Full Length Research Paper
ABSTRACT
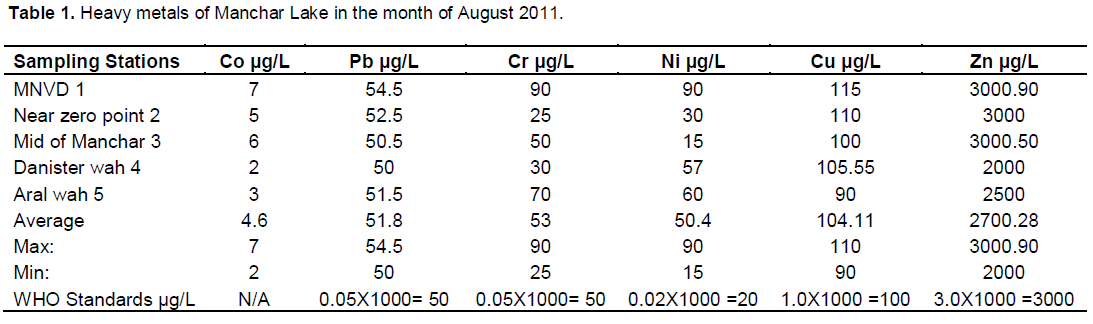
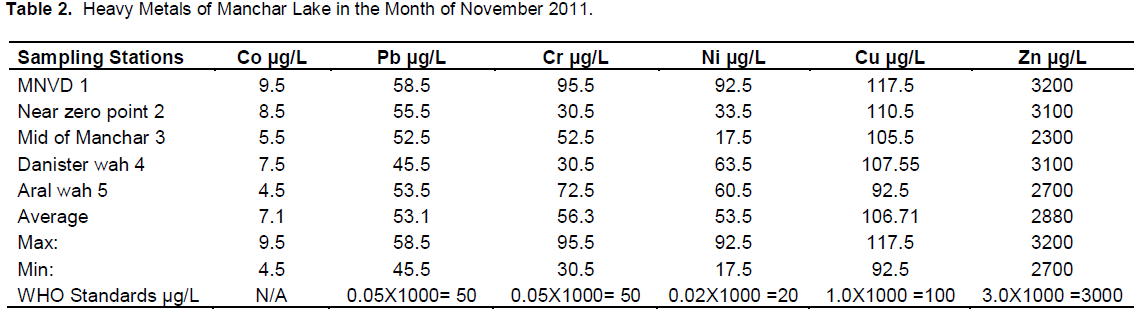
Manchar Lake is the largest fresh water lake in Pakistan, situated in Jamshoro District. It was created in the 1930 when the Sukkur Barrage was constructed on the river Indus. It is a vast natural depression flanked by Khirthar range in the west, Lakhi hills in south and river Indus in the east. On the north eastern side is the protective embankment. The lake is fed by two canals, the Aral Wah and the Danister from the river Indus. The lake also collects water from numerous small streams in the Khirthar Mountains. The area of the lake fluctuates during the flood season from 350 to 520 km2. The mean depth of the lake is at present 13 feet. Contamination of Manchar Lake is serious issue of aquatic pollution. Lake gets polluted by different waves although it is a second largest lake of Sindh province. Untreated damping of industrial liquid waste is one of the main causes of its pollution, hence for the justification of its contamination some trace metals, Lead, Copper, Zinic, Cobalt, Chromium and Nickel were analyzed by dual mode of analytical methods flame atomic absorption spectrometry (FAAS) and electro thermal atomic absorption spectrometry (ETAAS) by multi element stranded solution. The highest concentration of Lead, Copper, Zinic, Cobalt, Chromium and Nickel were 54.5 and 58.5 µg/L, 115 and 117.5 µg/L, 3000 and 3200 µg/L, 7 and 9.5 µg/L, 90 and 95.5 µg/L, 90 and 117 µg/L in month of August and November, 2011 respectively (Pb, Cu, Zn, Co, Ni, and Cr) were selected for study.
Key words: Contamination, waste, pollution, environment, fresh water.
INTRODUCTION
MATERIALS AND METHODS
RESULTS AND DISCUSSION
CONCLUSION
CONFLICT OF INTEREST
REFERENCES
|
Aamir I, Tahir S (2003). Study of trace elements water in the vicinity of Palosi Drain, Peshawar. Pak J. Biol. Sci. 6(1):86-91. Crossref |
||||
|
Abdul QS, Tasneem GK, Muhammad BA, Muhammad KJ, Hassan IA, Nusrat J, Jamil AB Ghulam AK (2009). Accumulation of arsenic in different fresh water fish species – potential contribution to high arsenic intakes. Food Chem. 112(2):520–524. Crossref |
||||
|
Anazawa K, Kaida Y, Shinomura Y, Tomlyasu T, Sakamota H (2004). Heavy metals distribution in river water and sediments around a "Firefly village" Japan: application of multivariate analysis. Anal. Sci. 201:79-84. Crossref |
||||
|
Ansari TM, Marr IL, Tariq N (2004). Heavy metals in Marine pollution perspective. A Mini Rev. J. App. Sci. 4(1):1-20. Crossref |
||||
|
Arain MB, Kazi TG, Baig JA, Jamali MK, Afridi HI, Shah AQ, Jalbani N, Sarfraz RA (2009). Determination of arsenic levels in lake water, sediment, and foodstuff from selected area of Sindh, Pakistan: Estimation of daily dietary intake, Food. Chem. Toxicol. 47(1):242–248. Crossref |
||||
|
Arain MB, Kazi TG, Jamali MK, Jalbani N, Afridi HI, Shah A (2008). Total dissolved and bioavailable elements in water and sediment samples and their accumulation in Oreochromis mossambicus of polluted Manchar Lake. Chemosphere 70(10):1845–1856. Crossref |
||||
|
Arain MB, Kazi TG, Jamali MK, Jalbani N, Afridi HI, Sarfraz RA, Baig JA, Shah AQ (2009). Assessment of water quality of polluted lake using multivariate statistical techniques, A case study. Ecotox. Environ. Safety. 72(2):301–309. Crossref |
||||
|
Borgmann U, Norwood WP, Clarke C (1993). Accumulation, regulation and toxicity of copper, zinc, lead and mercury. Hyalella azteca Hydrobiologia. 259(2):79–89. Crossref |
||||
| Bradl H (2005). Heavy metals in the environment: Origin, Interaction and Remediation. Elsevier/Academic Press, London. | ||||
|
Coogan TP, Latta DM, Snow ET, Costa M (1989). Toxicity and carcinogenicity of nickel compounds, In: McClellan RO, editor. Critical reviews in toxicology. 19, Boca Raton, FL. CRC Press, pp. 341-384. Crossref |
||||
|
Das KK, Buchner V (2007). Effect of nickel exposure on peripheral tissues: Role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev. Environ. Health. 22:133-49. Crossref |
||||
| David AW, Pamela W (2002). Environmental Toxicology: Cambridge University Press. | ||||
| Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007). Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2(5):112-118. | ||||
| Elllis KN (1989). Surface water pollution and its control: Macmillan press ltd, hound mill, Basingstoke, Hampshire RG 21 London, 3-18:97-208. | ||||
|
Garrett RR (2000). Natural sources of metals to the environment, Hum. Ecol. Risk Assess. 6:945-963. Crossref |
||||
| Goyer R (1991).Toxic effects of metals. In: Amdur MO, Doull JD, Klaassen CD, editors. Casarett and Doull's toxicology, 4th ed. New York, Pergamon Press. pp. 623-680. | ||||
|
Haijian B, Yanhong W, Zhaobin S, Shuchun Y (2011). Historical trends of heavy metal contamination and their sources in lacustrine sediment from Xijiu Lake, Taihu Lake Catchment, China, J. Environ.Sci. 23(10):1671–1678. Crossref |
||||
| Hari OS, Nepal MS, Aryo, Sindh N (1994). Combined effect of waste of distillery and sugar mill on seed germination, seeding growth and biomass of okara, J. Environ. Bio. 3(15):171-175. | ||||
| IARC (1990). Monograph on the evaluation of carcinogenic risks to humans, 49, Lyans, France. 318-411. DHHS (1994). Seventh annual report on carcinogens: Summary 1994. Research Triangle Park, NC, USA: DHHS, National Inst. Environ. Health Sci. pp. 262-269. | ||||
| Korai AL, Sahato GA, Kazi TG, Lashari KH (2008). Lead concentration in Fresh water, Muscle, and Liver of Catla Catla (Hamilton) from Keenjhar lake. Pak. J. Anal. Environ. Chem. 9(1):11-19. | ||||
|
Lashari KH, Sahato GA, Korai ALS, Habib N, Palh ZA, Urooj N (2012). Heavy metals burden of Keenjhar lake, District Thatta, Sindh, Pakistan. Afr. J. Biotechnol. 11 (59):12305-12313. Crossref |
||||
|
Ley QC, Zavala NAA, Espinosa-Carreon TL, Peckham H, Marquez-Herrera C, Campos-Villegas L (2011). Baseline heavy metals and metalloid values in blood of loggerhead turtles (Caretta caretta) from Baja California Sur, Mexico, Marine Poll. Bull. 62(9):1979–1983. Crossref |
||||
| Malle KG, Zink in der Umwelt (1994). Acta Hydrochim. Hydrobiol. 20:196-204. | ||||
| Mason CF (1998). Biology of Fresh water Pollution. Longman Scientific and Technical. | ||||
|
Miranzadeh IB, Heidari M, Mesdaghinia AR, Yousesain M (2011). Survey of microbial quality of drinking water in rural areas of kashan Iran. Pak. J. Bio. Sci. 14(1):59- 63. Crossref |
||||
| Prabu PC (2009). Impact of Heavy Metal Contamination of Akaki River of Ethiopia on Soil and Metal Toxicity on Cultivated Vegetable Crops. Elec. J. Environ. Agric. Food Chem. 8(9):818-827. | ||||
|
Rippey B, Rose N, Yang H, Harrad S, Robson M, Travers S (2008). An assessment of toxicity in profundal lake sediment due to deposition of heavy metals and persistent organic pollutants from the atmosphere. Environ, Int. 34(3):345–356. Crossref |
||||
| Sharma RK, Aqrawal M (2005). Biological effects of heavy metals: an overview, J. Env. Bio. 26(2):301–313. | ||||
| Shi B, Zhaoyu, Lu Z (2008). Environmental Pollution and Human Health, China Environmental Press. | ||||
| Taub FB (2004). Fish 430 lectures (Biological Impacts of Pollutants on Aquatic Organisms), University of Washington College of Ocean and Fishery Sciences, Seattle, WA. | ||||
| UNICEF, WHO (2009). Diarrhea: Why Children are still dying and what can be done. APHA (American Public Health Association), Standard methods for the examination of water and waste water. 1992. | ||||
| Waheed T, Kausar T (1987). Quality of drinking water in Lahore. Pak. J. Med. Res. 26:162-165. | ||||
| WHO/UNICEF (2004). Meeting the MDG drinking water and sanitation: a mid-term assessment of progress, Geneva: Switzerland. | ||||
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0