Full Length Research Paper
ABSTRACT
The study analysed the fatty and amino acid compositions of chia seeds and the physicochemical characterisation of chia seeds oil. The quantification of amino and fatty acids was done using high-performance liquid chromatography (HPLC) and gas chromatography equipped with a flame ionisation detector (GC-FID). The amino acids from chia seeds were leucine, valine, phenylalanine, lysine, isoleucine, threonine, histidine, and methionine with 1.63, 1.37, 1.34, 1.14, 0.98, 0.98, 0.88 and 0.81 g/100 g, respectively. Saturated and unsaturated fatty acids were 3.95 and 30.48%, respectively. Meanwhile, the mean ratio of Omega 6/Omega 3 fatty acids was 0.333. The established physicochemical characteristics of CSO were acid value 2.63 mg KOH/g, peroxide value 6.23 meq active oxygen/kg, iodine value 200.721 g I2/100 g, saponification value 193.345 mg KOH/g, refractive index 1.454 at 40°C relative density 0.8824 g/cm3 at 20°C and specific gravity 0.882 at 40°C. The fatty acid and amino acid profiling of chia seeds revealed the presence of an appreciable amount of polyunsaturated fatty acids (PUFA) and essential amino acids. The physicochemical characteristics of CSO constitute a drying oil category that requires timely usage and proper storage condition to reduce rancidity from air or light exposure.
Key words: Chia seeds, chia seeds oil, amino acids, fatty acids, physicochemical characteristics.
INTRODUCTION
The human body needs protein as one of the macronutrients to help the body to function correctly. Proteins play many crucial roles in the human body, which include building and repairing body tissues, coordination of other body functions, and many others. The building blocks of proteins are amino acids (AA). There are 22 different AA, with 9 being essential AA. Essential amino acids need ingestion since they cannot be synthesised by the body (Tessari et al., 2016).
Chia seeds have been introduced in East Africa recently and have been gaining popularity due to their nutrient composition, mainly the AA and fatty acids (FA). Chia seeds contain fat (30 to 33%) and proteins (15 to 25%) (Ixtaina et al., 2008). More than 60% of its fat contains polyunsaturated fatty acids (PUFA), mainly Omega 3 and 6 fatty acids (Di Marco et al., 2020; Ghafoor et al., 2020; Grancieri et al., 2019; Rajaram, 2014; Segura-Campos et al., 2014; Shen et al., 2018) are vital to human health throughout one's lifespan (Gazem et al., 2017; Wu et al., 2016).
Various researchers have reported various oil content of chia seeds (Suri et al., 2016), Brazilian chia seeds with 31.2 g/100 g, and Argentina, Bolivia, Colombia and Peru with an average of 28.5 to 32. 7 g/100 g (Shen et al., 2018). This variation may affect the physicochemical properties of chia seeds oil (Suri et al., 2016; Shen et al., 2018). Also, chia seeds contain 18 amino acids a human body needs and all 9 essential amino acids (Suri et al., 2016; Ullah et al., 2016). Ayerza and Coates (2004), Ixtaina et al. (2011), and Suri et al. (2016) reported that the protein and fat content of chia seeds varies from one place to another in the world due to differences in climatic conditions, soil and agronomic activities. Due to the nutritional potential of chia seeds, the incorporation of chia seeds in food products has gained popularity in dairy (Kibui et al., 2018) and bakery products (Romankiewicz et al., 2017). In all these applications, incorporating chia seeds has contributed to improved texture and nutritional composition in terms of protein content and fatty acids (Segura-Campos et al., 2013) in addition to extending the shelf life (Valdivia-López and Tecante, 2015) of the final product. Protein from chia seeds tends to form plastic films, which have low permeability to oxygen, which prevents food products from undergoing deterioration due to the presence of oxygen and reduces or inhibits the rate of colour change, flavour change and nutrient deterioration (Valdivia-López and Tecante, 2015).
To detect amino acids effectively by high-performance liquid chromatography (HPLC), AAs should be chemically modified into derivatives that absorb or fluoresce ultraviolet light using HPLC (Calull et al., 1991). Reagents commonly used for derivatisation to improve the analytical capability of AA are phenylisothiocyanate (PITC) to obtain PTC-amino acids and ortho-phthalaldehyde (OPA) to derive OPA-amino acids (Calull et al., 1991; Checa?Moreno et al., 2008). However, many other reagents are used for the derivatisation of amino acid analysis but are associated with different shortcomings (Calull et al., 1991; Checa?Moreno et al., 2008; González-Castro et al., 1997). The OPA method has greater sensitivity but is limited to primary amino acids (Checa?Moreno et al., 2008). The PITC is reported to react with all essential amino acids, and the process of derivatisation is simple, rapid and used for precolumn derivatisation (Calull et al., 1991; Checa?Moreno et al., 2008; González-Castro et al., 1997). For fatty acid profiling, gas chromatography equipped with a flame ionisation detector (GC-FID) has commonly been used by researchers (Nitrayová et al., 2014; Uzunova et al., 2019). This study, therefore, analysed the fatty and amino acids compositions and identified the physicochemical characteristics of chia seeds oil from chia seeds grown in Kagera, Tanzania.
MATERIALS AND METHODS
Chia seeds sample
Chia seeds (Salvia hispanica L.) were collected from the Karagwe district in the Kagera region on the western shores of Lake Victoria in Tanzania. Freshly-harvested chia seeds of about 10 kg were cleaned by winnowing to remove debris. Cleaned seeds were wrapped in polyethylene bags and immediately placed in dark storage at a temperature ranging from 25 to 30°C before analysis. Before analysis, raw chia seeds were ground using a laboratory-scale grinding machine (Waring Commercial, made in the USA) and sieved to obtain a uniform particle size of 0.2 mm.
Amino acid (AA) profiling
Free amino acids were determined using phenylisothiocyanate as a derivatising agent using high-performance liquid chromatography with Ultraviolet Detection (Klikarová et al., 2021). The analysis was conducted using laboratory facilities of the Department of Food Science and Technology at the University of Dar es Salaam, the Tanzania Bureau of Standards (TBS) for preliminary analysis, and SGS Tanzania Superintendence Company Limited in Dar es Salaam.
Standard and reagents
Methanol (HPLC grade), phenylisothiocyanate (PITC), trimethylamine (TEA), acetic acid, tetrahydrofuran (HPLC grade), acetonitrile (HPLC grade), sodium hydroxide (anhydrous), sodium acetate and amino acid standards for protein hydrolysate were bought from Sigma Co. (St. Louis, MO, USA). The study used ultra-purified water produced in the laboratory with a pore size of 0.068 µS/cm (Evoqua Water Technologies, made in the USA).
Preparation of standard
A stock solution of AA was prepared from a standard solution containing 0.5 µmol/mL except for L-cystine at 0.25 µmol/mL in 0.2 N sodium citrate. Five-point calibration points of AA were prepared in a range of 0.0238 to 0.2143 µmol/mL, except L-cystine, which was 0.0119 to 0.10715 µmol/mL. The calibration curve was used to calculate AA concentration in chia seeds.
Sample preparation
Ten grams of chia seeds flour were inserted into a 250 mL beaker, followed by 40 mL of extracting solvent (75% methanol in ultra-purified water). The mixture was homogenised for 2 min using a homogeniser machine (Ultra Turrax, IKA T 18, made in Germany). The extract was then transferred to a 100 mL volumetric flask, and the beaker was rinsed four times with 15 mL of extracting solvent.
Extracting solvents were added to a glass bottle to make a standard volume of 100 mL stored at 4°C for 60 min (Antoine et al., 2001). The content was transferred to centrifuge tubes and centrifuged at 27,000 ×g (4,000 rpm) for 60 min (Rotofix 32A, Germany). The supernatant was filtered using filter paper (Whatman, 90 mm diameter, GE Healthcare UK Limited). Filtered sample extract solutions were stored in a fridge at 4°C. Before HPLC injection, an aliquot of this solution was filtered through 0.45 µm pore size (HPLC grade, Nagel Germany made) into 1.5 mL HPLC vials. The spiking method helped to determine the accuracy of the method. Meanwhile, for the recovery study, the sample was fortified with the amino acid standard to attain a concentration of 0.1 µmol/mL for all AA except L-cystine, which was 0.05 µmol/mL.
Mobile phase preparation
The study used the method of González-Castro et al. (1997) with modifications. Mobile phase A was an aqueous buffer prepared by adding 0.5 ml/L TEA to 0.14 M of sodium acetate, and the pH of this mixture was adjusted to 6.2 with acetic acid. Mobile phase B comprised acetonitrile and ultra-purified water (60:40). The mobile phases were degassed (3210 BRANSON, Nagtech, UK) for 10 min to remove any air bubbles.
Amino acid derivatisation procedure
Amino acid derivatisation outlined by González-Castro et al. (1997) was followed with modifications. 100 µl of amino acid standard solution or sample extract were pipetted into 2 mL vials (Agilent Technologies), followed by the addition of 400 µL of derivatising reagent (methanol: TEA: PITC: water at 7:1:1:1). The mixture of the sample, or amino acid standard solution and derivatising reagent was blended using a vortex machine (Thermo scientific, assembled in China) for 3 s. Finally, the mixture was injected into the HPLC system to detect and quantify amino acids.
Chromatographic procedure
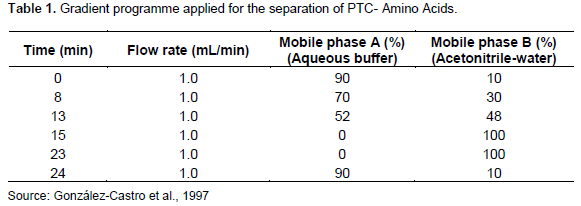
The study used the method of González-Castro et al. (1997) with modifications. HPLC (SHIMADZU Nexera X2, Japan) was used for analysing amino acid profiling. HPLC instrument was equipped with a pump (LC – 30AD), a membrane degasser (DGU – 20A3R), an autosampler (SIL -30AC), a diode array detector (SPD-M30A) and a column oven (CTO-20AC). Agilent Zorbax Eclipse plus C18 100A (4.6 mm × 150 mm × 5 µm) was used to separate AA. The analysis was carried out at 30°C, a flow rate of 1.0 mL/min, an injection volume of 25 µL, and a wavelength of 254 nm. Lab Solution software version 5.86 (200 -2016 Shimadzu, Corporation) was used for data acquisition, processing and quantification. The gradient programme applied for separating PTC-amino acid is shown in Table 1. After separating the amino acid chromatogram (peaks), integration and quantification were carried out using the standard curve of peak areas obtained from known concentrations of the amino acid standard mixtures.
Fatty acid profiling analysis
Fatty acids profiling was conducted using gas chromatography equipped with a flame ionisation detector (GC-FID) as different researchers (Nitrayová et al., 2014; Uzunova et al., 2019) have commonly applied. The analysis was conducted using the Tanzania Bureau of Standards (TBS) laboratory facilities for preliminary analysis and SGS Tanzania Superintendence Company Limited, based in Dar es Salaam.
Standards and reagents
Pyrogallic acid (AR grade), ethanol (AR grade), hydrochloric acid (AR grade), diethyl ether (AR grade), petroleum ether (30 - 60°C, HPLC grade), toluene, n-hexane (AR grade), triglyceride internal standard solution (C11:0) mixture of 37 fatty acid methyl ester standard (FAMEs) and 9 single standard methyl esters of C16:0, C16:1, C17:1, C18:0, C18:1tran, C18:2cis, C18:3(GLA), C18:3(ALA) and C20:0 (Sigma Co., St. Louis - MO, USA). Chloroform (AR grade), boron trifluoride reagent (14% BF3 in MeOH) and anhydrous sodium sulphate (Na2SO4 - AR grade) are manufactured by LOBA Chemie PVT LTD, India. The response factor, the ratio of peak area to the concentration of known standard, was used to calculate the concentration of FA in chia seeds.
Acid hydrolysis
Chia seed flour was hydrolysed with methanolic sulphuric acid (1 M) before profiling the fatty acids present to facilitate the extraction of fats. Sample preparation and acid hydrolysis were conducted following ISO (2017) with some modifications. About 0.5 g of chia seeds flour was weighed using Laboratory analytical balance (Explorer Pro, Switzerland). The weighed sample was placed in a volumetric flask, followed by the addition of 100 mg of pyrogallic acid, 2 mL of 5 g/L triglycerides internal standard solution (C11:0), 2 mL of ethanol, 3 mL of ultra-purified water and 7 mL of hydrochloric acid (37%) and mixed thoroughly. The flasks were kept in a water trough at 75 to 80°C for 40 min and shaken every 10 min. The mixture was cooled at room temperature, and 10 mL of ethanol was added and shaken gently to facilitate fat extraction.
Fat extraction
About 25 mL of diethyl ether was added to the volumetric flask with mixtures from the acid hydrolysis stage and shaken for 5 min. The process was repeated by adding 25 mL of petroleum ether and left to stand until the upper layer was transparent. The upper liquid was then poured into a 250 mL flat bottom flask. The remaining bottom layer re-extraction of fat was repeated for two more times, until there was no more upper liquid separated after adding petroleum ether. All the portion of extract from the upper layer was put in the rotary evaporator to remove petroleum ether.
Methylation to FAMEs
Three millilitres of chloroform and 3 mL of diethyl ether were added to the extracted fat residues and placed in a 15 mL reaction tube. The mixture evaporated to dryness at 40°C in a sample concentrator (TechneTM FSC4NCS, Fisher Scientific UK) with a nitrogen purge system. After that, 2 mL of 14% BF3 in MeOH and 1 mL of toluene were added and closed with a lid and vortex for 1 min. The blend was heated for 45 min at 100°C and shaken every 10 min. The tube was then left to cool down to room temperature, and 5 mL of ultra-purified water, 1 mL of hexane and 1 g of anhydrous sodium sulphate were added. Again, the tube vortex for 1 min until layers separated and the top layer transferred to another vial containing 1 g of anhydrous sodium sulphate. Also, the top layer was transferred to another vial in readiness for analysis in GC-FID. In addition to the oil sample, a sample with a known concentration (B?PEA 20 - 408) of FA was run in parallel with the sample to determine the method's accuracy.
GC-FID setup and quantification
Gas chromatography equipped with flame ionisation detector (GC-FID) Agilent Technologies (Awuchi et al., 2019) equipped with Intuvo 9000 GC system was used to analyse the fatty acid profiling. The GC-FID instrument was equipped with a GC column (diameter 0.25 mm, film thickness 0.2 µm of 100 m length). The analysis of fatty acids was carried out based on ISO (2015). The analysis was carried out at a flow rate of 1.4 mL/min, hydrogen gas flow at 40 mL/min, air flow of 400 mL/min, nitrogen gas at 40 mL/min and oven temperature programmed at 50°C, gradient 5°C/min to 130°C, gradient 25°C/min to 175°C, for 7 min, gradient 2°C/min to 215°C for 1 min, gradient 2°C/min to 230°C hold 10 min. The total run time was 35 min. The sequence of analysis started with the blank (hexane), standard, control sample, sample and standard, respectively.
Chia seed oil characterisation
Extraction of chia seeds oil (CSO)
Chia seeds oil was extracted using automated Soxhlet equipment (Model EV 16, Gerhardt Bonn, Germany) and petroleum ether as extraction solvent (Horwitz, 2010). About 20 g of chia seeds flour was weighed, placed in extraction thimbles, and set in the Soxhlet machine. A ratio of 1:10 of sample and extraction solvent was used. About 200 mL of petroleum ether was added, and the extraction process was left to continue for about 4 h. After the extraction, petroleum ether was recovered using a rotary evaporator, and the flask was placed in an oven at 105°C for 30 min to dry the fat.
Determination of chia seeds oil yield
Chia seeds oil yield was determined by considering the mass of the extracted oil and the mass of the seeds flour used for oil extraction by the method described by Timilsena et al. (2017), as shown by the equation:

Physicochemical characterisation of chia seeds oil
The chia seeds oil physicochemical parameter of acid value (AV) was determined according to ISO (2009). The peroxide value (PV) was determined according to the method described by Animal (2007), the iodine value (IV) (Yuan et al., 2020), the saponification value (SV) (Gu et al., 2017), and the refractive index (RI) following Dumitru (2020) using Abbe refractometer at 40°C (RFM 860, Bellingham UK). The relative density (RD) and specific gravity (SG) were measured by density metre (Mettler Toledo, Switzerland).
Data analysis
All the analyses were carried out in triplicates for amino acids, fatty acids, and physicochemical characterisation of chia seeds oil. Descriptive statistics were done using Microsoft Excel (15.0.5285 of 2013, Microsoft Inc. CA, USA). In addition, analysis of variance was used to determine statistical differences between types of AA or FA analysed using R statistical software (4.0.3 of 2020). Turkey's honest significance test was applied to find the means that are significantly different from each. Subsequently, the results were expressed as mean ± standard error of the mean and p-values of less than 0.05 were significant.
RESULTS AND DISCUSSION
Quality control results for amino acids and fatty acids
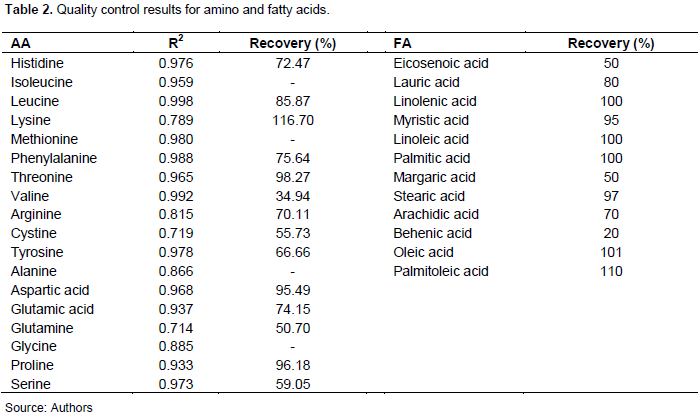
The correlation coefficient between peak area and AA concentration ranged from 0.719 to 0.992. The values for leucine, phenylalanine and valine fall within the acceptable range, that is, greater than 0.998 (Bartolomeo and Maisano, 2006), as Table 2 illustrates. The method for extraction and quantification of AA was found accurate for leucine, lysine, threonine, aspartic acid and proline since their recoveries values were 90 to 110% (Reason, 2003). Poor recoveries of other amino acid result from the destruction of some amino acids (tryptophan and cysteine) during acid hydrolysis. In contrast, other amino acids can interfere with each other (threonine and glycine) (Bartolomeo and Maisano, 2006). Table 2 also shows recovery percentage (quality control) results for fatty acids.
Amino acid profiling
The amino acid composition of chia seeds (Table 3) showed the presence of 18 amino acids of different quantities, including essential amino acids such as leucine, valine, phenylalanine, isoleucine and threonine. In this study, chia seeds were found to have significantly high levels of glutamic acid (4.41 ± 0.023) and arginine (3.73 ± 0.009) in g/100 g. Similarly, other researchers reported comparable high levels of glutamic acid and arginine. For example, Kulczy?ski et al. (2019) reported values of 3.5 and 2.14, Dautant et al. (2007) reported values of 7.08 and 4.23, and Valdivia-López and Tecante (2015) reported values of 12.3 and 8.06 in g/100 g for glutamic acid and arginine, respectively.
All the values are expressed in mean ± standard error, n=3. Different small-scale letters within amino acids indicate a statistical difference at a 5% significant level according to Tukey's HSD multiple ranks test, whereas different capital letters within average AA indicate a statistical difference according to the student t-test at a 5% significant level.
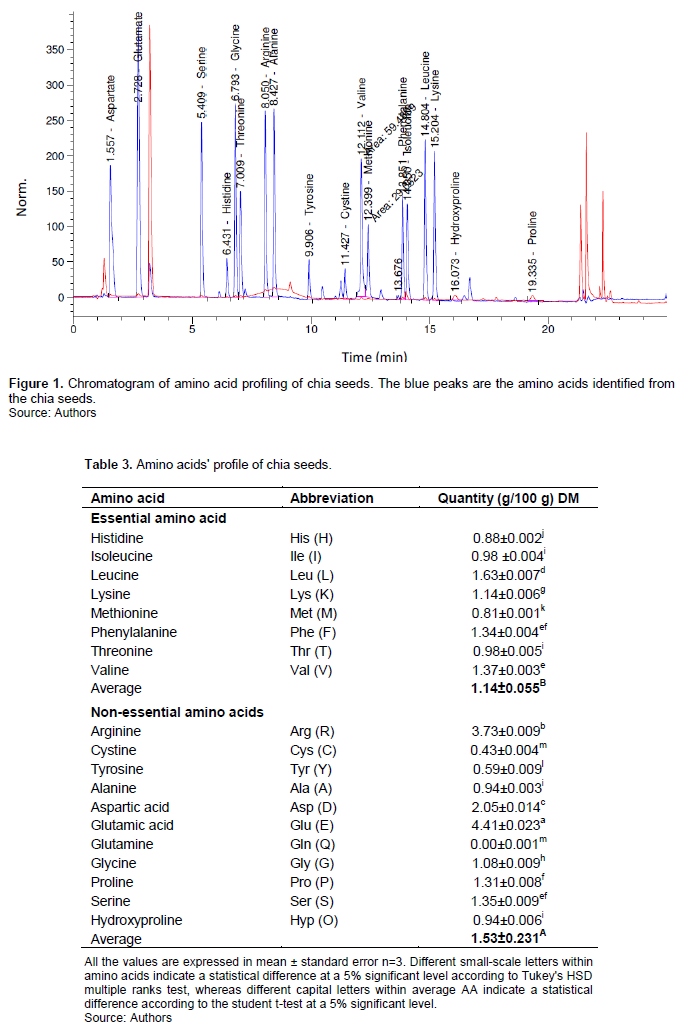
The essential amino acid, leucine 1.63 g/ 100 g, had the highest amount, followed by valine (1.37) and phenylalanine (1.34) in g/100 g. For non-essential amino acids, glutamic acid had the highest quantity (4.41), followed by arginine (3.73) and aspartic acid (2.05), all in g/100 g. The chromatograms of amino acid profiling are presented in Figure 1, indicating the peak area of each amino acid detected.
In general, chia seeds were found to have a high amount of non-essential amino acids; however, 43% (that is 1.14 g/100 g) of the total AA found in chia seeds was essential AA, as Table 3 illustrates. Chia seeds from Kenya (Kibui et al., 2018) reported lower values of essential amino acids: leucine 1.02, valine 0.62, phenylalanine 0.75, lysine 0.73, isoleucine 0.54, threonine 0.4, histidine 0.33, and methionine 0.52 in g/100 g than the one reported in the present study. Nitrayová et al. (2014) reported chia seeds from Poland with 1.42, valine 0.79, phenylalanine 1.16, lysine 0.93, isoleucine 0.74, threonine 0.54, histidine 0.61 and methionine 0.67 in g/100 g, which are lower than the one reported in the present study but higher than what Kibui et al. (2018) reported. These variations might be attributable to different geographical conditions, agronomic practices occurring during the cultivation of chia seeds and seed maturation (Ayerza, 2019), and the application of different analytical techniques. However, all the results conclusively show that chia seeds contain various amino acids in varying quantities (Table 3). As Table 3 illustrates, these results indicate that the chia seeds contain 18 AAs out of 22 amino acids the human body needs as building blocks for proteins for different functions in the body (Kulczy?ski et al., 2019; Nitrayová et al., 2014). These amino acids play different roles and functions in the body, which include blood sugar regulations, body tissue growth, muscle growth, energy production, immunity functioning of the body and brain functioning (Kulczy?ski et al., 2019; Nitrayová et al., 2014).
Fatty acid profiling
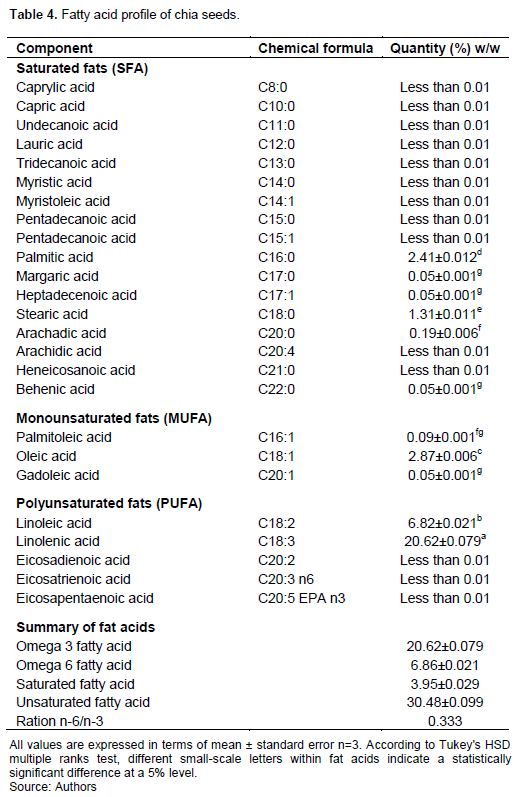
Analysis of fatty acids profiling of chia seeds revealed a significantly high amount of unsaturated fatty acids, 30.48% attributable to linolenic and linoleic acid, as compared to saturated fatty acids of 3.95% (Table 4). The monounsaturated fat acids (MUFA) were palmitoleic and oleic, whereas polyunsaturated fatty acids (PUFA) were linoleic and linolenic acids, as Table 4 illustrates.The fatty acid profile composition of chia seeds is presented in Table 4. The detection limit for the fatty acids was 0.01%. The saturated fatty acids recorded (Table 4) were palmitic, margaric, heptadecenoic, stearic, arachidic, and behenic. Mono-unsaturated acids were palmitoleic, oleic, and gadoleic acids. On the other hand, polyunsaturated fatty acids were linoleic and linolenic acids. The saturated fatty acids, palmitic acid (2.41%) and stearic acid (1.31%) were predominantly present. The ratio of Omega 6/Omega 3 fatty acid was 0.333, comparable with other research studies of chia seeds from Argentina/ Guatemala, Mexico and Australia, respectively (Ixtaina et al., 2011; Segura-Campos et al., 2014; Timilsena et al., 2017). The nutritious richness of chia seeds is popular due to the high amount of polyunsaturated fatty acids, particularly the Omega 3 and 6 fatty acids. In recent years, people have suffered from non-commutable diseases such as diabetes, cardiovascular diseases, and various cancers, which are highly associated with eating processed foods that are mostly less nutritious and have high carbohydrate and fat content. These foods mostly contain saturated fats and less polyunsaturated fatty acids, which have biologically active functions for human health (Swanson et al., 2012). Omega 3 and 6 are linked with human health benefits, which during pregnancy are associated with proper foetal development and reduction of cardiovascular diseases (Swanson et al., 2012). Emerging knowledge of chia seeds, especially their composition, including fatty acids, has opened the possibility of improving health using vegetarian sources through which chia seeds are cultivated. Doing so would reduce the burden of over-dependence on sea foods for sourcing Omega 3 and 6, particularly because sea foods are increasingly becoming scarce. Still, they are also associated with risks of environmental degradation in the sea (Kaale and Eikevik, 2014). In developing countries such as Tanzania and most sub-Saharan African nations, malnutrition is still one of the causative factors of child stunting. This causation might be associated with low income resulting in poor health. As chia seeds are a cultivated crop rich in polyunsaturated fatty acids, the crop could be introduced to farmers and used at different levels in the household to combat this problem. Omega 3 and 6 fatty acids reportedly contribute positively to one's lifespan in terms of brain functioning and cognition (Rahmawaty and Meyer, 2020).
Chia seeds oil physicochemical characterisation
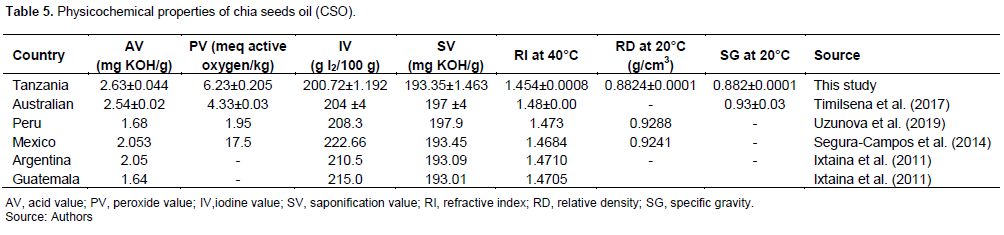
In this study, chia seeds oil yield was 27.4±0.128%. Researchers reported chia seeds oil (CSO) yield varies between 26 and 36% (Segura-Campos et al., 2014; Timilsena et al., 2017). The variation in oil yield may be attributable to agronomic and geographical conditions (Ayerza, 2019) and the method and extraction solvent applied (Ixtaina et al., 2011). Table 5 shows the physicochemical characteristics of chia seeds oil obtained using solvent extraction from different geographical locations, including results from this study.
The abbreviation of acid value (AV) is expressed in mg KOH/g, peroxide value (PV) is expressed in meq active oxygen/kg, iodine value (IV) is expressed in g of I2/100 g, saponification value (SV) is expressed in mg KOH/g, refractive index (RI) measured at 40°C, relative density (RD) measured at 20°C in g/cm3, and specific gravity
measured at 20°C.
In this study, the oil was characterised based on its physicochemical properties. Different parameters were established as follows: the acid value of 2.63 mg KOH/g, peroxide value of 6.23 meq active oxygen/kg, iodine value of 200.72 g I2/100 g, the refractive index of 1.454 at 40°C, the relative density of 0.8824 g/cm3 at 20°C and specific gravity of 0.882 at 20°C, as detailed in Table 5.
The chia seeds used in this analysis were dark with some slight whitish seeds and CSO characterised by golden colour. The colour of the seeds mostly depends on the level of maturity of the seeds, from dark seeds to black/black spotted. In all chia seeds, a few white seeds were mixed with dark/black spotted ones, sometimes not fully matured (white seeds). The oil extracted from grounded chia seeds flour was golden yellow (Figure 1).
The physicochemical characteristics commonly identify the oil type are saponification value (SV), iodine value, refractive index, and relative density. The saponification value refers to potassium hydroxide (KOH), which should be in milligrams to convert 1 g of fat into soap (Ibeto et al., 2012). The SV of chia seeds oil of 193.345 mg KOH/g showed a high number of fatty acids. Other oils, such as shea-nut oil and jatropha oil, were reported to have SV of 195 and 193.55, respectively (Ibeto et al., 2012). Meanwhile, the SV for chia seeds ranged from 193 to 198 mg KOH/g. The high value of SV in CSO shows an increased number of fatty acids but also signals the possibility of using the oil for cosmetic purposes in producing soap-like products.
The AV of CSO measured was 2.63±0.044 mg KOH/g. Any oil's AV represents the rancidity level as it usually occurs during the decomposition of triglycerides. The lower the AV, the better the oil quality. The acid value and PV commonly serve as the parameter to describe oil quality. The PV of CSO was 6.23 meq active oxygen/kg, which is within the acceptable limit of ≤ 10 meq active oxygen/kg for most edible oils and fats (Balley, 1982). Table 5 also shows the values of AV and PV of CSO that ranged from 1.6 to 2.6 mg KOH/g and 4.3 to 17.5 meq active oxygen/kg, indicating the CSO's easiness of undergoing rancidity. Implicitly, this suggests the possibility of CSO undergoing proper refining or protection from oxidation after extraction. Moreover, immediate usage should be recommended due to the instability of oil, which can be associated with shorter shelf life than other types of oils. Furthermore, the oil should be stored at a low temperature and dark to avoid oxidation and rancidity.
CSO's IV was 200.721 I2/100 g. The IV of oil explains the oil's identity but also classifies the degree of unsaturation (Knothe, 2002). The IV of oils with a value < 125 is described as non-drying (e.g. olive), IV of 125 to 150 as semi-drying (e.g. sunflower oil), whereas IV of >150 are described as drying oils (Knothe, 2002). The CSO values (Table 5) ranged from 200 to 222 I2/100 g, which signals that all the CSO from different locations is drying. On the other hand, non-drying oils are good oils which can be used in sauces and dressing as they will not harden when exposed to air (e.g., olive oil). On the other hand, drying oils have a high level of unsaturation which reveals the instability of oil.
This study established a refractive index of 1.454 at 40°C for CSO. The values of CSO established from different studies (Table 5) ranged from 1.45 to 1.48. The RI of oil is also used to show the rancidity levels of oil as it measures the passage of light rays traversing through the material. The RI is measured at a specific temperature. At every degree of temperature, the change of RI is at a rate of 0.000385 (Shahidi, 2005). The RI of 1.454 for CSO is still comparable for most common oils as the values range from 1.447 to 1.482 (Shahidi, 2005). This value of RI, PV, AV and IV of CSO has shown a high level of unsaturation of the oil. Due to the unsaturation level of fatty acids present in chia seeds oil, the same trend was noted for the RD value. The relative density of oil serves as an identification criterion of the oil type, but the higher RD value also shows an unsaturation level of fatty acids (Segura-Campos et al., 2014). This study's relative density values and specific gravity of chia seeds oil were lower than previously reported values (Table 5). The oil's specific gravity (SG) is an essential factor when improving the oil's process and quality control (Timilsena et al., 2017). Both SG and RD also facilitate the identification of the type of oil.
CONCLUSION
Chia seeds' fatty acid and amino acid profiling revealed the presence of appreciable amounts of PUFA and essential amino acids, which offer significant health benefits to the human diet. The physicochemical characteristics of CSO showed it is a drying oil category necessary for timely usage and proper storage condition to reduce rancidity from exposure to either air or light. Chia seeds grown in Kagera, Tanzania, can thus be used to improve human health through different means of consumption. Therefore, the study recommends the scaled-up usage of chia seeds due to their amino and fatty acids richness.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The authors thank the Tanzania Bureau of Standards (TBS) for its financial support and providing access to their laboratory to analyse samples.
REFERENCES
|
Animal I (2007). Vegetable fats and oils-Determination of peroxide value. ISO 3960:2007. |
|
|
Antoine F, Wei C, Littell R, Quinn B, Hogle A, Marshall M (2001). Free amino acids in dark?and white?muscle fish as determined by o?phthaldialdehyde precolumn derivatization. Journal of Food Science 66(1):72-77. |
|
|
Awuchi CG, Igwe VS, Echeta CK (2019). The functional properties of foods and flours. International Journal of Advanced Academic Research 5(11):139-160. |
|
|
Ayerza R (2019). Antioxidants, protein, oil content and fatty acids profiles of chia seeds (Salvia hispanica L.) genotype Tzotzol growing in three tropical ecosystems of Bolivia, Ecuador and Paraguay. International Journal of Agriculture Environment and Food Sciences 3(3):191-196. |
|
|
Ayerza R, Coates W (2004). Composition of chia (Salvia hispanica) grown in six tropical and subtropical ecosystems of South America. Tropical Science 44(3):131-135. |
|
|
Balley A (1982). Industrial Oil and Fat Product. John Wiley-Interscience, New York, NY, USA. |
|
|
Bartolomeo MP, Maisano F (2006). Validation of a reversed-phase HPLC method for quantitative amino acid analysis. Journal of Biomolecular Techniques 17(2):131. |
|
|
Calull M, Fábregas J, Marcé R, Borrull F (1991). Determination of free amino acids by precolumn derivatization with phenylisothiocyanate. Application to wine samples. Chromatographia 31(5-6):272-276. |
|
|
Checa?Moreno R, Manzano E, Mirón G, Capitán?Vallvey LF (2008). Revisitation of the phenylisothiocyanate?derivatives procedure for amino acid determination by HPLC?UV. Journal of Separation Science 31(22):3817-3828. |
|
|
Dautant F, Simancas K, Sandoval A, Müller A (2007). Effect of temperature, moisture and lipid content on the rheological properties of rice flour. Journal of Food Engineering 78(4):1159-1166. |
|
|
Di Marco AE, Ixtaina VY, Tomás MC (2020). Inclusion complexes of high amylose corn starch with essential fatty acids from chia seed oil as potential delivery systems in food. Food hydrocolloids 106030. |
|
|
Dumitru MG (2020). Water activity role on dried Agaricus bisporus L. lipid oxidation during storage time. Revista de Chimie 71(5):125-131. |
|
|
Gazem RAA, Puneeth HR, Shivmadhu C, Madhu ACS (2017). In vitro anticancer and anti-lipoxygenase activities of chia seed oil and its blends with selected vegetable oils. In Vitro 10(10). |
|
|
Ghafoor K, Ahmed IAM, Özcan MM, Al-Juhaimi FY, Babiker EE, Azmi IU (2020). An evaluation of bioactive compounds, fatty acid composition and oil quality of chia (Salvia hispanica L.) seed roasted at different temperatures. Food Chemistry 333:127531. |
|
|
González-Castro M, López-Hernández J, Simal-Lozano J, Oruña-Concha M. (1997). Determination of amino acids in green beans by derivatization with phenylisothiocianate and high-performance liquid chromatography with ultraviolet detection. Journal of Chromatographic Science 35(4):181-185. |
|
|
Grancieri M, Martino HSD, Gonzalez de Mejia E (2019). Chia seed (Salvia hispanica L.) as a source of proteins and bioactive peptides with health benefits: A review. Comprehensive Reviews in Food Science and Food Safety 18(2):480-499. |
|
|
Gu LB, Pang HL, Lu KK, Liu HM, Wang XD, Qin GY (2017). Process optimization and characterization of fragrant oil from red pepper (Capsicum annuum L.) seed extracted by subcritical butane extraction. Journal of the Science of Food and Agriculture 97(6):1894-1903. |
|
|
Horwitz W (2010). Official methods of analysis of AOAC International. Volume I, agricultural chemicals, contaminants, drugs/edited by William Horwitz. Gaithersburg (Maryland): AOAC International, 1997. |
|
|
Ibeto CN, Okoye COB, Ofoefule AU (2012). Comparative study of the physicochemical characterization of some oils as potential feedstock for biodiesel production. International Scholarly Research Notices 2012. |
|
|
ISO (2009). ISO 660: Animal and vegetable fats and oils: determination of acid value and acidity. ISO Geneva. |
|
|
ISO (2017). ISO 12966?2: 2017. Gas chromatography of fatty acid methyl esters-Part 2: Preparation of methyl esters of fatty acids) with some modifications. Developed by ISO/TC 34/SC 11: Animal and vegetable fats and oils. International Organization for Standardization Geneva. |
|
|
ISO U (2015). 12966-4. Animal and Vegetables Fat and Oils. Gas Chromatography of Fatty Acid Methyl Esters. Part 4: Determination by Capillary Chromatography. International Organization for Standardization: Geneva, Switzerland. |
|
|
Ixtaina VY, Martínez ML, Spotorno V, Mateo CM, Maestri DM, Diehl BW, Nolasco SM, Tomás MC (2011). Characterization of chia seed oils obtained by pressing and solvent extraction. Journal of Food Composition and Analysis 24(2):166-174. |
|
|
Ixtaina VY, Nolasco SM, Tomas MC (2008). Physical properties of chia (Salvia hispanica L.) seeds. Industrial crops and products 28(3):286-293. |
|
|
Kaale LD, Eikevik TM (2014). The development of ice crystals in food products during the superchilling process and following storage, a review. Trends in Food Science and Technology 39(2):91-103. |
|
|
Kibui AN, Owaga E, Mburu M (2018). Proximate composition and nutritional characterization of Chia enriched yoghurt. African Journal of Food, Agriculture, Nutrition and Development 18(1). |
|
|
Klikarová J, ?eslová L, Fischer J (2021). Rapid analysis of phenyl isothiocyanate derivatives of amino acids present in Czech meads. Journal of Chromatography A. 1644:462134. |
|
|
Knothe G (2002). Structure indices in FA chemistry. How relevant is the iodine value? Journal of the American Oil Chemists' Society 79(9):847-854. |
|
|
Kulczy?ski B, Kobus-Cisowska J, Taczanowski M, Kmiecik D, Gramza-Micha?owska A (2019). The chemical composition and nutritional value of chia seeds-Current state of knowledge. Nutrients 11(6):1242. |
|
|
Nitrayová S, Brestenský M, Heger J, Patráš P, Rafay J, Sirotkin A (2014). Amino acids and fatty acids profile of chia (Salvia hispanica L.) and flax (Linum usitatissimum L.) seed. Potravinarstvo Slovak Journal of Food Sciences 8(1):72-76. |
|
|
Rahmawaty S, Meyer BJ (2020). Stunting is a recognized problem: Evidence for the potential benefits of ω-3 long-chain polyunsaturated fatty acids. Nutrition 73:110564. |
|
|
Rajaram S (2014). Health benefits of plant-derived α-linolenic acid. The American Journal of Clinical Nutrition 100(suppl_1):443S-448S. |
|
|
Reason AJ (2003). Validation of amino acid analysis methods. Protein sequencing protocols (pp. 181-194). Springer. |
|
|
Romankiewicz D, Hassoon WH, Cacak-Pietrzak G, Sobczyk M, Wirkowska-Wojdy?a M, Cegli?ska A, Dziki D (2017). The effect of chia seeds (Salvia hispanica L.) addition on quality and nutritional value of wheat bread. Journal of Food Quality 2017. |
|
|
Segura-Campos MR, Ciau-Solís N, Rosado-Rubio G, Chel-Guerrero L, Betancur-Ancona D (2014). Chemical and functional properties of chia seed (Salvia hispanica L.) gum. International Journal of Food Science 2014. |
|
|
Segura-Campos MR, Salazar-Vega IM, Chel-Guerrero LA, Betancur-Ancona DA (2013). Biological potential of chia (Salvia hispanica L.) protein hydrolysates and their incorporation into functional foods. LWT-Food Science and Technology 50(2):723-731. |
|
|
Shahidi F, 2005. Quality Assurance of Fats and Oils. Bailey's Industrial Oil and Fat Products, vol. 6 of Edited by F. Shahidi. John Wiley & Sons. |
|
|
Shen Y, Zheng L, Jin J, Li X, Fu J, Wang M, Guan Y, Song X (2018). Phytochemical and biological characteristics of Mexican chia seed oil. Molecules 23(12):3219. |
|
|
Suri S, Passi SJ, Goyat J (2016). Chia seed (Salvia hispanica L.)-A new age functional food, 4th International Conference on Recent Innovations in Science Engineering and Management. pp. 286-299. |
|
|
Swanson D, Block R, Mousa SA (2012). Omega-3 fatty acids EPA and DHA: health benefits throughout life. Advances in nutrition 3(1):1-7. |
|
|
Tessari P, Lante A, Mosca G (2016). Essential amino acids: master regulators of nutrition and environmental footprint? Scientific reports 6(1):1-13. |
|
|
Timilsena YP, Vongsvivut J, Adhikari R, Adhikari B (2017). Physicochemical and thermal characteristics of Australian chia seed oil. Food Chemistry 228:394-402. |
|
|
Ullah R, Nadeem M, Khalique A, Imran M, Mehmood S, Javid A, Hussain J (2016). Nutritional and therapeutic perspectives of Chia (Salvia hispanica L.): A review. Journal of Food Science and Technology 53(4):1750-1758. |
|
|
Uzunova G, Perifanova-Nemska M, Petkova Z, Minkova S, Nikolova K (2019). Physicochemical characteristic of chia seed oil from Peru. BULGARIAN CHEMICAL COMMUNICATIONS 217. |
|
|
Valdivia-López MÁ, Tecante A (2015). Chia (Salvia hispanica): A review of native Mexican seed and its nutritional and functional properties. Advances in Food and Nutrition Research 75:53-75. |
|
|
Wu H, Sung A, Burns?Whitmore B, Jo E, Wien M (2016). Effect of Chia Seed (Salvia hispanica, L) Supplementation on Body Composition, Weight, Post?prandial Glucose and Satiety. The FASEB Journal 30:lb221-lb221. |
|
|
Yuan Q, Tu M, Gao P, Hu C, He D (2020). Comparative analysis of rapeseed oils prepared by three different methods. Journal of Oleo Science 69(12):1641-1648. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0