 3.5; the must also was chaptalized with table sugar to increase the °Brix from 6 to 20 concentration. However, following protocol, no acid (tartaric and citric acids) was used to adjust pH level of the must. Also, sugar cane liquid was used for the juice chaptalization in the protocol. Sulfiting was employed with a pill of Campden tablet pre-dissolved in 3 ml of distilled water and added to the five (5) liters of clear juice for sterilisation. The set-up was left on the laboratory bench for 24 h at room temperature (25°C) before being pitched with the yeasts.
Yeasts conditioning, pitching and fermentation
The modified protocol by Darman et al. (2010), coupled with the manufacturer’s protocol, was followed for the starter culture conditioning. The yeasts were used in the rehydrated form as the fermentation agent. Averagely, 3 g of each yeast was rehydrated in an aliquot (30 ml) of the must to make a slurry and allowed to stand for about 10 min before being pitched with the fermentable must. The fermentation proceeded for 7 days at room temperature (25°C) in airtight high-density polyethylene (HDPE) plastic fermenters.
Wine clarification and fining
After seven days, the fermentation process was terminated by flocculating with a yeast cell agent (Bentonite). The bentonite slurry was prepared by mixing 3 tablespoons of the bentonite with a pint of warm water (45°C) in a saucepan per the manufacturer’s protocol. Averagely, two tablespoons of bentonite slurry was added to five litres of the wine and stirred vigorously while adding the slurry. According to the manufacturer’s protocol, the lees was allowed to settle for about 12 h. After 12 hrs, the wine sample was siphoned. The wine was racked twice at 12 hrs before bottling and maturation. Before the bottling and maturation, samples were taken for phytochemical analyses and consumer acceptability evaluations.
Maturation and compositional data collection
After the thorough fining of the wine, the samples were filled into sterilized glass bottles and tightly corked. The bottled wine samples were then kept for three months in a dark cabinet in the Family and Consumer Science Food laboratory to mature. The data collection of this study was done in three phases: The first phase of data was obtained on the Young wine (before maturation) on the phytochemical contents, antioxidant characteristics and consumer acceptability. The second phase of data (phytochemical contents and antioxidant characteristics) was done when the wines were subjected to ageing for three months at room temperature (25°C), of which aliquots of the samples were drawn every 30 days’ interval for phytochemical quantification. Finally, the last phase of data (phytochemical and consumer acceptability) was obtained after the maturation period (3 months).
Phytochemical determination
The phytochemical contents quantified were as follows: Phenolics, anthocyanins, flavonoids and antioxidant characteristics as described following. Also, the sensory attributes evaluated include colour, aroma, taste, mouthfeel and overall acceptability.
Determination of total phenolic content
The phenolic content of the wine was quantified using the Folin-Ciocalteu procedure published (Prior et al., 2005). About 0.5 ml of the wine samples was mixed with 0.5 ml of Folin-Ciocalteu phenol reagent (Sigma Chemical Co., St. Louis, Mo., U.S.A.) and 7.5 ml of deionized water. The mixture was held at room temperature for 10 min, and 1.5 ml of 20% sodium carbonate (w/v) was added. The mixture was then heated at 30°C in a water bath for 20 min and cooled in an ice bath before measuring the absorbance at 760 nm using the UV/Vis spectrophotometer (JENWAY). A standard curve of gallic acid (ranging from 0 to 100 mg/l) was prepared, and the results were determined from a regression equation;
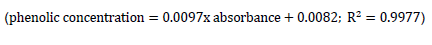
was then expressed as mg gallic acid equivalents per litre of wine (GAE/mg/g).
Determination of total flavonoid content
The total flavonoid content of the watermelon wine was quantified by a colourimetric method followed by Jia et al. (1999). Approximately 125 µl of the wine was added to 75 µl of 5% Sodium Nitrite (NaNO2) solution, and the mixture was allowed to stand for 6 min. Afterwards, 150 µl of aluminium trichloride (10%) was added and then incubated for 5 min, followed by an addition 750 µl of NaOH (1 M). The final volume of the mixture was then adjusted to 2500 µl with distilled water. The mixture was allowed to incubate for another 15 min before the absorbance was read at 510 nm wavelength against a prepared blank using a spectrophotometer. The flavonoid content was determined using a standard curve of catechin (0 to 100 mg/l), and the results were determined from a regression equation;
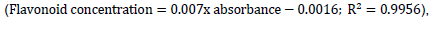
was expressed as mg catechin equivalents per litre of wine (CTE/mg/g).
Total anthocyanin content determination
The collaborative study methodology published by Lee et al. (2005) was followed to quantify the total anthocyanin content. The method employs pH differential on the structural change of the anthocyanin chromophore between pH values of 1.0 and 4.5. Anthocyanins have a maximum absorbance at a wavelength of 510 nm at a pH of 1.0. The coloured oxonium form predominates at a pH of 1.0, and the colourless hemiketal forms at a pH of 4.5. An aliquot of the wine (1ml) was added to 4 ml of buffer (KCl, 0.025 mol/l) with a pH of 1.0. The pH was adjusted with HCl (0.20 mol equi/l). One millilitre of the wine was also placed into a 10 ml volumetric flask, and 4 ml of the buffer (CH3CO2Na, 0.4 mol/l) with a pH of 4.5 was added. The pH was adjusted with HCl (0.20 mol equi/l). Absorbance was measured with a spectrophotometer at 510 and 700 nm. The total anthocyanin content (TAC) was calculated as the milligram equivalent of cyanidin 3-glucoside per litre as follows:
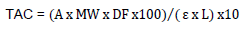
Where, A = (A520 nm - A700 nm) pH 1.0 - (A520 nm - A700 nm) pH 4.5; Molecular weight (MW) = 449.2 g/mol; Dilution factor (DF) = 10; L (path length in cm) = 1; ε (molar extinction coefficient) = 26,900 l/mol cm
Determination of antioxidant activity-DPPH assay
The antioxidant activity of the wine was determined using the 1, 1-diphenyl-2-picrylhydrazyl (DPPH) assay described by Yu et al. (2002). Approximately 1 ml of the wine sample each was mixed vigorously with 3 ml of 0.06 μM DPPH dissolved in methanol and was incubated at 25°C in darkness for 20 min instead of the 60 min as used in the stated protocol. Afterwards, the decrease in the absorption of DPPH after the wine sample was measured at 517 nm instead of 515 in the stated protocol. The DPPH radical scavenging activity (RSA) was calculated as follows:
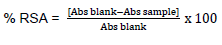
Where; Abs sample is the absorbance of the sample (wine), and Abs blank is the absorbance of the control (Distilled water)
Total antioxidant capacity determination
The antioxidant capacity of the treatments were determined using the protocol by Prieto et al. (1999). First, an aliquot of the watermelon wine (500 μl, diluted at 1:20) was combined with 5 ml of reagent solution (0.6 M H2SO4, 28 mM NaH2PO4, and 4 mM ((NH4)2MoO4) in a 15 ml test tube. The reaction mixture was incubated at 95°C for 90 min and then cooled at room temperature, and absorbance was measured at 695 nm. The total antioxidant capacity (TAC) was expressed as tannic acid equivalent (TAE).
Sensory evaluation of watermelon wine
Sensory analysis was conducted on wine samples from Lalvin EC 1118 wine, Lalvin QA 23 wine, Redstar premier rouge wine and Redstar premier classique wine following fermentation and ageing. The sensory evaluation was conducted on the following attributes; aroma, colour, taste, mouthfeel (astringency) and overall acceptability using a five-point hedonic scale described by Stone and Sidel (2004) and shown on the scale as 5 representing like very much, 4 representing like, 3 representing neither like nor dislike, 2 representing dislike and 1 representing dislike very much. Fifty-six (56) untrained panellists familiar with wine were used for the sensory evaluation. The untrained panellists were between 18 and 45 years comprising students (50) and staff (6) at the Nyankpala campus.
Statistical analysis
The data obtained was subjected to a two-way analysis of variance (ANOVA) using Genstat eighteenth edition and Tukey Pair Wise comparison used for mean separation at the confidence level of p < 0.05.
RESULTS AND DISCUSSION
Phytochemical characteristics of watermelon wine
The phytochemical characteristic of wine is of great concern in enology because of its contribution to clinical studies aside from its sensory influence. The watermelon wine’s phytochemical characteristics include total phenolics, anthocyanins, flavonoids, and antioxidant activity quantified before and after the maturation/ageing (Table 1).
Total phenolic content
The total phenolic content of the watermelon wine varied significantly (p=0.003) between treatments in both the young (sample after 7 days of fermentation) and the matured (90 days after initial fermentation) wine. In the young wine, the total phenolic content was statistically higher in the Redstar premier classique sample (105.33 µg/g) and lower in the Lalvin EC 1118 (98.69 µg/g). Similar phenolic concentrations were also recorded in the matured wine samples, with the least concentration of the phenolics in the Lalvin EC1118 (156.08 µg/g) and the highest in Redstar premier classique (275.99 µg/g). The maturation process impacted the concentration level of the phenolic content in all the treatments (Table 1). The phenolic concentration in the young wines was lower than the phenolic concentrations recorded in the matured wine samples.
This observation was contrary to what was reported by Baiano et al. (2016), that mostly phenolic concentration diminished with oak chip treatment in the course of maturation. Other authors reported that low evolution of anthocyanins and tannins during ageing decreases total phenolic concentration (Tzachristas et al., 2020). However, the samples were completely devoid of anthocyanins (Table 1). The Redstar premier classique and Redstar premier rouge samples recorded significantly higher concentrations in the young and matured wine samples (Table 1). The increase in phenolic concentrations could be linked to the evolution of additional compounds from the oak chips used for wine maturation (Jean-Louis et al., 2020). This disparity in phenolic contents confirms the earlier research that alcoholic medium extracts phytochemicals better (Mojzer et al., 2016).
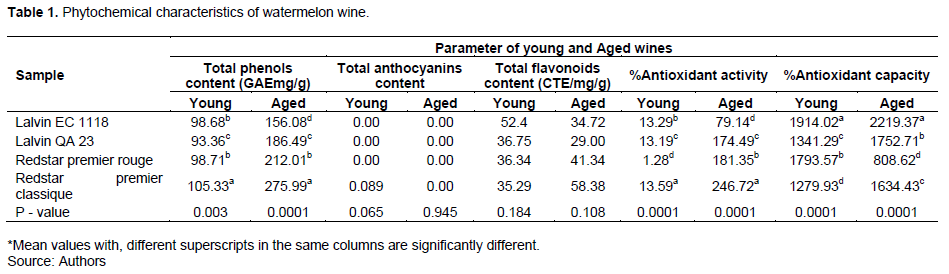
Total anthocyanins content
In the young wine, anthocyanins concentration was not detected in all the wine samples except in Redstar premier classique wine sample that recorded a minute concentration (0.089). However, anthocyanin was not detected in the matured wine samples. The absence of anthocyanins could be attributed to the report that yeast cells can adsorb anthocyanins during wine fermentation (Morata et al., 2019). Therefore, treatment did not show a significant difference in the young wines (p=0.065) and the matured/aged samples (p=0.945), respectively, as presented in Table 1 for the anthocyanins concentration.
Total flavonoids content
The mean concentrations of the flavonoids recorded in the young wines were 35.29 in the Redstar premier classique, 52.48 in the Lalvin EC 1118, 36.75 in the Lalvin QA 23 and 36.64 in that the Redstar premier rouge. After the maturation phase, the flavonoids quantified in the wines after the maturation period revealed that the least mean flavonoids concentration was observed in the Lalvin QA 23 (29.00), while Redstar premier classique (58.38) recorded the highest mean for total flavonoids content. However, Lalvin EC 1118 and Redstar premier rouge recorded 34.72 and 41.34 flavonoids concentration, respectively. The quantified flavonoid content appeared to correlate with the anthocyanins content in the watermelon wine samples. Generally, the flavonoid concentration was low in the watermelon wine samples (Mariya et al., 2020). The flavonoid concentration quantified in watermelon wines could be due to much of the flavonoids concentrated in the rind rather than the pulp since most of the polyphenols are localized in the skin of fruits, as reported by Mariya et al. (2020). Treatment Lalvin EC 1118 and Lalvin QA 23 samples recorded reduced flavonoid content after storage. This is in line with the report of Bala and Kocher (2017), who recorded a decrease in phenolic content in muskmelon wine during bottle storage. However, the trend was the opposite of the Redstar premier rouge and Redstar premier classique samples. Although this opposite flavonoid concentration pattern may be attributed to the yeast species involved, it was noticed that the reduction in the flavonoid concentration was recorded in the Lalvin species, while the Redstar premier species recorded an increment in flavonoid concentrations.
Antioxidant activity and capacity
The scavenging activity of the watermelon wines showed a significant difference (p=0.001) in both the young and matured wine samples (Table 1). The study revealed an increase in the percentage inhibition activity of the treatment as the samples matured for 90 days (3 months). The least percentage scavenging activity in the young wine was experimentally noticed in the Redstar Premier rouge wine sample (1.28), while the Redstar premier classique recorded the highest potential free radical scavenging activity of 13.59. The percentage scavenging activity of Lalvin EC 1118 (13.59) was statistically higher than that of the percentage scavenging activity recorded in the Lalvin QA 23 (13.19). Again, in the matured samples, Redstar premier classique still recorded the highest percentage of inhibition activity (246.72), followed by the Redstar premier rouge (181.35) and then the Lalvin QA 23 (174.49), while the least recorded in the Lalvin EC 1118 (79.14). It was indicating that maturation is a significant factor influencing the level of radical scavenging activity in the watermelon wine samples. Generally, the antioxidant activity improved in the young wines from 1.28 to13.59 to 79.14 to 246.72% after the maturation period (90 days). An increase in the radical scavenging activity observed in the matured wine samples could be attributed to the concentration of the phenolic compounds in the wines. This observation supports reports that wine scavenging ability depends on its phenolic content (Bertelli et al., 2021; Innocent and Matenda 2018).
The samples' antioxidant capacity, which measures the thermodynamic conversion efficiency of an oxidant probe upon reaction with an antioxidant, of the samples was significantly different (p=0.001) among the treatments. In the young wine samples, the lowest mean antioxidant capacity was observed in Redstar premier classique (1279.93), while the highest mean percentage (1914.02) antioxidant capacity was recorded in Lalvin EC 1118 sample. The antioxidant capacity of the Redstar premier (1793.57) was significantly higher than that of the Lalvin QA 23 (1341.29). Accordingly, the Lalvin EC 1118 sample retained the greatest mean percentage (2219.37) of antioxidant capacity, while Redstar premier rouge recorded the least mean antioxidant capacity percentage (808.62) in the aged wines. Generally, the percentage antioxidant capacity increased significantly in all the samples across the maturation time (Table 1), indicating maturation time impacted the antioxidant capacity of the wine samples. This increment in antioxidant capacity could be due to adding oak chips during maturation. This affirms the report by Jean-Louis et al. (2020) that extracts from oak (phenolic compounds) increase the antioxidant capacity of matured wines.
Consumer acceptability of the watermelon wine
The results of the sensory attributes of wines produced from watermelon fruit are shown in Table 2. The wine samples of both young and matured (aged) were evaluated. Interestingly, the treatments did not show any significant difference (p > 0.05) in the sensory attributes evaluated (Table 2). The sensory attributes evaluated on the wine were colour, aroma, taste, mouthfeel and overall consumer acceptability.
Colour
The mean rank of the measured sensory attributes on the young wine samples did not show a significant difference from each other. However, Redstar premier classique sample ranked the highest (63.71), the next ranked sample was the Lalvin QA23 (60.00), followed by Lalvin EC 1113 (52.00), and the Redstar premier rouge recorded the least rank (50.29). However, considering the matured samples, interestingly, Redstar premier rouge sample scored the maximum (63.11), Lalvin EC1118 ranked second best (60.29), the third highest scored sample was Redstar premier classique (52.50), and the Lalvin QA 23 (50.11) ranked the least in terms of colour for the matured samples. The maturation period, therefore, influenced the sensory characteristics of the wine samples. From the panels’ choice of colour among the young wines, Lalvin EC 1118 and Redstar premier rouge were preferred compared to the Lalvin QA 23 and Redstar premier classique. However, the opposite happened after the wines matured; thus, Redstar premier rouge and Lalvin EC 1118 were the most preferred. The colour improvement in the Redstar premier rouge and Lalvin EC 1118 samples agreed with other research reports that sensory properties improve with maturation when exposed to oak products (Tchabo et al., 2017).
Aroma
The mean values obtained for the aroma of young wine samples ranged between 51.93 to 61.95. Though no significant difference was observed in the mean rank of aroma in the young wines (p=0.0692), Lalvin QA23 recorded the highest mean rank aroma (61.95), followed by Redstar premier classique (56.80), Redstar premier rouge (55.68) and Lalvin EC 1118 (51.90). Similarly, the aroma concentrations of the samples were not affected by maturation time since there were no significant differences in the mean values of aroma among the samples (p=0.253). However, the Redstar premier rouge scored higher (62.98) in terms of an aroma than the Redstar premier classique (59.21), followed by Lalvin EC 1118 (56.82), and the lowest rank was recorded in the Lalvin QA 23 (46.98). The aroma of the wines was similar irrespective of the treatments within the young wines and after maturation. The similarities in the aroma of the wine samples may result from little to no variation in the yeast strains used for the fermentation.
Taste
In the young wine samples, the Redstar premier rouge was ranked first (60.50), followed by Lalvin QA 23 (55.89), Redstar premier classique (55.07) and Lalvin EC 1118 (54.54). For the matured wine, Redstar premier rouge was ranked the highest (64.23), followed by Redstar premier classique (59.32), Lalvin EC 1118 (53.59), and Lalvin QA 23 (48.86) in that order. Maturation factor did not affect the taste of the wine samples because changes in taste mean ranks were almost the same for both young and aged wine. This observation could be linked to the negligible change in apparent oBrix measured during the maturation period. It is discernible that a change in apparent oBrix could directly affect the taste of the sample. The maturation time did not greatly influence the taste due to the stability of the ethanol concentration in the samples throughout the maturation period. This observation was earlier reported by Villamor et al. (2013) that the taste of wine could be influenced by its alcohol concentration instability.
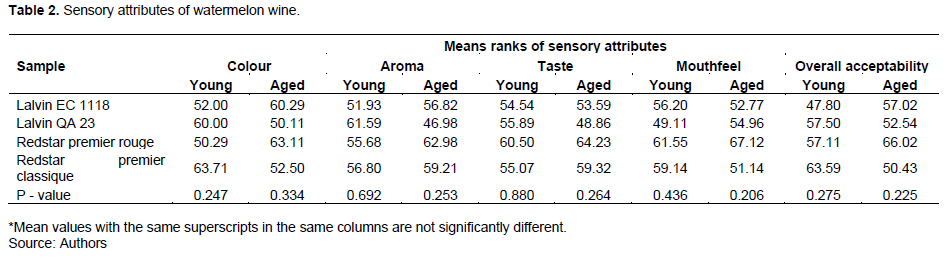
Mouthfeel (astringency)
The Redstar premier rouge sample was ranked highest (61.55) as compared to the mean ranks of Redstar premier classique (59.14), Lalvin EC 1118 (56.20) and Lalvin QA 23 (49.11) among the young wine samples as presented in Table 2.
However, astringency in the wine samples decreased after maturation, indicating maturation factor influenced wine astringency content. The astringency level increased in Redstar premier rouge (67.12), followed by Lalvin QA 23 (54.96). However, the astringency levels of Lalvin EC 1118 (52.77) and Redstar premier classique (51.14) declined after the samples matured and hence ranked the lowest (Table 2). Changes in the individual phytochemical compound compositions could explain the instability in the astringency.
Overall acceptability
The mean values for consumer acceptability of young wines ranked from 47.80 to 63.59. Even though the overall acceptability revealed similarities in the sensory characteristics among the wine samples, the Redstar premier classique was ranked the most accepted sample (63.59), followed by Lalvin QA 23 (57.50), Redstar premier rouge (57.11) and the Lalvin EC 1118 (47.80) before maturation. In the matured samples, the Redstar premier rouge was ranked most preferred (66.02), followed by Lalvin EC 1118 (57.02), Lalvin QA 23 (52.54), and Redstar premier classique (50.43). The panelists’ choice of the samples at the young and mature stages was the Redstar premier classique ranked highest among the samples.
CONCLUSION
The study significantly revealed that watermelon is a potential tropical fruit for winemaking based on its high moisture and sugar content. The research further showed that the phytochemical characteristics of the yeast strains exhibited different patterns during maturation. The phytochemical concentrations were generally higher in the matured wine samples compared to the young wine samples indicating that adding oak chips improves phytochemical contents during maturation. The research findings also showed that anthocyanin was not detected in the watermelon wine samples except the Redstar premier classique, which recorded negligibly low concentration in the young wine. The flavonoid concentrations in the wines were also low, both in the young and matured wines.
The wine samples showed great potential for free radical scavenging and antioxidant activity with the artificial radical reagent (DPPH). The antioxidant activity of the matured wine samples, regardless of the yeast strain, increased as a function of maturation time. The antioxidant capacity of the watermelon wines generally increased in the matured wines except in the Redstar premier rouge, which drastically declined. However, the Lalvin EC 1118 recorded the highest antioxidant capacity among the young and matured wine samples. The antioxidant activity of the matured wines improved due to the addition of oak chips as the main agent for the maturation of the wine samples in the glass bottles.
From the data obtained, it can be concluded that any of the four commercial yeast strains used in this study was suitable for making watermelon wine. However, it manifested that the Redstar premier classique produced the most desirable results, followed by the Lalvin EC 1118 yeast strains. Furthermore, the antioxidant activity and capacity revealed the clinical potential of the watermelon wines.
CONFLICT OF INTERESTS
The authors declared that they have no conflict of interest.
FUNDING
This research received no specific grant from the public, commercial or non-profit funding agencies.
ACKNOWLEDGEMENTS
The authors sincerely thank Mr. Solomon K. Chikpah and Dr. Edem Benard Dzramedo for their assistance.
REFERENCES
|
Addo JK, Osei MK, Mochiah MB, Bonsu KO, Choi HS, Kim JG (2015). Assessment of farmer level postharvest losses along the tomato value chain in three agro- ecological zones of Ghana. International Journal of Research in Agriculture and Food Sciences 2(9):15-23. |
|
|
Baiano A, Gianni A, Mentana A, Quinto M, Centonze D Nobile MA (2016). Effects of the treatment with oak chips on color ? related phenolics, volatile composition, and sensory profile of red wines?: the case of Aglianico and Montepulciano. European Food Research and Technology 242(5):745-767. |
|
|
Bala J, Kocher GS (2017). Preparation of an alcoholic beverage from muskmelon (Cucumis melo L. var. Punjab Sunheri). International Journal of Current Microbiology and Applied Sciences. 6(5):1373-1383. |
|
|
Bertelli A, Biagi M, Corsini M, Baini G, Cappellucci G, Miraldi E (2021). Polyphenols: From theory to practice. Foods 10(11):4-5. |
|
|
Darman RD, Ngang JE, Etoa F, (2010). Development of water melon (Citrullus vulgaris L.) red wine. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 38(2):73-77. |
|
|
Innocent K, Matenda RT (2018). Postharvest technology and value addition of watermelons (Citrullus lanatus): an overview. Journal of Postharvest Technology 6(2):75-83. |
|
|
Jean-Louis P, Philippe R, Jérôme BA, Farid S, Michel M (2020). Determination of ellagitannins in extracts of oak wood and in distilled beverages matured in oak barrels. Association of Officiating Analytical Chemists 73(4):498-501. |
|
|
Jia Z, Tang M, Wu J (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry 64(4):555-559. |
|
|
Lee J, Durst RW, Wrolstad RE (2005). Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. Journal of AOAC International 88(5):1269-1278. |
|
|
Lingua MS, Fabani MP, Wunderlin DA, Baroni MV (2016). From grape to wine: Changes in phenolic composition and its influence on antioxidant activity. Food Chemistry 208:228-238. |
|
|
Mariya A, Haruna AH, Babangida AZ (2020). Phytochemical Analysis of Some Selected Indigenous Fruits Collected from Lokogoma-Abuja, Nigeria. Journal of Diseases and Medicinal Plants 6(2):50-55. |
|
|
Mojzer EB, Hrn?ci?c MK, Škerget M, Knez Ž, Bren U (2016). Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 21:901. |
|
|
Morata A, Escott C, Loira I, Del Fresno JM, González C, Suárez-Lepe JA (2019). Influence of Saccharomyces and non-Saccharomyces yeasts in the formation of pyranoanthocyanins and polymeric pigments during red wine Making. Molecules 24(24):44-90. |
|
|
Morrison A, Rabellotti R (2017) Gradual catch up and enduring leadership in the global wine industry. Research Policy 46(2):417-430. |
|
|
Mundaragi A, Thangadurai D (2017). Process optimization, physicochemical characterization and antioxidant potential of novel wine from an underutilized fruit Carissa spinarum L. (Apocynaceae). Food Science and Technology 38:428-433. |
|
|
Pavlidou E, Mentzorou M, Fasoulos A, Tryfonos C, Petridis D, Giaginis C (2018). Wine: An aspiring agent to promoting longevity and preventing chronic diseases. Diseases 6(3):73. |
|
|
Prieto P, Pinda M, Anguilar M (1999). Spectrophotometric quantification of antioxidant capacity through the formation of phosphomolybdum complex: Specific application to the determination of vitamin E. Analytica Biochemistry 269(2):337-341. |
|
|
Prior RL, Wu X, Schaich K (2005). Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. Journal of Agricultural and Food Chemistry 53(10):4290-4302. |
|
|
Przybylska S (2020). Lycopene-a bioactive carotenoid offering multiple health benefits: A review. International Journal of Food Science and Technology 55(1):11-32. |
|
|
Raposo R, Chinnici F, Ruiz-Moreno MJ, Puertas B, Cuevas FJ, Carbú M, Guerrero RF, Somovilla VO, Rojas JMM, Cantos-Villar E (2018). Sulfur free red wines through the use of grapevine shoots: Impact on the wine quality. Food Chemistry 243:453-460. |
|
|
Romdhane, MB, Haddar A, Ghazala I, Jeddou KB, Helbert CB, Ellouz-Chaabouni S (2017). Optimization of Polysaccharides Extraction from Watermelon Rinds: Structure, Functional and Biological Activities. Food Chemistry 216:355-364. |
|
|
Saranraj P, Ramesh CR (2019). Tropical Fruit Wines: Health Aspects. International Journal of Microbiological Research 10(3):94-110. |
|
|
Saranraj P, Sivasakthivelan P, Naveen M (2017). Fermentation of fruit wine and its quality analysis: A review. Australian Journal of Science and Technology 1(2):85-97. |
|
|
Stone H, Sidel J (2004). Sensory Evaluation Practices. 3rd edition. New York: Academic Press 338 p. |
|
|
Tchabo W, Ma Y, Kwaw E, Zhang H, Xiao L, Tahir HE (2017). Aroma profile and sensory characteristics of a sulfur dioxide-free mulberry (Morus nigra) wine subjected to non-thermal accelerating aging techniques. Food Chemistry 232:89-97. |
|
|
Tzachristas A, Pasvanka K, Calokerinos A, Proestos C (2020). Polyphenols: Natural Antioxidants to Be Used as a Quality Tool in Wine Authenticity. Applied Sciences 10(5908):15-28. |
|
|
Umeh SO, Ikele MO, Okeke BC, Nwiyi IU (2021). Investigation in the use of a Yeast Specie Isolated from a Fermented Beverage for Mixed Fruit Wine Production. International Journal of Trend in Scientific Research and Development 5(5):538. |
|
|
Veli? D, Veli? N, Klari? DA, Klari? I, Tominac VP, Košmerl T, Vidrih R (2018). The production of fruit wines - a review. Croatian Journal of Food Science and Technology 10(2):4-7. |
|
|
Villamor RR, Evans, MA, Ross CF (2013). Effect of ethanol, tannin, and fructose concentrations on sensory properties of model red wines. American Journal of Enology and Viticulture 3:342-348. |
|
|
Wang J, Shi Y, Zhang H, Deng X, Wang Y, Ma Y, Zhao X, Zhang C (2018). Characterization and Comparison of Unfermented and Fermented Seed-Watermelon Juice. Journal of Food Quality 2:1-9. |
|
|
Wong A, Chernykh O, Figueroa A (2016). Chronic L-citrulline supplementation improves cardiac sympathovagal balance in obese postmenopausal women: A preliminary report. Auton. Neuroscience Basic Clinical 198:50-53. |
|
|
Yakubu VA, Salifu A, Moses KAM, Ibrahim NI (2018). Means of transportation affects the physical qualities of watermelon (Citrullus lanatus [Thunb]) fruit within the Tamale Metropolis in the Northern Region of Ghana. Journal of Biology, Agriculture and Healthcare 8(4):50-59. |
|
|
Yu L, Haley S, Perret J, Harris M, Wilson J, Qian M (2002). Free radical scavenging properties of wheat extracts. Journal of Agricultural and Food Chemistry 50(6):1619-1624. |
|
|
Yusufu MI, John PG, Ahemen SA (2018). Production and quality evaluation of wine from watermelon juice and ginger extract. Journal of Human Nutrition and Food Science 6(1):1-7. |
|
|
Zhou Y, Zheng J, Li Y, Xu DP, Li S, Chen YM (2016). Natural polyphenols for prevention and treatment of cancer. Nutrients 8(8):2-35. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0