ABSTRACT
Phenylethyl alcohol is used as an antimicrobial preservative in many pharmaceutical products, especially nasal sprays. A simple and accurate reverse phase high performance liquid chromatographic method was developed to assay of phenylethyl alcohol in budesonide nasal spray preparations. A waters C18 symmetry column chromatographic system (150 × 4.6 mm, 5 µm particle size) was performed with a 50:50 (%V/V) mixture of water and acetonitrile as a mobile phase. The detection of the phenylethyl alcohol was carried out at 220 nm and flow rate was employed 1.0 ml/min. The retention time of phenylethyl alcohol was about 2.8 min. Linearity was established in the concentration range of 173.28 to 259.92 mg/ml (80 to 120% of the target concentration), with a regression coefficient of 0.9991. Specificity was tested in the presence of placebo; no interference was detected at the retention time of phenylethyl alcohol. The results of the analysis were validated statistically and recovery percentage studies confirmed the accuracy and precision of the proposed method.
Key words: Phenylethyl alcohol, budesonide, nasal spray, reversed-phase high-performance liquid chromatography (RP-HPLC), preservative.
Allergic rhinitis is a common disease and refers to inflammation of the nasal passages including sneezing, itching, nasal congestion and runny nose. Intranasal corticosteroids are among the most effective treatments for permanent allergic rhinitis. Some individuals unable to tolerate aerosols may prefer an aqueous nasal spray (Mygind, 1993).
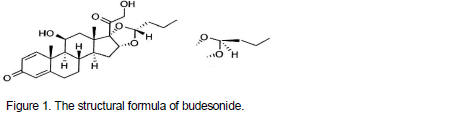
Budesonide, the active component of budesonide nasal spray is a corticosteroid designated chemically as (RS)-11β, 16α, 17, 21-Tetrahydroxypregna-1, 4-diene-3, 20-dione cyclic 16, 17-acetal with butyraldehyde. Budesonide is provided as a mixture of two epimers (22R and 22S). The empirical formula of budesonide is C25H34O6 and its molecular weight is 430.5. Its structural formula was shown in Figure 1.
Budesonide is a white to off-white, tasteless, odorless powder that is practically insoluble in water and in heptanes, sparingly soluble in ethanol, and freely soluble in chloroform. Its partition coefficient between octanol and water at pH 7.4 is 1.6×103. Budesonide is an anti-inflammatory corticosteroid that exhibits potent glucocorticoid activity and weak mineralocorticoid activity. In standard in vitro and animal models, budesonide has approximately a 200-fold higher affinity to the glucocorticoid receptor and a 1000-fold higher topical anti-inflammatory potency than cortisol. As a measure of systemic activity, budesonide is 40 times more potent than cortisol when administered subcutaneously and 25 times more potent when administered orally in the rat thymus involution assay (Rice-Thomas et al., 2009).
Antimicrobial preservatives are included in preparations to kill or inhibit the growth of microorganisms inadvertently introduced during manufacture or use. They are used in sterile preparations such as eye-drops and multidose injections to maintain sterility during use and in cosmetics, foods, and non-sterile pharmaceutical products such as oral liquids, creams, inhalations and nasal sprays to prevent microbial spoilage. The choice of a suitable preservative for a preparation depends on pH, compatibility with other ingredients, the route, dose and frequency of administration, partition coefficients with ingredients and containers or closures, degree and type of contamination, concentration required, and rate of antimicrobial effect (Thomas et al., 1989).
Phenylethyl alcohol is an excipient of budesonide nasal spray. It is an antimicrobial preservative designated chemically as 2-Phenylethanol. The empirical formula of phenylethyl alcohol is C8H10O (Figure 2) and its molecular weight is 122.17. Phenylethyl alcohol is a clear, colorless liquid with an odor of rose oil. It has a burning taste that irritates and then anesthetizes mucous membranes (Rowe et al., 2009; O'Neil et al., 2001). Phenylethyl alcohol is very soluble in alcohol, in fixed oils, in glycerin, and in propylene glycol, and sparingly soluble in water and slightly soluble in mineral oil Franson et al., 2012).
Phenylethyl alcohol in relatively low concentrations (1:400) exerts an effective inhibitory action on Gram-negative bacteria and may thus be used for differential inhibition (Lilley and Brewer, 1953; Hodges et al., 1996). Assay and detection of phenylethyl alcohol in nasal sprays is one of the important experiments during manufacturing process in quality control laboratory. Therefore the aim of this study was finding a fast and valid measurement method to assess phenylethyl alcohol in budesonide nasal sprays.
High performance liquid chromatography (HPLC) is one of the most powerful analysis methods. In recent years the use of reversed-phase high-performance liquid chromatography (RP-HPLC) method for determination of drug substances is very common and HPLC instruments and RP-HPLC solvents are available in most pharmaceutical laboratories. For this reasons, we developed a RP-HPLC method for determination of phenylethyl alcohol in Budesonide nasal spray and similar formulations which this analysis method is simple, fast, short response time, cheap price, with high accuracy and high precision. This analysis method was fully validated and can be done easily in any laboratories.
HPLC grade acetonitrile was procured from Merck Company (Germany), pure standard of phenylethyl alcohol (99.9 % w/w) was obtained from LGC Company (England) and HPLC grade water was prepared by using Millipore Milli Q plus purification system (USA). The 0.45 µm nylon filter was obtained from Millipore Company (USA) and L1 columns were procured from both Waters Company (USA) and Agilent Company (USA). All other chemicals were analytical grade and commercially available.
Chromatography (Waters HPLC system, USA) was performed with a 1525 separation module through inherent manual injector jointed to 2487 UV detector and also a 2695 separation module with inbuilt auto injector and 2996 photodiode array detector. Waters C18 symmetry column (150×4.6 mm, 5 µm particle size) and Agilent C18 column were used for chromatographic separation under isocratic elution. Detection was carried out using an UV spectrophotometric detector at 220 nm and Waters Breez and Empower software was used. The mobile phase was a 50:50 (% v/v) mixture of prepared water and acetonitrile. Mobile phase was sonicated and degassed before use. The flow rate of mobile phase was adjusted at 1.0 ml/min. The column temperature was maintained at ambient conditions. The injection volume was 20 µl and total run time was 5 min. The phenylethyl alcohol was identified by retention time of the standard phenylethyl alcohol peak. Also in specificity test, phenylethyl alcohol peak was identified against standard compound peak in the presence of placebo.
Validation of the method
Based on previous and similar chromatographic methods, the best system for determining of phenylethyl alcohol was selected (Harris, 1991; Skoog et al., 1991; Moffat et al., 2011). The method is intended to assay phenylethyl alcohol in budesonide nasal spray during analytical method validation. The method was validated, in accordance with ICH guidelines (Authors Group, 2005) and other similar works (Rao et al., 2010; Blanco et al., 1999). All validation factors such as linearity, specificity, accuracy, precision, repeatability, reproducibility and robustness were assessed.
Linearity
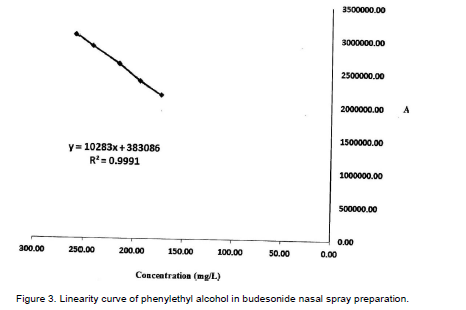
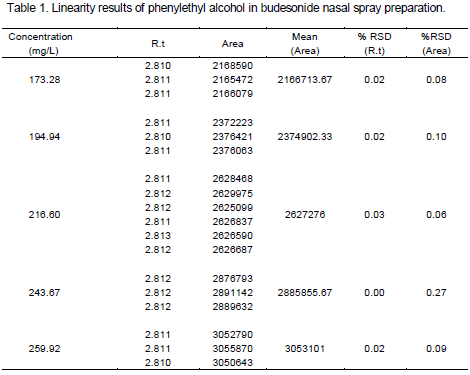
Linearity was obtained with the concentration range of 173.28 to 259.92 mg/L for phenylethyl alcohol. Linearity was performed with different dilutions. Calibration solutions were 80 to 120% of the target concentration. Calibration graph was plotted on the basis of analysis of calibration solutions. The coefficient of regression was obtained 0.9991 and the slop of 10283 was achieved (Figure 3). Standard stock solution of phenylethyl alcohol (2.166 g/L) was prepared by dissolving it in water. From this stock, concentrations of 173.28, 194.94, 216.60, 243.67, 259.92 mg/L were prepared in acetonitrile. Each solution was injected three times except that the target solution (216.60 mg/L) was injected six times. The results are shown in Table 1 and Figure 3.
Specificity
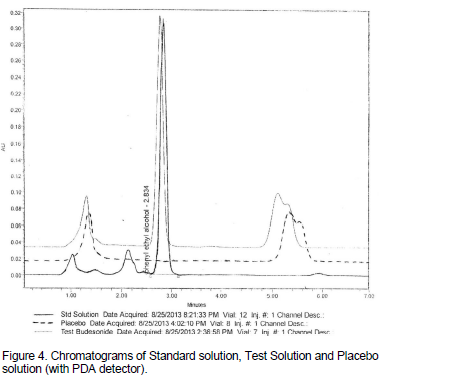
Specificity was tested against standard compound and against potential interferences in the presence of placebo. As Figures 4, 5 and 6 depict, no interference was detected at the retention time of phenylethyl alcohol in placebo solution.
Accuracy
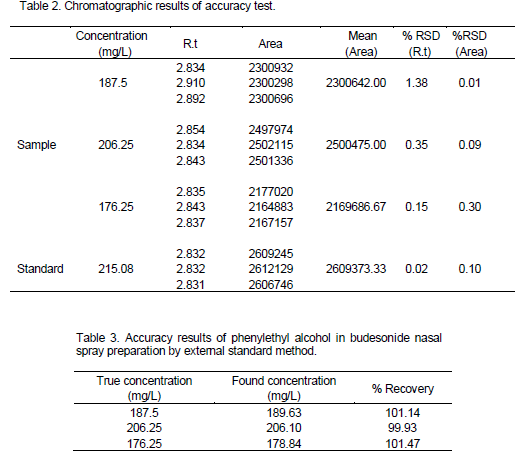
Accuracy was determined by the two methods including: 1) Spiking the phenylethyl alcohol standard in placebo. 2) Using linearity curve. Standard stock solution of phenylethyl alcohol (2.1508 g/L) was prepared in water. 1.0 ml of this solution was transferred into 10 ml volumetric flask and diluted with acetonitrile to achieve
final concentration of 215.08 mg/L. Concentrations of 187.5, 206.25, 176.25 mg/L with acetonitrile were prepared from budesonide nasal spray.
Spiking the phenylethyl alcohol standard in placebo
Accuracy was evaluated by spiking the phenylethyl alcohol standard in placebo at three different concentrations level and were calculated the recovery percentages with external standard method. Results are presented in Tables 2 and 3. Recovery percentages were in the range of 98.0 to 102.0% that show this method has suitable accuracy.
Using linearity curve
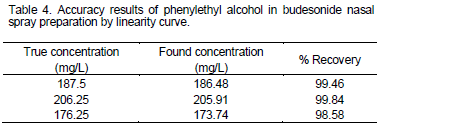
Accuracy was estimated by this method at three different concentration levels and recovery percentages were
calculated with use linearity curve. In this method concentrations were obtained by a linear equation and recovery percentages were in the range of 98.0 to 102.0% that demonstrate this method has suitable accuracy. These results are represented in Table 4. Spiking in placebo and using linearity curve overall confirm which accuracy of this method is in the series of very high-quality.
Precision
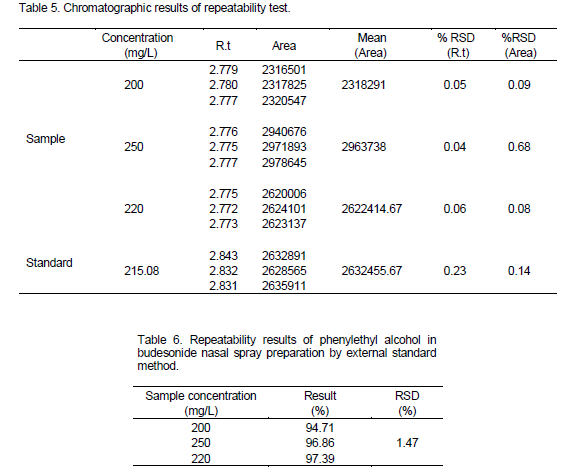
Precision was studied in the three levels including: Repeatability (Intra-assay precision), ruggedness and solution stability (Intermediate precision) and reproducibility. Standard stock solution of phenylethyl alcohol (2.1508 g/L) was prepared in water. 1.0 ml of this solution was moved to 10 ml volumetric flask and diluted with acetonitrile to achieve a final concentration of 215.08 mg/L. Concentrations of 200, 250 and 220 mg/L with acetonitrile were prepared from budesonide nasal spray.
Repeatability
Repeatability was studied at three different concentration levels and relative standard deviations of results were calculated. Results in Tables 5 and 6 were found less than 2.0%.
Ruggedness and solution stability
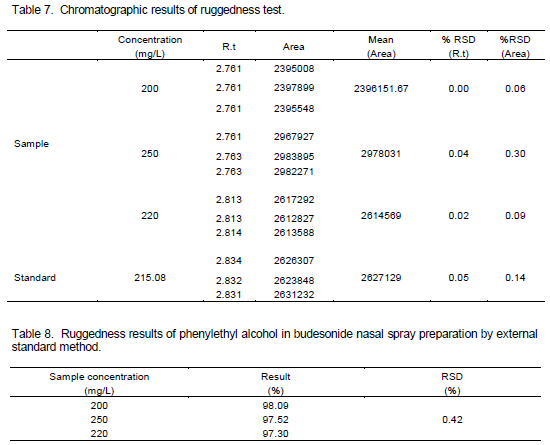
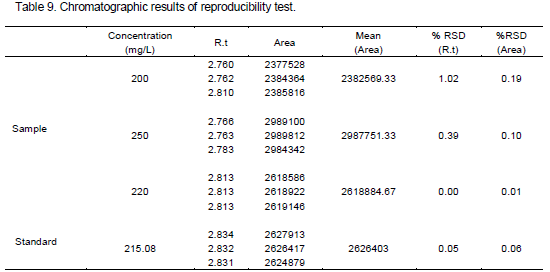
The ruggedness of the method was studied on three different days with different analysts. The relative standard deviations of results were found less than 2.0%. To demonstrate the stability of both standard and sample solutions during analysis, both solutions were analyzed over a period of 48 h at room temperature. The results showed that for all solutions, the retention times and peak areas of phenylethyl alcohol remained almost unchanged (RSD<2.0%) which indicating that no significant degradation occurred within this period. Both solutions were stable for at least 48 h. These results are presented in Tables 7 and 8.
Reproducibility
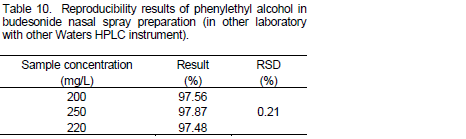
The reproducibility of method was studied in the two different laboratories. The percentage relative standard deviations of results with other Waters HPLC Instrument and in other laboratory were found less than 2.0%. The results are given in Tables 9 and 10.
Robustness
The robustness of the method was determined by making slight changes in the chromatographic conditions that is, mobile phase ±5%, flow rate ±0.1 ml/min (Woolfson et al., 2014). Also this method was done with another C18 column (150×4.6 mm, 5 µm, Agilent Company), and finally similar results were obtained.
The purpose of this study was development a method to determination of phenylethyl alcohol in budesonide nasal spray and other similar nasal anti allergic formulations. The mixture of water and acetonitrile in different ratios was examined as a mobile phase and lastly a mixture of water and acetonitrile in the ratio of 50:50 (V/V) and flow rate of 1.0 ml/min was selected. The optimum wavelength for detection was considered at 220 nm (because of no interfering and suitable shape). After obtaining these final conditions of the chromatographic system, validation of the method was performed.
(i) Linearity was recognized in the concentration range of 173.28 to 259.92 mg/ml (80 to 120% of the target concentration) with a regression coefficient of 0.9991.
(ii) Specificity was experienced in the presence of placebo; no interference was detected at the retention time of phenylethyl alcohol.
(iii) Accuracy was determined by the two technique of spiking and linearity curve. Recovery percentages were calculated and results were in the range of 98.0 to 102.0%.
(iv) Precision was studied in the three levels including; repeatability, ruggedness and reproducibility.
Percentage relative standard deviations of the results were calculated that were less than 2.0%. The results of the analysis were validated statistically and confirmed the accuracy and precision of the proposed method.
We concluded that proposed RP-HPLC method for determination of phenylethyl alcohol in budesonide nasal spray is simple, precise, specific, and highly accurate and this method is very less time consuming in quality control laboratories. So, this method can definitely be used in phenylethyl alcohol drug substance analysis and determination of phenylethyl alcohol in budesonide nasal spray and other similar nasal anti-allergic formulations such as Fluticasone nasal spray, Beclometasone nasal spray, Mometasone nasal spray and etc. The advantages of this method over other old methods are short retention time for determination of phenylethyl alcohol (about 2.7 min), simple mobile phase, economical and practical procedure to assay phenylethyl alcohol in other similar pharmaceutical products.
The authors of this paper declared no conflict of interest.
The authors are grateful to Ms. Leila Gholampour who helped sympathetically and also special thanks to Dr. Lida Khalafi and Dr. Mohammad Rafiei because of their advices on writing this paper. In the end, authors are appreciated from Research and Development Department of Jaber Ebne Hayyan pharmaceutical Company.
REFERENCES
Mygind N (1993). Glucocorticosteroids and rhinitis Allergy. Eur. J. Allergy Clin. Immunol. 48(7):476–490.
Crossref |
|
|
|
Rice Thomas F, Santarpia D, Caine CY, Lagow B, Struble S, Shenouda N (2009). Physicians Desk Reference® (PDR®) 63th editions: 654. |
|
|
|
Thomas WJ, Wade A, Reynolds James EF (1989). Martindale 29 th editions: 1355. |
|
|
|
Rowe CR, Sheskey JP, Quinn EM (2009). Handbook of Pharmaceutical Excipients, 6th editions: 490-491. O'Neil Maryadele J, Smith A, Heckelman Patricia E, Obenchain Jr JR, Gallipeau Jo Ann R, Arecca MAD (2001). The Merck Index, An Encyclopedia of chemicals, Drugs, and biologicals, Volume 2, 14 th edition, published by Merck Research Laboratories, Whitehouse station: 1296. |
|
|
|
Franson TR, Bravo RH, Courtney JE, Demars SS, Kirking DM, Williams RL (2012). United State Pharmacopeia 35 (National formulary 30), 1:1136. |
|
|
Lilley BD, Brewer JH (1953). The selective antibacterial action of phenylethyl alcohol. J. Am. Pharm. Assoc. Sci. 42:6-8.
Crossref |
|
|
Hodges NA, Denyer SP, Hanlon GW, Reynolds JP (1996). Preservative efficacy tests in formulated nasal products: reproducibility and factors affecting preservative activity. J. Pharm. Pharmacol. 48:1237-1242.
Crossref |
|
|
|
Harris CD (1991). Quantitative chemical analysis, 3th editions, W. H. Freeman and Company, New York: 666-680. |
|
|
|
Skoog AD, West MD, Holler JF (1991). Fundamentals of analytical chemistry, 6th editions, Saunders college publishing, Toronto: 712-737. |
|
|
|
Moffat CA, Osselton DM, Widdop B (2011). Clarke's analysis of drugs and poisons, 4th editions, published by Pharmaceutical Press, London: pp. 597-598. |
|
|
|
Authors Group (2005). ICH Guidelines Q2 (R1) – Validation of Analytical Procedures: Text and Methodology: pp. 1-13. |
|
|
|
Rao KLN, Reddy KP, Babu KS, Raju KS, Rao KV, Shaik JV (2010). Simultaneous estimation of Fluticasone propionate, Azelastine hydrochloride, Phenylethyl alcohol and Benzalkonium chloride by RP-HPLC method in nasal spray preparations. J. Res. Pharm. Sci. 1(4):473-480. |
|
|
Blanco M, Serrano D, Bernal JL (1999). UV-spectrophotometric determination of beclometasone dipropionate and phenylethyl alcohol in a nasal spray by inverse least-squares. Talanta 50(3):527-532.
Crossref |
|
|
|
Woolfson D, Fenton-May VI, Buckton G, Cairns DB, Cook GD, Davidson A, Duffy TD, Goddard C, Helliwell K, Horder R, Matthews BR, Tsang L (2014). British Pharmacopoeia, Appendix III, Volume 5: V-A189-190. |