The oil and raffia palm species are indigenous to West and Central Africa, and their exploitation both at industrial and artisanal scale generate important quantities of renewable fibrous biomass that can be converted to high and low value bio-based products. Fibre samples were prepared from three raffia palm species drawn from three locations and Oil Palm Empty Fruit Bunch (OPEFB) from an industrial palm oil mill, all in the South West Region of Cameroon. The samples were tested for chemical composition using wet chemical methods and an elemental analyzer. The functional groups were determined by Fourier Transform Infrared Spectrometer (FT-IR) spectroscopy while morphological analysis and crystallinity were evaluated by scanning electron microscopy and powder X-ray diffraction respectively. The results obtained show that extractives-free raffia palm fibres are similar to OPEFB with respect to lignin, hemicellulose and cellulose contents, morphology, occurrence of silica bodies on the surface of fibres and internal porosity. Scanning Electron Microscopy (SEM) indicated that the diameter of raffia fibre were larger than those of OPEFB stalk and spikelet fibres. The content of extractives determined by gravimetric method showed significantly lower values for raffia biomass compared to EFB fibres. The response of the fibres to hypochlorite treatment as revealed by FT-IR and morphological studies was very similar. The raffia fibres reflected significantly lower ash content and the powder X-ray studies showed slight differences in crystallinity index between raffia and OPEFB. The variability in key characteristics of biomass from the different species of palm are within the limits of the variability shown by biomass from the same species of plants. This leads to the conclusion that, the two types of palm which are both indigenous to the West and Central Africa can serve as source of blended renewable biomass. Promotion of cultivation and conservation of existing raffia plantations presents as a strategy for a more sustainable supply of biomass feedstock for bio-based products.
An increasing importance of bio-based products derived from biomass is driving research aimed at gaining a better understanding of the composition, structure and reactivity of biomass from traditional and novel sources. The sustained interest in biomass is because biomass is considered a renewable feedstock (Azman et al., 2010). It can be transformed to a wide range of value-added products ranging from chemicals, pharmaceuticals and nutraceuticals at the high-value end, through liquid biofuels and materials, to solid biomass fuels and soil amendments at the low-value end using the integrated biorefinery concept (Dale et al., 2011; Wan et al., 2010). A combination of physical, chemical and biotechnological processes are used in a biorefinery concept to transform biomass, through various stages of pretreatment (deconstruction, separation, purification and conversion of major components into the value-added products). A steady supply of biomass with well-defined characteristics and proximity of source to transformation facilities are key considerations for sustainability. Ongoing research and developmental efforts are aimed at improving the sustainability of the integrated biorefinery concept which is by proposing a suitable blend sourced locally, and ensuring a smart collection and temporary storage solutions (Sommersacher et al., 2015; Zhixue et al., 2017; Rainer et al., 2009). The blending of locally available biomass from diverse agricultural and forestry residues presents as an attractive option for national economies that are exporters of primary goods from the agriculture and forestry sectors (OECD/IEA, 2010; William et al., 2009). Successful biomass blending and bio-refinery operations require comprehensive baseline data on the characteristics of each component. On the issue of sustainability, multipurpose tree crops are of great interest given the role of forests in enhancing sustainable livelihoods in many national and rural economies, especially in Africa (Aggrey et al., 2011) Palms are typically multipurpose plants, and they are exploited in various ways by different communities for food, shelter, textiles, decoration/landscaping and medicine. Several palm species such as the African oil palm (Elaeis guineensis), coconut palm (Cocos nucifera), date palm (Phoenix dactylifera) and the betel nut palm (Areca catechu) are already domesticated and exploited on an industrial scale.
Empty Fruit Bunches (EFB) constitute one of the major waste streams from the production of palm oil and have been extensively studied as feedstock for the production of natural fibres, cellulose, organic fertilizers, soil amendments and adsorbents (Abdullah et al., 2011; Yusoff, 2004). Palms of the genus Raffia have socioeconomic importance mainly in the areas where they are native, but the most important members of this genus such as Raffia hookeri and Raffia vinifera, have been classified by the Food and Agricultural Organization (FAO) as having important development potential, deserving of domestication efforts (Dennis, 1998). Important Raffia palm products exploited by local communities in West and Central Africa include palm sap and wine, building materials, textiles, substrate for the cultivation of edible beetle larvae, materials for handicraft, medicine and food (Obahiagbon, 2009). The leaf stalk or ‘raffia bamboo’ is the most versatile part of the raffia palm because fibres can be extracted from its leaflets. It also finds a variety of other uses such as; production of furniture, building and packaging materials, raw material for craftwork and as fuel (Dennis, 1998; Obahiagbon, 2009). The base leaf can be harvested throughout the year and at different stages of development. Despite their potential contribution to sustainable diets and as source of renewable biomass for multiple applications, there are no initiatives to promote the cultivation and conservation of raffia palms. Scientific knowledge of Raffia palms is limited to the physicochemical characteristics and nutritive value of the sap (or palm wine), and the physical and mechanical characteristics of fibres from the leaflets and ‘bamboo’ (Abdullah et al., 2011; Foadieng et al., 2014; Sikame et al., 2017). This paper presents the results of a comparative study based on the physicochemical properties and morphology of fibrous biomass of the base leaf from selected raffia species and fibers from empty fruit bunch of the African oil palm. The aim is to establish the feasibility of blending raffia bamboo biomass with EFB, as a strategy to improve the exploitation of raffia palms and to make biomass feedstock available.
Extraction of fibres and determination of extractives content
The raffia palm fibres were collected from four different locations and de-fibered manually. The fibres were air-dried and cut into small sizes to pass a mesh sieve size of about 0.2 - 0.4 cm and 15 g of the fibres was weighed and used for the extraction process. 200 mL of distilled ethanol was measured and transferred into a 500-mL round bottom flask and the extraction was done by mounting a soxhlet setup with the 15 g fibres and 200 mL distilled ethanol. The set-up was left to run for 8 h ensuring that distilled ethanol was steadily boiling at a temperature of 90°C. At the end of the extraction process, the fibres were air-dried for several hours to remove the extractives. Samples were coded and subjected to different treatments and Table 1 gives a summary of the codes and description of the samples that were used.

Characterization of extractives-free fibres
Determination of ash content
Ash content was determined by the gravimetric method in a muffle furnace. 5.0 g of the fibre samples was placed inside a Pyrex beaker and dried in an oven for 4 h at 105°C, then removed and cooled in a desiccator. Furthermore, 2.0 g of the sample was weighed into a 50-mL Pyrex beaker and placed on a tray into the muffle furnace, and the temperature raised to 600°C for 6 h (ashing). The ashed sample was allowed to cool overnight inside the furnace then removed and weighed immediately.
FT-IR analysis
The FT-IR spectra of the biomass samples were obtained by using PerkinElmer, FT-IR spectrum GX instrument. 2 mg of fiber was impregnated in 200 mg of dried potassium bromide (KBr) and the mixture compressed for preparation of pellets. Each spectrum was the average of 64, co-addition of scans with a total scan time of 15 s in the IR range of 4000-400cm-1.
Powder X-Ray diffraction
The XRD diffractogram of the fibre was recorded on a Bruker D8 Advance X-ray diffractometer, using a Cu K-radiation source (= 0.15406 nm, 40 kV, and 40 mA). Scans were taken over the 2θ range from 10 to 100°C in steps of 0.01°C at room temperature in open quartz sample holders.
Scanning electron microscopy
The surface morphology of the samples was examined by a Scanning Electron Microscope (SEM) (JEOL JSM-5600 or TESCAN VEGA III XMU, Q150 TL). Prior to examination, samples were prepared by mounting about 3 fibers threads (units) onto a 5 mm × 5 mm double sided carbon tape, on an aluminum stub then sputter-coated for 40 s with gold.
Elemental and proximate analysis
Samples were analyzed for their ultimate content (carbon, hydrogen, nitrogen and sulphur). The common organic elements such as C, H, N, S and O were analyzed in a PerkinElmer Elemental CHNSO analyzer (1.0 mg of the sample was used in a tin boat assortment for percentage composition by mass analysis of C, H, N and S while the percentage composition by mass analysis of O was determined by difference) while the cation composition was determined using atomic absorption spectrophotometer.
Determination of lignin
Lignin was determined according to the Klaxon method. The fibres were dried at 105°C and treated with 72% by weight sulphuric acid (H2SO4) in the ratio of 1 g fibre per 15 ml of solution with frequent stirring using a glass rod at a temperature of 4°C in a refrigerator over a period of 5 min (when the sample completely soaks in the acid). The samples were hydrolyzed for 2 h at room temperature while stirring every 5 min to ensure complete mixing and wetting. The residue was transferred into a 1000-ml flask and diluted to a 3% acid concentration with 560 ml of distilled water. This was placed on a heating mantle, attached to a reflux condenser and allowed to heat for 4 h. Finally, the hydrolysis solution was removed, filtered through a sintered glass, washed thoroughly with hot distilled water and the residue collected was dried and weighted.
Determination of holocellulose
To determine the holocelluse content, the fibres were heat treated at 90 - 95°C for 90 min with 0.7% NaClO2 solution buffered at pH 4 while maintaining the fibre liquor ratio at 1:50. Finally, 2% sodium metabisulphite solution was added to the fibres and allowed for 15 min (to reduce the chlorite action), washed thoroughly with distilled water, dried in an oven for 2 h and weighed. The fibres treated with sodium chlorite are called chlorite holocellulose or bleached fibres.
Determination of hemicellulose and cellulose
The dried chlorite holocellulose was treated in 24% KOH solution for 4 h while occasionally stirring in the liquor (ratio of 1:100). By this treatment, hemicelluloses goes into solution and the -cellulose is separated by filtration, washed thoroughly with 2% acetic acid solution then with distilled water. The product was dried and the amount of -cellulose obtained by weight difference between the holocellulose and the hemicelluloses.
Chemical composition of the fibre samples
The chemical composition of the fibres samples is shown in Table 2 and it is observed that Raffia species have lower extractives content than EFB. These interfere with some components analysis mostly lignin, thus lignocelluloses with high extractive contents are extracted first to ensure good results (Sikame et al., 2017). Hemicellulose content is slightly higher in EFB (23 to 24%) while Raffia species show a higher percentage composition of cellulose (40 - 53%) suggesting a relatively higher sugar yield. The lignin content for raffia palm (20 to 33%) and OPEFB (25 to 28%) are comparable, and are intermediate between hard woods and grasses. The cellulose, lignin and hemicellulose content of biomass for all palm species investigated compares well with the typical composition of biomass in literature (Abdul et al., 2006). Raffia species have a higher; cellulose, a lower ash, potassium and extractives contents relative to EFB. The higher cellulose content of raffia suggest higher sugar yields for bioethanol production while the lower; ash, potassium and extractive content suggest better combustion characteristics. Lignin content is variable and provides no indication of differences in lignin-related recalcitrance. These results suggest that using raffia and EFB as a blend in biomass feed stock could lead to no significant chemical changes in the property of the final product.

FT-IR spectroscopy
The FT-IR results from Figure 1 and Table 3 show that the spectra for the various samples are similar with respect to the number of bands and their frequencies especially in the fingerprint region. This result suggests that the composition of the biomass from Raffia and EFB samples are similar, notably with respect to the lignin, hemicellulose and cellulose content. The band in the range 3341 cm-1 to 3271 cm-1 expressed in all the samples corresponds to OH stretching vibrations attributed to alcohol and phenolic hydroxyl groups while the broadening of the bands indicates the presence of hydrogen bonding. The shoulder near the OH stretching vibration at 2940 – 2900 cm-1 is attributed to asymmetric vibration (VasCH2) characteristic of hemicellulose, cellulose and lignin (Justo et al., 2009; Khalil et al., 2001; Adel et al., 2011) while the band at 2165 – 2161 cm-1 is the stretching vibrations of C=C=O (ketones) and indicates the presence of ketenes. The absorption bands in the range 1750 - 1700 cm-1 for Raffia species is characteristic of pectinous material (lignin and hemicellulose) and their absence in OPEFB suggests the removal of pectinuous material and possibly some hemicellulose due to the steam sterilization of full fruit bunches that takes place during the extraction of palm oil in modern mills.
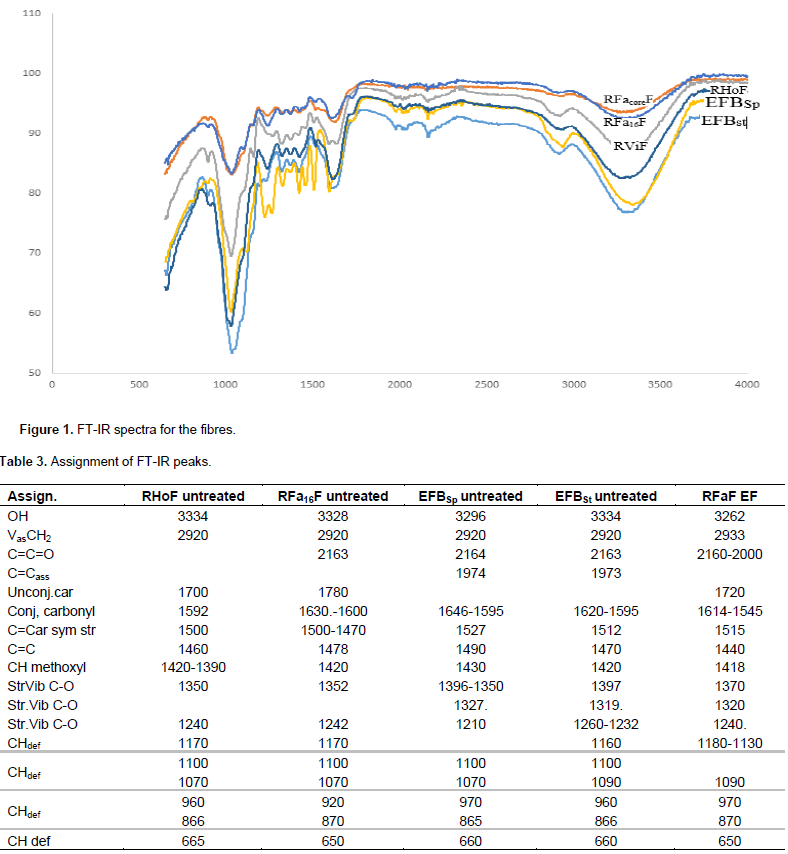
The bands in the region 1665 – 1615 cm-1 are related to the stretching vibration common to carbonyls (unconjugated carbonyls) while the bands at 1622 – 1592 cm-1 are attributed to aromatic ring vibrations earlier reported in literature (Ivan, 2008; Bermello et al., 2010). The other bands at 1505 cm-1 and the range 1461 – 1420 cm-1, are attributed to the aromatic ring vibrations (1501±3) (1461 cm-1, while bands at 1420 cm-1 are attributed to C=C aromatic ring) (Bermello et al., 2010). The bands in the range 1325 – 1319 cm-1 and 1242 – 1222 cm-1 are characteristic of the stretching vibration of lignin (syringyl rings), stretching vibrations of C-O bonds, the lignin (guaiacyl rings) and stretching vibrations of C-O bonds respectively. These results reveal the presence of two most commonly occurring building units of lignin, namely guaiacyl (G) and syringyl (S) (Sjöström, 1981). The S/G ratio varies with wood type (hardwoods, softwoods and grasses) and has been used for the classification of lignin (Ruan et al., 2004). The G/S ratios from this study (as estimated from the intensities of the peaks at 1070 cm-1 and 1090 cm-1) indicates that the lignin from Raffia and EFB fall within the same range and closer to hardwood lignin (Faix, 1992) indicating that both palm species have similar physicochemical properties.
XRD results
The X-ray diffractograms of samples are shown in Figure 2 and the fibre samples reveal crystallinity with four peaks at 2θ: 17.83, 19.33, 23.3, and 34.5, corresponding to lattice planes (101, 002 and 040) which have been earlier reported in literature (He et al., 2008). In order to examine the intensities of the diffraction bands, and further establish the crystalline and amorphous areas more exactly, the diffractograms were deconvoluted using Gaussian profiles. The results revealed that both the peak intensities and peak broadening differ from one species to another. The more pronounced difference occurred at the peak range between 2θ, 21.90° and 23.30° reflection assigned to the crystallographic plane of cellulose. The crystallographic planes are labeled according to the native cellulose structure as described by Wada and Okano (2001). The diffraction peaks in the range; 2θ, 14.5° – 15.3° are assigned to the (‾110) crystallographic plane, those in the range 15.7° – 16.30° 2θ reflection are assigned to the (110) crystallographic plane and 18.30° – 18.40° assigned to the amorphous phase while the 21.90° – 22.20° 2θ reflection are related to the (200) crystallographic plane of cellulose I (Popescu et al., 2011).

The degree of cellulose crystallinity is one of the most important crystalline structural parameters that determines strength, reactivity and recalcitrance. The crystallinity index calculated according to the Herman’s Equation (2) for all the fiber samples (Wada and Okano, 2001; Popescu et al., 2011) is presented in Table 4.
Where Cr.I. Is the crystallinity index; A (cryt.) is the sum of crystalline band areas; and A (total) is the total area under the diffract gram. The raffia fibres have lower crystallinity indices and are thus expected to show lower recalcitrance than EFB fibres; consequently, they are mechanically more workable. However, blending feedstock from the two palm species will modify the mechanical strength and the recalcitrance of the final product.

Morphological analysis
The SEM micrographs for the fibres are shown in Figure 3. The SEM images of empty fruit bunch OPEFB and raffia fibre surfaces shown in Figure 3 revealed that silica bodies were partly embedded in circular craters which are located in extracellular cavities within the hypodermal layer of cells (between the parenchymentous layers). The parenchymentous layer of raffia has a diameter of about 500 µm while that of the OPEFB is about 150 µm) and spread relatively uniformly over the strand’s surface (Figure 3a and 3f). The geometry of the protrusions was circular with the occurrence of spikes, which is consistent with the findings of Law et al. (2007) and Shamsudin et al. (2012). The silica bodies are dispersed on the surface of the fibre in aligned arrangements, as shown in Figure 3a to 3f. A similar aligned arrangement of silica bodies was observed by Sreekala et al. (1997) and Isroi et al. (2012) while a random arrangement was observed by Hamzah et al. (2011). The shape, size and distribution pattern of silica bodies observed on fiber strands of all the Raffia species studied in this work and OPEFB of Elaeis guineensis are strikingly similar to those in the epidermis of oil palm leaf (Syagrus coronata) as reported by Lins et al. (2002). These researchers also reported that silicon and oxygen are two elements present in the silica bodies. There is diversity in silica bodies with respect to the morphology, size, location, and composition, which vary with species and genera (Prychid et al., 2003).

The association of the silica bodies and fibre matrix of the OPEFB has not yet been fully elucidated (Omar et al., 2014) and neither has that of raffia palm been initialized. The deposition of silica bodies in biologically well engineered craters is a unique feature contrasting with the usual extra- and intra-cellular silica deposition (Neumann and De Figueiredo, 2002; Richmond and Sussman, 2003). The presence of these craters (Yakum et al., 2015; Khalil et al., 2001) with perforated bottoms (Figure 2c) suggests that the deposition of silica on fibre surfaces is a predetermined biological process, not a random occurrence. Such genetic arrangement underscores the biological need of raffia and oil palm trees, the purpose of which might be multifunctional and beyond the nutritional requirement. Currie and Perry (2007), Fang and Ma (2006) and Lins et al. (2002) reported in previous works that silica bodies act as a defensive barrier that protects against bacterial and fungal attacks. It is commonly understood that these biological attacks may only take place when the hydrolase enzymes attach the exposed amorphous region of the fibre. The removal of silica-bodies revealed that the bottom of the silica-crater is perforated (Figure 2c), which could suggest that the formation of silica might have originated from the interior of the fibrous strand through the probable siliceous pathways. Therefore, the removal of silica bodies could open up the siliceous pathway and expose more of the amorphous region of the fibres, resulting in better enzymatic hydrolysis performance and enhance chemical penetration in pulping. Silicon and oxygen will be taken up in the form of silicic acid, a water-soluble and uncharged monomeric molecule that is commonly transported through xylem sap tissue (Ma and Yamaji, 2006; Prychid et al., 2003). With the aid of transpiration, precipitation, and polymerization, the accumulated silicic acids will then be concentrated, resulting in the formation of colloidal silicic acid and silica gel, where finally intra and extracellular silica bodies will be formed (Neethirajan et al., 2009). Silica bodies commonly found in the epidermis or in the sheath cells of vascular bundles are also known as opaline phytoliths.
The number of silica bodies per cell varies while the location is highly dependent on the location of the cells and the plant species. Prychid et al. (2003) reported that the silica bodies infill the cell lumens and bind to cellulose in the cell walls. This will form a silico-cellulose membrane, where the cell wall is silicified and the strength will be increased. This study shows that the silica bodies are located within the cell wall or between the cellulose wall and plasma membrane which suggests that silica bodies could act as an initial protector of the cell wall components. Therefore, the absence of silica bodies may increase the cellulosic accessibility to the enzymes, resulting in good digestibility and hydrolysis performance. This is in agreement with Yunus et al. (2010) and Omar et al. (2014), where the authors claim that the silica bodies can impede penetration into a lignocellulosic matrix. Silica bodies have also been suspected to act as a barrier to enzymatic hydrolysis, as they hinder enzyme penetration into the inner layer of the OPEFB (Bahrin et al., 2012; Hamzah et al., 2011). A study by Najafpour et al. (2007) also confirmed that the removal of silica bodies from OPEFB fibre could expose the active sites of cellulose and thus increase sugar yield. In a research project by Isroi et al. (2012), fungal communities were observed to be concentrated on the empty silica body sites. From this phenomenon, it is likely that the removal of silica bodies can be a key point in exposing the cellulose or hemicellulose materials because bacterial and fungal colonisation actions are very substrate-specific. Removal of silica bodies may be performed using a number of methods, such as physical treatments (Law et al., 2007), physico-chemical treatments (Bahrin et al., 2012), and biological treatments (Isroi et al., 2012; Mohamad et al., 2013). There is a great need to remove silica bodies effectively for lignocellulosic materials from OPEFB and raffia to be used as various bioresources and value-added bioproducts. These results suggest that raffia fibres are more resistant to chemical treatment relative to the OPEFB.
The mineral and extractives content of raffia palm fibres for all species in this study are significantly lower than those of OPEFB. Lignin, cellulose and hemicellulose contents shows differences without a particular trend, and within limits of variability that have been observed for biomass from the same species, or even part of the same plant. The lignin content and the S/G ratios place both OPEFB and raffia fibre biomass closer to hard woods than soft woods. This indicates that, raffia palm fibres can be blended with OPEFB for production of paper pulp. The response of all fibres to sodium chlorite pulping did not show significant differences. This result is consistent with the expected recalcitrance, based on lignin content and cellulose crystallinity. Silica bodies were observed on all raffia fibres but the pattern of arrangement of fibres appears to be slightly different from that observed for OPEFB. Raffia fibres had larger diameters and the alignment of cellulose micro fibrils and fibre axis appears to be more linear than that in OPEFB. Crystallinity data does not distinguish between OPEFB. From the results of the chemical composition, the blending of OPEFB with raffia palm fibres should improve cellulose yields, reduce losses to effluents and improve pyrolysis/combustion properties of biomass feedstock.
The authors have not declared any conflict of interests.