Full Length Research Paper
ABSTRACT
Diet based on whole cereal flours is associated with a high prevalence of iron deficiency anemia and zinc deficiency in low/middle-income countries. Such flours contain high content of phytate that chelates minerals such as iron and zinc, making them unavailable for absorption by humans. To improve the mineral absorption, a phytate:iron molar ratio <1 and a phytate:zinc molar ratio <5 is needed to be achieved. This study aimed to improve the phytate degradation in composite wheat-cassava-whole sorghum flour bread by adding a phytase releasing yeast Pichia kudriavzevii TY13 in baking, preincubated or not, with addition of yeast extract. The phytate and mineral contents were measured by high-performance ion chromatography. Addition of P. kudriavzevii TY13 to the composite flour dough and fermentation for 2 h at room temperature resulted in a 98% phytate degradation. However, the same phytate reduction in the composite bread was achieved after 1 h fermentation at room temperature with addition of preincubated P. kudriavzevii TY13 plus yeast extract. Increasing the fermentation temperature to 30°C, the phytate content was equally low after fermentation for 1 h with P. kudriavzevii TY13 (preincubated or not) plus yeast extract. In conclusion, a faster reduction of phytate in composite bread was obtained by increasing the fermentation temperature, and addition of P. kudriavzevii TY13 (preincubated or not) with added yeast extract. The phytate to iron molar ratio was then 0.2 and the phytate to zinc molar ratio 0.6, which strongly indicates an improved bioavailability of both minerals from such a bread.
Key words: Phytate, phytase, Pichia kudriavzevii TY13, yeast extract, wheat flour, cassava flour, sorghum flour, bread making.
INTRODUCTION
Globally, about 2 billion people are affected by anemia and it is estimated that iron deficiency is the major cause of about half of all cases. In particular, iron deficiency is highly prevalent in many low-income countries (WHO, 2015) where cereals constitute the major staple food (Taylor et al., 1995; Tatala et al., 1998; Hurrell et al., 2002). The generally accepted explanation for this situation is that legume-cereal based meals are rich in phytate (myo-inositol hexaphosphate), an antinutrient whose phosphate groups strongly chelates divalent metal ions of iron, zinc, and calcium making them unavailable for absorption by the human body (Hurrell et al., 2003; Prasad., 2013; Gupta et al., 2015).
Wheat bread is an important staple food in Mozambique, but the country only produces about 5% of its needs of wheat flour and therefore needs to rely on imported wheat at a high cost for the country (FAOStat, 2020). However, Mozambique produces other types of cereals and starchy roots that can successfully replace wheat flour to a large extent in bread making (Eduardo et al., 2014). In such local varieties of composite bread, it is essential to know how and when it is necessary to reduce the phytate content to improve mineral nutrition in Mozambique. In order to improve iron and zinc absorption, the phytate content needs to be enzymatically degraded by phytase. Phytases are a subgroup of phosphatases which catalyze a stepwise dephosphorylation of phytate to lower phosphoric esters of myo-inositol, releasing soluble inorganic phosphate and nonchelated minerals which becomes available for human intestinal absorption (Konietzny and Greiner, 2002). To improve iron absorption, it is recommended to degrade the phytate content to a phytate: iron molar ratio <1 (Hurrell et al., 2002) and for an improved zinc absorption the phytate:zinc molar ratio needs to be <15 (Nävert et al., 1985). In practice, this means that in cereal-based foods a phytate reduction of more than 95% needs to be achieved.
Several techniques have been applied to decrease the phytate content in cereal-legume based products. Examples are soaking, germination, fermentation, and addition of phytate degrading enzymes (phytases). These techniques are either based on activation of intrinsic phytases or by addition of exogenous phytase in the form of microorganisms synthesizing phytase or commercial phytases extracted from Aspergillus niger.
Soaking cereal flours of rye, maize, and sorghum at optimal conditions for phytase activity (55°C and pH 5) has been found to almost completely degrade the phytate content (Sandberg and Svanberg, 1991) and similar results were obtained in cereal-legume-based complementary foods soaked at optimal conditions for intrinsic cereal phytases (Egli et al., 2003). Soaking of milled wholegrains of sorghum and maize in de-ionised water without pH adjustment resulted in reduction of the phytate:iron molar ratio, however not to a level below one (Kruger et al., 2014). During fermentation, the production of organic acids (mainly lactic acid) causes a reduction in pH to levels at which the endogenous phytase in the cereal flours more efficiently degrades phytate. Studies have shown that a natural lactic acid fermentation of sorghum flours may reduce the phytate content up to 70% (Matuschek et al., 2001), and in combination with presoaking the sorghum flour an almost complete degradation of the phytate content was achieved (Svanberg et al., 1993). Kruger et al. (2012) succeeded to reduce the phytate content in a lactic fermented porridge of a genetically modified low phytate sorghum by 90%, however, still with a phytate:iron molar ratio >1.
In an attempt to optimize the baking conditions for phytate degradation by activation of intrinsic phytase, extended proofing time (2 h) at 37°C and pH adjusted to pH 4.5, Türk et al. (1996) succeeded to reduce up to 96% of the phytate content in whole wheat bread. Addition of a commercial microbial phytase from A. niger has been shown to completely degrade the phytate content in preparation of cereal-based complementary porridges (Hurrell et al., 2003). However, adding phytase from A. niger in baking of whole wheat bread has been less successful in reducing the molar ratio of phytate:iron to lower than one (Penella et al., 2008; Haros et al., 2001; Rosell et al., 2009). It would also be difficult in Mozambique to implement the strategy of adding a commercial phytase, since no such food grade product is up to now available in the country. It would thus be an advantage if the phytase could be naturally administered with the yeast in the baking process.
In the present study, a natural yeast Pichia kudriavzevii TY13 isolated from lactic fermented Tanzanian maize gruels (togwa) (Hellström et al., 2010) was applied with the aim to produce a composite bread with a phytate:iron molar ratio <1. Yeasts from the genera P. kudriavzevii has got GRAS status (Kurtzman et al., 2011) and the TY13 strain has shown a high capacity to synthesize and release phytase to the surrounding medium (Hellström et al., 2015; Qvirist et al., 2017). To achieve the aim, different strategies to improve the biosynthesis and release of phytase from P. kudriavzevii TY13 in the bread dough was applied, such as addition of growth promoting yeast extract, preincubation of the yeast and increased fermentation temperature and time.
MATERIALS AND METHODS
Ingredients
The ingredients used for the composite flours were wheat (Triticum aestivum) flour of lower extraction rate of 72%, which is a commercial white flour from Sweden (Frebago 1050 Bagerivetemjöl), cassava flour (Manihot esculenta Crantz) and non-tannin white whole sorghum flour (Sorghum bicolor) from Inhambane province in Mozambique (harvested in 2015). Cassava roots were peeled, washed, cut in pieces, and sun-dried for 4 days. During the drying period, the cassava pieces were flipped from time to time to ensure no mold contamination and then milled, packed, and stored. The sorghum grains were harvested, then washed, and damaged grains sorted out. After sun-drying for 2 days, the grains were milled at 100% extraction rate and finally packed and stored.
Bread making procedure
The bread was prepared by mixing 100 g of wheat flour, 50 g of cassava flour and 50 g of whole sorghum flour for 1 min in a kitchen aid (Artisan, Model 5KSM 150, USA), and then 135 mL of water and the rest of the ingredients were added (sugar 4 g, salt 2 g, baking yeast 4 g, margarine 6 g and ascorbic acid 0.1 g). The mixture was then blended for 2 min at speed level 2 followed by 3 min at speed level 4. At the mixing stage, 2 g of P. kudriavzevii TY13 was also added (with or without 2 g of yeast extract) or preincubated P. kudriavzevii TY13, and the pH of the dough was adjusted to 4.0 by adding a 20% lactic acid solution (~20 mL). Preincubation of 2 g P. kudriavzevii TY13 was done in 100 mL water of 30°C adjusted to pH 4.0 with lactic acid (with or without 2 g of yeast extract) and then the mixture was kept in a heating cabinet at 30°C for 1 h. The incubated yeast mixture was then added to the dry bread ingredients with an additional 35 mL of water. The dough was then covered with cotton cloth and left to ferment for 1 h at room temperature (~21°C) or at 30°C. After weighing, the dough was divided in four round shaped 80 g pieces and placed into baking pans and left to ferment for another 1 h and then baked as rolls for 8 min at 250°C in a kitchen oven. During the whole bread making process samples were withdrawn at all stages; after mixing, after fermentation (1 or 2 h) and at 30 min after baking and cooling. The withdrawn samples were placed into a plastic test tube and immediately frozen at -20°C. Finally, the samples were transferred to the freeze drier and dried for three days.
Phytate extraction and analysis
The phytate content was determined using an HPIC method developed by Carlsson et al. (2001). A milled freeze-dried sample of 0.5 g was extracted with 10 mL of 0.5 M HCl for 3 h at room temperature (22°C) under magnetic stirring. The extracts were frozen overnight, thawed, and centrifuged at 12000 rpm corresponding to 13400 × g for 5 min, and the supernatants were then decanted and 50 µL of supernatants injected and analyzed by HPIC with an Omni Pac PAX-100 (4 × 250 mm) analytical column and a PAX-100 (4 × 50 mm) guard-column (Dionex Corp., Sunnyvale, CA, USA). The separated inositol phosphates were detected after a post-column reaction with Fe(NO3)3×9H2O, using UV detection at 290 nm (Waters 486, tunable absorbance detector, Massachusetts, USA). The concentrations of phytate (myo-inositolhexakisphosphate, InsP6) were calculated using a standard curve created by external standards of InsP6. All the reagents were of analytical grade (Sigma-Aldrich Co, St. Louis, MO, USA), and de-ionized water was used. The concentrations are presented on dry weight (DW) basis as the mean ± SD µmol/g.
Mineral extraction and analysis
For mineral extraction and analysis, the procedure according to Fredrikson et al. (2002) was followed. Approximately 250 mg of freeze-dried and ground sample was digested with 0.75 mL of nitric acid and 0.15 mL of concentrated hydrogen peroxide in Teflon vessels in an Ethos Plus microwave reaction system (model Multiwave PRO, Anton Paar Co., Ashland, VA, USA). After digestion to a transparent solution, samples were cooled to room temperature and diluted to a final volume of 10 mL with deionized water. Then 0.1 mL of ascorbic acid solution (20 g/L) was added to a sample volume of 0.9 mL to reduce Fe3+ to Fe2+ to avoid two peaks from the same element. A volume of 50 µL was injected and analysed by ion chromatography equipped with an IonPac CS5A column (250 × 4 mm, Dionex Corp., Sunnyvale CA) coupled with UV-vis detection at 500 nm, based on the formation of mineral complexes by pyridine-2,6- dicarboxylic acid in the mobile phase. Standard mineral solutions between 5 and 1000 ppb of iron and zinc were prepared by diluting an original concentration of 1000 ppm of the minerals in 0.05 M HCl and 2 g of ascorbic acid/L. For quality control in each batch of microwave digestion one vessel was used as a blank analysis, containing the nitric acid and peroxide hydrogen but not the samples.
Phosphate analysis
The content of dissolved inorganic phosphate (orthophosphate) in the yeast extract (Lot 105522, Scharlau Microbiology, Scharlab S.L., Spain) and the composite flour components was determined according to the HPIC method described by Qvirist et al. (2015). Each sample (~0.1 g) was mixed in 10 mL deionized water with pH adjusted to ~4.0 with added lactic acid, left to stand under agitation for 1 h and then centrifuged at 5000 g for 5 min. Aliquots of the supernatant were diluted in deionized water to achieve concentrations between 1 and 20 mg/L phosphate. The chromatograph consisted of a Dionex GS50 gradient HPLC pump equipped with a PAX-100 OmniPac guard and analytical column (Dionex Corp., Sunnyvale CA) and an anion self-regenerating suppressor (ASRS-300, 4 mm) at 50 mA (Dionex Corp.). The phosphate was eluted at a flow rate of 0.8 mL/min, using a gradient elution ranging from 2 to 49% of NaOH (0.2 M) with H2O as counter eluent and a constant 2% isopropanol solution (50% in H2O). Phosphate was detected using a conductivity detector (CD20, Dionex Corp.). Total run time for each sample was 35 min. Standard phosphate solutions of 1 to 10 µg/mL was used and the phosphate concentration was quantified by integrating the peak using the software Chromeleon (Dionex Corp).
Determination of dry matter
The dry matter was determined by a moisture balance encompassing the Precisa 310M mass balance and HA300 dryer (Precisa, Dietikon, Switzerland). A temperature of 70°C was used and initial sample weight was approximately 0.5 g.
Determination of pH
The pH was measured using Mettler Toledo MA 235 pH/Ion Analyzer. A 16 g dough piece was weighed and put into a flask tube and 8 g of water was added, then stirred using an electromagnetic plate (Retsch, Germany) and stirrer bar for 5 min. Finally, the pH was read using the pH meter probe.
Chemicals
The used chemicals were hydrochloric acid from Scharlau (Scharlab S.L. Spain), concentrated nitric acid from Fisher Chemical (Sweden), lactic acid from Scharlau (Scharlab S.L., Spain), deionized water, iron nitrate, ascorbic acid, peptone from (BactoTM, USA), glucose from Sigma Aldrich (Merck, Germany) and agar from Oxoid (Thermo Fisher, UK). The yeast extract was obtained from Scharlau Microbiology (Lot 105522, Scharlab S.L. Spain) that according to the manufacturer had a total nitrogen content of 10.9% corresponding to a crude protein content of 68.1 g/100 g out of which free amino acids constituted about 32 g/100 g. The total content of B-vitamins was about 140 mg/100 g. The phosphate content in the yeast extract measured by the HPIC method was 14.80 mg/g.
Preparation of yeast culture
A non-genetically modified strain of a wild-type P. kudriavzevii (TY13) (Qvirist et al., 2017) was used in the present study. The P. kudriavzevii TY13 was long term stored in 15% glycerol at -80°C and short term stored during the experimental periods on YPDA plates (yeast extract 10 g, peptone 20 g, glucose 20 g and agar 20 g in 1 L H2O) at 4°C. As precultures, 5 mL of YPD in Falcon tubes were inoculated with TY13 from fresh YPD plates and incubated in a rotating carousel for 24 h at 30°C. The precultures were inoculated into yeast biomass production flasks: a set of 250 mL shake flasks each containing 200 mL YPD which were incubated for 24 h at 30°C under rotary shaking. To collect the yeast biomass, cultures were centrifuged at 4500 × g for 10 min using a Hereaus Multifuge (Kendro, Osterode, Germany), the supernatants were discarded, and the compressed yeast pellets were stored in a cold room (+4°C) until used within a few days.
Statistical analyses
Data are presented as mean values ± standard deviation of at least 3 replications. All statistical analyses were performed using SPSS (version 15.0, SPSS Inc., Chicago, IL) software. Mean values were compared by analysis of variance, and determination of significant differences between variables was made with Tukey´s HSD post hoc multiple range test. Differences were considered to be significant at p<0.05.
RESULTS
Phytate and mineral content
The cassava flour had a lower content of minerals, 3.8 µg/g for iron and 4.6 µg/g for zinc, compared with the wheat and much lower compared with whole sorghum flours (Table 1). In the composite flour with a mixture of the three flours, thus a significantly higher mineral content was obtained, for iron 26.3 µg/g and zinc 8.2 µg/g. The inorganic phosphate content in the three composite flours was for wheat 1.59 mg/g, for sorghum 1.32 mg/g, and for cassava 1.23 mg/g. In the yeast extract the phosphate content was significantly higher, 14.80 mg/g.
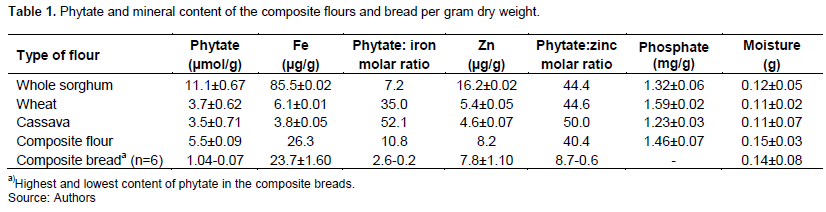
The highest phytate content was found in whole sorghum flour (11.1 µmol/g) with significantly lower content in wheat (3.7 µmol/g) and cassava flours (3.5 µmol/g). The phytate to iron molar ratios were high in all the flour types including the composite flour, from 7 in the whole sorghum flour to 52 in the cassava flour and about 11 in the composite flour. Moreover, the phytate to zinc molar ratios were also high in the flours, ranging between 40 and 50.
Phytate degradation at different stages in the baking process
Figures 1 to 4 show the phytate content at different stages in the baking process; after the mixing stage, after fermentation for 1 and 2 h at either room temperature or 30°C, and in the final composite bread. The pH was adjusted to 4.0 at the mixing stage and 2.0 g (wet weight) of P. kudriavzevii TY13 was added, preincubated or not, and with and without the addition of 2.0 g yeast extract. A composite bread without the addition of P. kudriavzevii TY13 was used as a reference bread.
Dough mixing step
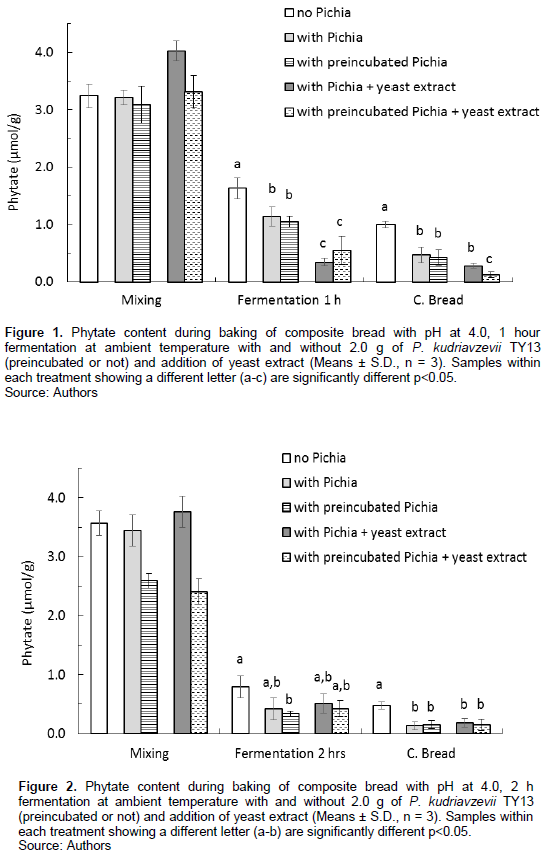
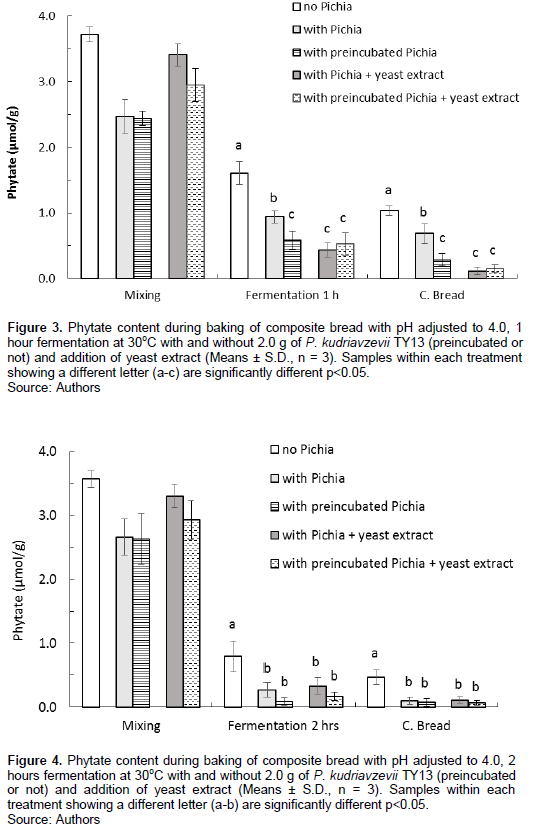
Already at the mixing stage at room temperature, there was a significant reduction of the phytate content, between 27 and 42% in comparison with the phytate content in the composite flour. There was, at this stage, no difference between the doughs with and without added P. kudriavzevii TY13 (Figures 1 and 2). There was an additional degradation in the doughs with added preincubated P. kudriavzevii TY13 (p<0.05), with a phytate reduction up to 56% observed when mixing was done at room temperature (Figure 2). However, there was no further enhanced phytate degradation by adding yeast extract in the preincubation with P. kudriavzevii TY13. Dough mixing at the higher temperature of 30oC (Figures 3 and 4) resulted in phytate reductions between 38 and 56% with addition of P. kudriavzevii TY13.
Dough fermentation step
The fermentation step resulted in a continued phytate degradation, and both preincubation of P. kudriavzevii TY13 and addition of yeast extract had a significant effect. After fermentation for 1 h, both at room temperature and 30°C, with added P. kudriavzevii TY13, the phytate content was about 1.0 µmol/g and significantly lower, about 0.40 µmol/g with addition of yeast extract (Figures 1 and 3). There was an additional phytate reducing effect by adding preincubated P. kudriavzevii TY13 at the higher fermentation temperature (30oC), the phytate content being 0.58 µmol/g compared with 0.94 µmol/g (p<0.05) in the dough with non-preincubated P. kudriavzevii TY13 (Figure 3). By increasing the fermentation time to 2 h at 30oC, the lowest phytate content of 0.09 µmol/g was achieved in the dough with preincubated P. kudriavzevii TY13 and no additional effect was at these conditions observed with addition of yeast extract (Figure 4).
In composite bread
By increasing the fermentation time, additional degradation was achieved in the composite breads with P. kudriavzevii TY13, with and without yeast extract. After 2 h fermentation at either room temperature or 30oC, the phytate content was consistently low and reduced to between 0.07 and 0.18 µmol/g (Figures 2 and 4). This corresponds to a phytate:iron molar ratio of 0.2 to 0.4 and a phytate:zinc molar ratio of 0.6 to 1.5.
However, the same low phytate content was achieved in the composite bread fermented for 1 h at 30oC with P. kudriavzevii TY13, preincubated or not plus addition of yeast extract (Figure 3) as well as in the composite bread fermented for 1 h at room temperature with preincubated P. kudriavzevii TY13 and additional yeast extract (Figure 1).
DISCUSSION
Phytate and mineral content
Cassava has generally a lower amount of minerals than cereals and a major part of the minerals are found in the root peels (Montagnac et al., 2009). The highest content of phytate and minerals was in our work obtained in whole sorghum flour as a result of the bran fraction, which is rich in phytate as well as minerals (Hurrell, 2003). Since one main function of phytate in plant seeds is mineral storage, this was expected. On the other hand, the wheat and cassava flours used in our study had lower iron and zinc contents because of low extraction rate for the wheat (not fortified) and exclusion of peels for the cassava roots during flour preparation. The phytate content of the wheat and cassava flours were at the same level as previously reported (Lazarte et al., 2015). Of the bread flour ingredients, the whole sorghum flour had the lowest phytate to iron molar ratio of 7.2 due to its high content of iron, whereas the composite flour used for the baking experiments had a ratio of 10.8. As this is many-fold higher than 1, composite bread products prepared from such flours are expected to result in low intestinal absorption of iron in humans (Hurrell, 2004). However, this may be improved by strategies to reduce the phytate content during the baking process, to obtain phytate to iron molar ratios below 1. This would sufficiently decrease the relative fraction of chelated non-available Fe in favor of non-chelated Fe, available for intestinal uptake. The same initial problem was true for zinc; phytate to zinc molar ratio was higher than 15, demonstrating low zinc absorption (Nävert et al., 1985).
Phytate degradation at different stages in the baking process
The baking procedures were based on strategies to allow for a higher phytate degradation in the composite breads, that is, increased fermentation time and temperature, and the addition of the non-conventional yeast P. kudriavzevii TY13, in prior work shown to be superior in phytate degradation (Hellström et al., 2010, 2012, 2015; Qvirist et al., 2017). The yeast was added at the mixing stage, with or without preincubation, and addition of yeast extract. The rationale behind adding yeast extract was based on our earlier work demonstrating that addition of yeast extract to a synthetic yeast medium (YNB) increases phytase release to the surrounding (assessed as activity in cell free supernatant after centrifugation). In those experiments, it was shown that the observed increased activity was not a result of increased growth rate (compared with same medium without yeast extract) but by increased secretion of non-cell-bound phytase (Hellström et al., 2015).
In the present work, there was a significant phytate degradation at the mixing stage at room temperature, and especially so with addition of preincubated P. kudriavzevii TY13. With a higher temperature in the mixing stage (30°C) there was a significantly higher phytate degradation in the doughs with added P. kudriavzevii TY13, on average 50% compared with the doughs without addition of P. kudriavzevii TY13, ~35%. Thus, the degradation observed at the mixing stage suggests that prevailing conditions resulted in (i) activation of intrinsic flour phytases and (ii) production and release of active extracellular phytase by P. kudriavzevii TY13.
The composite doughs with P. kudriavzevii TY13 fermented for 1 h at room temperature showed a higher phytate reduction (79%) than the composite doughs without P. kudriavzevii TY13 (70%). This indicates that the released active phytase from the P. kudriavzevii TY13 contributed to the phytate degradation that has been achieved by the intrinsic phytases in the composite flours as earlier has been reported by Türk et al. (1996), Egli et al. (2003), and Vilanculos and Svanberg (2021). By increasing the fermentation time to 2 h, the composite doughs with P. kudriavzevii TY13 still showed higher phytate degradation (92%) than the composite dough with no P. kudriavzevii TY13 (85%).
In the composite breads with added P. kudriavzevii TY13 fermented for 1 h at room temperature, the trend was the same as in the doughs after fermentation; the phytate reduction was higher (92%) in the composite breads with P. kudriavzevii TY13 (preincubated or not) than in the breads without added P. kudriavzevii TY13 (80%). Adding yeast extract resulted in a further improved phytate degradation, 95%, that increased to 98% (p<0.05) with preincubated P. kudriavzevii TY13 (Figure 1). This effect could be explained by the higher production and release of extracellular phytase after preincubation of P. kudriavzevii TY13 plus yeast extract as previously shown by Hellström et al. (2015). Yeast extracts contain a mixture of amino acids, peptides, vitamins and carbohydrates that promotes growth of microorganisms such as yeast and fungi as well as production of secondary metabolites including phytase (K?osowski et al., 2018; Li et al., 2011; Sasirekha et al., 2012; Sørensen and Sondergaard, 2014). With respect to TY13, we have shown that the main effect is on increased biomass specific phytase activity released to the medium, rather than on general increased growth. Among tested nitrogen sources (yeast extract, meat extract and peptone from casein) in cultivation of lactic acid bacteria, the highest cell concentration and phytase biosynthesis was achieved in yeast extract cultivation (Raman et al., 2019). The same has been found true for our strain TY13 (Hellström et al., 2015).
However, high phosphate conditions are known to repress the synthesis of acid phosphatases and phytases, while limiting phosphate conditions result in their expression. A sharp decline in phytase production of A. niger was observed even at a phosphate concentration of 5 mM in the growth medium with no production at 10 mM and above (Vats and Banerjee, 2002). The yeast variety P. kudriavzevii TY13 has previously been shown to produce extracellular phytase in the presence of inorganic phosphate up to 5 mM concentration in lactic fermented Tanzanian maize gruels (Hellström et al., 2012) and in high phosphate synthetic medium (Qvirist et al., 2017). In conventional baker’s yeast, phytase activity is strongly repressed at such levels of phosphate (Andlid et al., 2004). In our study, the free inorganic phosphate concentration in the composite dough could be estimated to about ~23 mM and with addition of yeast extract to about 26 mM, and still production and release of phytase by P. kudriavzevii TY13 was observed. At the preincubation step of P. kudriavzevii TY13 plus addition of 2 g of yeast extract the inorganic acid phosphate concentration is about 3 mM which indicates no phosphate induced inhibition on phytase synthesis and secretion by P. kudriavzevii TY13.
By increasing the fermentation time to 2 h, however, all composite breads with P. kudriavzevii TY13 degraded the same high amount of phytate (98%) (Figure 2). Obviously, addition of yeast extract had no additional effect when fermentation time was prolonged at ambient temperature.
However, at a fermentation temperature of 30°C, close to optimal growth temperature for the yeast, the addition of yeast extract increased phytate degradation already after fermentation for 1 h. Most likely the yeast extract somehow induced an increased synthesis and release of phytase from the P. kudriavzevii TY13 (Hellström et al., 2015) at a higher temperature. Whether this is a result of a general higher amount of growth factors that promote yeast growth, or if yeast extract contains specific phytase biosynthesis promoting compounds is not known. No additional reduction of the phytate content was observed in the composite breads fermented for 2 h at 30°C (compared with 1 h), suggesting that all phytate available for the yeast phytase was degraded already at 1 h, where the reduction was close to 100%.
CONCLUSION
Our results show that optimized fermentation temperature and pH in the baking of composite flour bread with added phytase releasing yeast P. kudriavzevii TY13 and yeast extract as growth and/or phytase production/release promotor improves the phytate degradation in the final bread. A unique property of TY13 is its capacity to produce and release active phytase in presence of inorganic phosphate which is ubiquitous in most foods including the composite flours in this study. Adding the P. kudriavzevii TY13 with and without yeast extract directly to the dough at the mixing stage and fermentation for 2 h at room temperature resulted in an almost complete phytate degradation. The same effect was obtained in the composite bread with 1 h fermentation time at 30°C and addition of P. kudriavzevii TY13 (preincubated or not) with yeast extract. This corresponds to a phytate to iron molar ratio of 0.2 and a phytate to zinc molar ratio of 0.6. Both ratios are low enough to allow an improved absorption of both minerals in humans. Finally, the practical applications of our findings are that it is possible to obtain a composite flour bread with nearly zero phytate content by addition of P. kudriavzevii TY13 in combination with ordinary baker’s yeast plus yeast extract and baking off under optimal conditions that are possible to achieve in commercial bakeries.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Andlid TA, Veide J, Sandberg AS (2004). Metabolism of extracellular inositol hexaphosphate (phytate) by Saccharomyces cerevisiae. International Journal of Food Microbiology 97(2):157-69. |
|
|
Carlsson NG, Bergman EL, Skoglund E, Hasselblad K, Sandberg AS. (2001). Rapid analysis of inositol phosphate. Journal of Agricultural and Food Chemistry 49(4):1695-1701. |
|
|
Eduardo M, Svanberg U, Ahrné L (2014). Consumers' acceptance of composite cassava-maize-wheat breads using baking improvers. African Journal of Food Science 8(7):390-401. |
|
|
Egli I, Davidsson L, Juillerat MA, Barclay D, Hurrell R (2003). Phytic acid degradation in complementary foods using phytase naturally occurring in whole grains cereals. Journal of Food Science 68(5):1855-1859. |
|
|
FAOStat (2020). Food and Agriculture Organization of the United Nations. |
|
|
Fredrikson M, Carlsson NG, Almgren A, Sandberg AS (2002). Simultaneous and sensitive analysis of Cu, Ni, Zn, Co, Mn, and Fe in food and biological samples by Ion chromatography. Journal of Agricultural and Food Chemistry 50(1):59-65. |
|
|
Gupta RK, Gangoliya SS, Singh NK (2015). Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. Journal of Food Science and Technology 52(2):676-684. |
|
|
Haros M, Rosell CM, Benedito C (2001). Fungal phytase as a potential breadmaking additive. European Food Resources and Technology 213(4):317-322. |
|
|
Hellström A, Qvirist L, Svanberg U, Veide Vilg J, Andlid T (2015). Secretion of non-cell-bound phytase by the yeast Pichia kudriavzevii TY13. Journal of Applied Microbiology 118(5):1126-1136. |
|
|
Hellström AM, Almgren A, Carlsson NG, Svanberg U, Andlid TA. (2012). Degradation of phytate by Pichia kudriavzevii TY13 and Hanseniaspora guilliermondii TY14 in Tanzanian togwa. International Journal of Food Microbiology 153(1-2):73-77. |
|
|
Hellström AM, Vázques-Juárez R, Svanberg U, Andlid TA (2010). Biodiversity and phytase capacity of yeasts isolated from Tanzanian togwa. International Journal of Food Microbiology 136(3):352-358. |
|
|
Hurrell RF (2003). Influence of vegetable protein sources on trace element and mineral bioavailability. Journal of Nutrition 133(9):2973S-2977S. |
|
|
Hurrell RF (2004). Phytic acid degradation as a means of improving iron absorption. International Journal for Vitamin and Nutrition Research 74(6):445-452. |
|
|
Hurrell RF, Reddy MB, Burri J, Cook JD (2002). Phytate degradation determines the effect of industrial processing and home cooking on iron absorption from cereal-based foods. British Journal of Nutrition 88(2):117-123. |
|
|
Hurrell RF, Reddy MB, Juillerat MA, Cook JD (2003). Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. American Journal of Clinical Nutrition 77(5):1213-1219. |
|
|
K?osowski G, Mikulski D, Jankowiak O (2018). Extracellular phytase production by the wine yeast S. cerevisiae (Finarome strain) during submerged fermentation. Molecules 23(4):848. |
|
|
Konietzny U, Greiner R (2002). Molecular and catalytic properties of phytate-degrading enzymes (phytases). International Journal of Food Science and Technology 37(7):791-812. |
|
|
Kruger J, Taylor JR, Oelofse A. (2012). Effects of reducing phytate content in sorghum through genetic modification and fermentation on in vitro iron availability in whole grain porridges. Journal of Food Chemistry 131(1):220-224. |
|
|
Kruger J, Oelofse A, Taylor JR (2014). Effects of aqueous soaking on the phytate mineral contents and phytate: mineral ratios of wholegrain normal sorghum and maize and low phytate sorghum. International Journal of Food Sciences and Nutrition 65(5):539-546. |
|
|
Kurtzman CP, Fell JW, Boekhorst J (2011). The Yeasts, a taxonomic study. (5th edition).Volume 3. Amsterdam: Elsevier Science and Technology. |
|
|
Lazarte CE, Carlsson NG, Almgren A, Sandberg AS, Granfeldt Y (2015). Phytate, zinc, iron and calcium content of common Bolivian food, and implications for mineral bioavailability. Journal of Food Composition and Analysis 39:111-119. |
|
|
Li M, Liao X, Zhang D, Du G, Chen J (2011). Yeast extract promotes cell growth and induces production of polyvinyl alcohol-degrading enzymes. Journal of Enzyme Research 2011:1-8. |
|
|
Matuschek E, Towo E, Svanberg U (2001). Oxidation of polyphenols in phytate-reduced high-tannin cereals: Effect on different phenolic groups and on in vitro accessible iron. Journal of Agricultural and Food Chemistry 49(11):5630-5638. |
|
|
Montagnac JA, Davis CR, Tanumihardjo SA (2009). Nutritional value of cassava for use as a staple food and recent advances for improvement. Comprehensive Reviews in Food Science and Food Safety 8(3):181-194. |
|
|
Nävert B, Sandström B, Cederblad A (1985). Reduction of the phytate content of bran by leavening in bread and its effect on zinc absorption in man. British Journal of Nutrition 53(1):47-53. |
|
|
Penella JMS, Collar C, Haros M (2008). Effect of wheat bran and enzyme addition on dough functional performance and phytic acid levels in bread. Journal of Cereal Science 48(3):715-721. |
|
|
Prasad AS (2013). Discovery of human zinc deficiency: its impact on human health and disease. Advances in Nutrition 4(2):176-190. |
|
|
Qvirist L, Carlsson NG, Andlid T (2015). Assessing phytase activity - methods, definitions and pitfalls. Journal of Biological Methods 2(1):1-7. |
|
|
Qvirist L, Vorontsov E, Veide Vilg J, Andlid T (2017). Strain improvement of Pichia kudriavzevii TY13 for raised phytase production and reduced phosphate repression. Journal of Microbial Biotechnology 10(2):341-353. |
|
|
Raman S, Abdullah N, Azizi LJ, Mohamad R (2019). Improvement of phytase biosynthesis by new bacterial isolate Pediococcus pentosaceus C4/1A via continuous cultivation. Journal of Microbiology, Biotechnology and Food Sciences 8(5):1118-1124. |
|
|
Rosell CM, Santos E, Sanz-Penella JM, Haros M (2009). Wholemeal wheat bread: A comparison of different breadmaking processes and fungal phytase addition. Journal of Cereal Science 50(2):272-277. |
|
|
Sandberg AS, Svanberg U (1991). Phytate hydrolysis by phytase in cereals; effects on in vitro estimation of iron availability. Journal of Food Science 56(5):1330-1333. |
|
|
Sasirekha B, Bedashree T, Champa KL (2012). Optimization and partial purification of extracellular phytase from Pseudomonas aeruginosa p6. European Journal of Experimental Biology 2(1):95-104. |
|
|
Sørensen JL, Sondergaard TE (2014). The effects of different yeast extracts on secondary metabolite production in Fusarium. International Journal of Food Microbiology 170:55-60. |
|
|
Svanberg U, Lorri W, Sandberg AS (1993). Lactic fermentation of non?tannin and high?tannin cereals: Effects on in vitro estimation of iron availability and phytate hydrolysis. Journal of Food Science 58(2):408-412. |
|
|
Tatala S, Svanberg U, Mduma B. (1998). Low dietary iron availability is a major cause of anemia: A nutrition survey in the Lindi District of Tanzania. American Journal of Clinical Nutrition 68(1):171-178. |
|
|
Taylor PG, Mendez-Castellanos H, Martinez-Torres C, Jaffe W, Lopez de Blanco M, Landaeta-Jimenez M, Leets I, Tropper E, Ramirez J, Casal M, Layrisse M (1995). Iron bioavailability from diets consumed by different socioeconomic strata of the Venezuelan population. Journal of Nutrition 125(7):1860-1868. |
|
|
Türk M, Carlsson NG, Sandberg AS (1996). Reduction in the levels of phytate during wholemeal bread making; Effect of yeast and wheat phytases. Journal of Cereal Science 23(3):257-264. |
|
|
Vats P, Banerjee UC (2002). Studies on the production of phytase by a newly isolated strain of Aspergillus niger var teigham obtained from rotten wood logs. Process Biochemistry 38(2):211-217. |
|
|
Vilanculos SL, Svanberg U (2021). Degradation of phytate in composite wheat/cassava/sorghum bread by activation of intrinsic cereal phytase. African Journal of Food Science 15(1):1-9. |
|
|
World Health Organization (WHO) (2015). The global prevalence of anemia in 2011. World Health Organization: Geneva, Switzerland. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0