ABSTRACT
Synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) are toxic and carcinogenic, thus they induce DNA damage. This calls for the need to find alternative antioxidants from natural products. Tagetes minuta (Asteraceae) is an annual herb that belongs to the Asteraceae family. It is used in common medicine and grows in temperate regions of South America, some parts of Africa and Asia. Essential oil from T. minuta was obtained by hydrodistillation while solvent extracts were obtained using ethyl acetate and methanol. Antioxidative compounds of T. minuta were isolated both from the Ethyl acetate extract and the essential oil. It was done by determining the scavenging activity using 2,2-diphenyl-1-picrylhaydrazyl free radical (DPPH) using thin layer chromatography (TLC). The active compounds were tested quantitatively for their radical scavenging activity using the U-1100 UV-VIS Spectrophotometer. The active fractions were isolated using TLC and High performance liquid chromatography (HPLC) and later detected using both Gas chromatography mass spectrometry (GC-MS) and Nuclear magnetic resonance (NMR). One pure active compound was obtained from the ethylacetate extract (neophytadene) by a combintion of GC-MS and NMR. The essential oil contained a number of compounds among which are trans-ocimen 15.90%, I-verbanone 15% of limonene 8.02%, tegetone 3.56%, and 2-pinen-4-one 7.84%.
Key words: Antioxidant activity, essential oil, 2,2-diphenyl-1-picrylhaydrazyl free radical (DPPH), Tagetes minuta.
An antioxidant is any substance, if present at low concentrations in combination with an oxidisable substrate, significantly delays or prevents oxidation of the substrate. Based on the historical success of natural products, a number of medicinal plants have been evaluated for their antioxidant potential (Argolo et al., 2004; Burits et al., 2001; Helle et al., 2004).
Free radical mediated damage is connected with several diseases, and therefore its prevention can play an important role in the cure of those diseases (Kanwal et al., 2011; Kulisica et al., 2004; Sharma and Trivedi, 2002; Smith et al., 2007). For example, oxidative stress has been widely postulated to be involved in the development and progression of some chronic diseases such as cardiovascular disease, neuronal disease, cataracts, and several types of cancer (Gua et al., 2009). There is increasing search for antioxidants that remove occurring naturally in vegetables, fruits and functional herbs to replace synthetic antioxidants. It has been found out that some synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) have been revealed to be potentially toxic and carcinogenic, they have been found to induce DNA damage (Helle et al., 2004).
Tagetes minuta (Asteraceae) is an annual herb that belongs to the Asteraceae family. Its leaves are slightly glossy, green and pinnately dissected into 4–6 pairs of pinnae (Cerruti et al., 2010; Daizy et al., 2007). It is used in common medicine and grows in temperate regions of South America, some parts of Africa and Asia (Hamill et al., 2000; Kamatenesi-Mugisha and Oryem-Origa, 2007; Tabuti et al., 2003; Vasudevan et al., 1997a). Infusions of leaves from di?erent species of Tagetes have been used to treat stomach and intestinal diseases (Gakuya et al., 2013; Harris et al., 2002; Paul and Kasenene, 2007; Tabuti et al., 2003; Vasudevan et al., 1997a), and other species have been found to possess di?erent biological activities, such as, antimicrobial, antiin?ammatory, antioxidant and antiviral (Andreotti et al., 2013; Dharmagadda et al., 2005; Hamil et al., 2000; Paul and Kasenene, 2007).
T. minuta is a wild shrub in Uganda that thrives mostly in the rainy season (Tabuti et al., 2003). Tagetes species, commonly known as marigold are also grown as ornamental plants and thrive in varied agro-climates (Vasudevan et al., 1997b). Bioactive extracts of different Tagetes parts exhibit nematocidal, fungicidal and insecticidal activity (Vasudevan et al., 1997b). T. minuta has been used by the local people in Uganda to relieve a number of ailments (Hamil et al., 2000; Paul and Kasenene, 2007).
Previous work on Tagetes species, Tagetes maxima reavealed strong antioxidant properties of its ethylacetate extracts (Parejo et al., 2005). T. maxima was found to exhibit strong radical scavanging and antioxidant activities (Parejo et al., 2005) .There is a great possibility of similar activity in other Tagetes species. Antioxidant activity of T. minuta from Uganda has not been determined according to literature, but since it belongs to the same family as other Tagetes species with strong antioxidant properties, it was necessary to determine its potential as an antioxidant.
In this research, antioxidative compounds of T. minuta were isolated both from the Ethyl acetate extract and the essential oil. It was done by determining the scavenging activity using 2,2-diphenyl-1-picrylhaydrazyl free radical (DPPH). The active compounds were tested quantitatively for their radical scavenging activity.
Plant material
Fresh aerial parts of T. minuta were collected from Mabira Forest in the morning hours in the month of November 2012. The sample was transported to Makerere University, Department of Chemistry Laboratory. Essential oils from T. minuta were extracted on arrival in the Laboratory. The remaining plant material was dried under shade for 3 weeks, ground in a mortal to obtain fine powder. A voucher specimen (CK001) was deposited at Makerere University Herbarium.
Hydrodistillation
Essential oil from fresh
T. minuta was extracted by hydro-distillation in a Clevenger type apparatus for 3 h with a separated extraction chamber. The resulting essential oils were dried over anhydrous-sodium sulphate to extract the water. The oil was kept in refrigerated conditions at 8°C prior to the antioxidant activity determination and GC-MS analysis (
Conti et al., 2010;
Polatoglu et al., 2012).
Chemicals
All chemicals and reagents used in extraction, isolation and analysis of the active compounds were obtained from Sigma-Aldrich (Germany). These chemicals and reagents were of analytical grade. The standards were also purchased from sigma-Aldrich.
Solvent extraction- Cold extraction
T. minuta dry powder (1000 g) was extracted four times with 2000 ml of ethyl acetate at 40 to 45°C. The supernatant (extract) was separated from the residue by paper filtration (Whatman No. 1 filter, whatman paper Ltd., UK). It was dried in vaccum using a rotary evaporator at 40°C to remove all the ethyl acetate to give a residue. The powder was dried and re-extracted three times with 2000 ml methanol. The extract was combined and evaporated at 40°C to dryness. Both methanolic and ethyl acetate extracts were kept in a dry place for further testing (Gua et al., 2009).
DPPH assay
The capacities to donate hydrogen atoms/electrons by the essential oil and solvent extracts from the test samples were preliminarily detected using thin layer chromatography (TLC) and further measured spectrophotometrically.
TLC screening for antioxidants
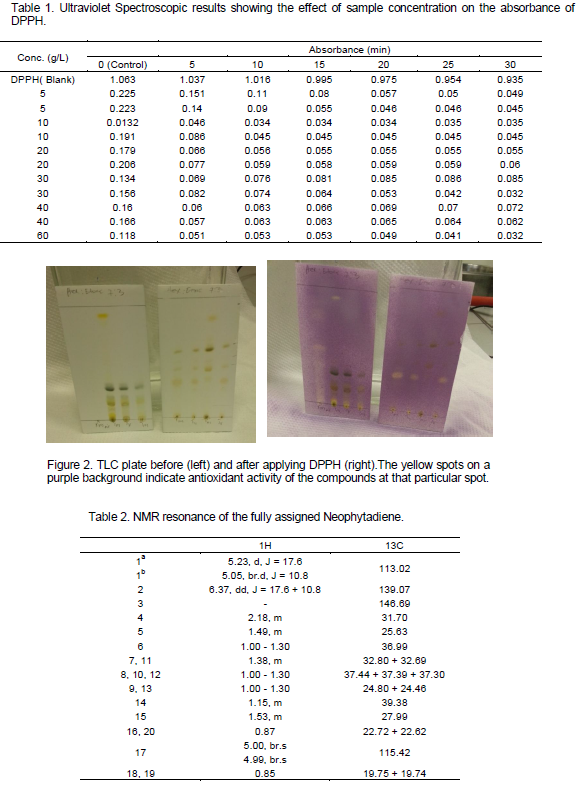
Dilutions of volatile oils (5 µl, 1:5 in hexane), and the crude extracts were spotted on silica gel sheets and developed in ethylacetate:hexane (7:3 v/v). The plates were sprayed with 0.2% solution of the stable radical, diphenylpicrylhydrazil ( DPPH) (Brand Williams et al., 1995; Burits et al., 2001; Helle et al., 2004; Xiao et al., 2010). Active spots were detected as yellow spots on a purple background. Zones where the colour changed within 30 min (after spraying) were taken as positive results (Burits et al., 2001).
DPPH spectrophotometric assay
This assay uses DPPH as a reagent (Argolo et al., 2004; Brand-Williams et al., 1995; Burits et al., 2001; Helle et al., 2004). 50 µl of various concentrations of the volatile oils were added to 5 ml of 0.004% methanolic solution of DPPH. After 30 min of incubation period at room temperature, the absorbance was read against the blank at 517 nm using a U-1100 UV-VIS Spectrophotometer (Hitachi Ltd; Tokyo Japan). The tests were carried out in duplicate. DPPH solution (1.0 ml; 0.3 mM) plus methanol (2.5 ml) was used as a negative control. After 30 min the absorbance values were measured at 517 nm and converted into the percentage antioxidant activity (AA) using the following formula:
%AA, which was, %aa=((AC(0) – AA(t))/AC(0))*100
Where A
C(0) is the AA for the control solution at t=o minutes, and A
A(ti) is the AA after the given time intervals, for I = 5, 10, 15, 20, 25, and 30 min (
Kulisica et al., 2004).
GC-MS analysis
GC-MS analysis was used to identify the compounds in the essential oil and solvent extracts that had antioxidant activity.The GC-MS results of T. minuta was already determined in a previous research (Kyarimpa et al., 2014).
High performance liquid chromatography
The active fractions were purified with HPLC. A Dionex Ultimate 3000 HPLC (Dionex) equipped with a diode array detector and operated by Chromeleon Version 6.80 SR9 software. 2.0 ml each of the active fractions was injected onto a 150 x 2.1 mm, 100 A, 2.6 µm Phenomenex Kinetex C18-column at 35°C. N-Hexane was used as a mobile phase. The flow rate was 237 µl/min. To selectively detect antioxidants, the detector recorded the signal at 520 nm (Application Note 281, Dionex Corporation, Sunnyvale, CA, USA). HPLC was carried out only samples with antioxidant activity and the solvent system was chosen based on Rf values of the TLC experiments.
Nuclear magnetic resonance (NMR) spectroscopy
Nuclear magnetic resonance (NMR) spectroscopy is (arguably) the most powerful tool available for determining the structure of organic compounds. It is used to identify and/or elucidate detailed structural information about chemical compounds. In this case it was used to determine the structure of the pure active compound in the sample.
All NMR spectra were recorded on a Bruker Avance II 400 (resonance frequencies 400.13 MHz for 1H and 100.63 MHz for 13C) equipped with a 5 mm broadband observe probe head (BBFO) with z–gradients at room temperature with standard Bruker pulse programmes. The sample was dissolved in 0.6 ml of CDCl3 (99.8% D). Chemical shifts are given in ppm, referenced to residual solvent signals (7.26 ppm for 1H, 77.0 ppm for 13C). 1H NMR data were collected with 32k complex data points and apodized with a Gaussian window function (lb = −0.3 Hz and gb = 0.3 Hz) prior to Fourier transformation. 13C-jmod spectra with WALTZ16 1H decoupling was acquired using 64k data points. Signal-to-noise enhancement was achieved by multiplication of the FID with an exponential window function (lb = 1Hz). All two-dimensional experiments were performed with 1k × 256 data points, while the number of transients (2–8 scans) and the sweep widths were optimized individually. The resulting FIDs were zero-filled to a 2k × 1k data matrix and apodized with a sine function for COSY in both the ω1 and ω2 dimensions prior to Fourier transformation.
Heteronuclear spectra were zero-filled only in F1 to a 1k × 512 data matrix, and apodized in both dimensions with a shifted sine function. The heteronuclear single quantum coherence (HSQC) experiment was acquired using adiabatic pulse for inversion of 13C and GARP-sequence for broadband 13C-decoupling, optimized for 1J(CH) = 145 Hz.
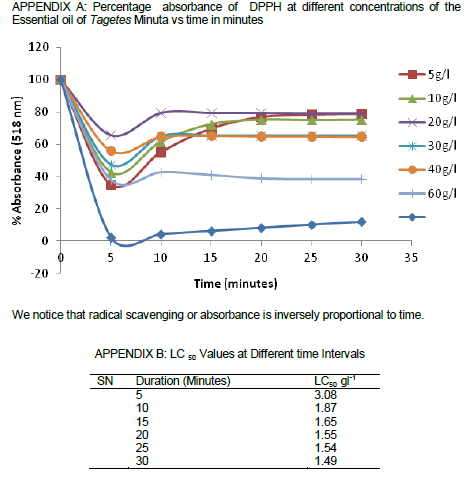
The essential oil from tagets minuta exhibited strong antioxidant activity on TLC. Some components of the crude extract were also found to be active (Figure 2). Track 1 on the first TLC plate from the left (Figure 2) was the essential oil, the other tracks were different extracts from T. minuta and Tephrosia Vogelli. It was noted that some of the components of the two plants had strong antioxidant activity. These fractions were isolated using Column Chromatography, HPLC. The pure fractions were analysed using NMR spectroscopy. Track 1 had very strong antioxidant activity as shown by the DPPH reaction on the TLC plate (Figure 2). This oil was later used for quantitative measurement again using DPPH and Ultra Violet Spectrophotometer (Table 1). The total antioxidant capacity revealed that the essential oil from T. minuta had a high antioxidant activity. Free radical scavenging activity of the extracts was assesed using the stable free radical DPPH. Plant extracts which reduce DPPH by donating hydrogen ions are considered as antioxidants having free radical scavenging activity. The results from Table 1, were used to calculate the amount of DPPH scavenged over a period of time according to the formula {%AA, which was , %aa=((AC(0) – AA(t))/AC(0))*100}, and the LC50 was determined (Appendix 1B). DPPH solution alone served as control (A0). It is evident from the study, that the investigated extracts and essential oil have the ability to quench free radicals. This indicates that T. minuta is a potential source of natural antioxidants.
Nuclear magnetic resonance (NMR) Spectroscopy
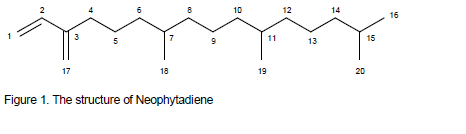
One active pure compound was identified using both the proton NMR and the carbon NMR as shown in Figure 1 and Table 2. The 1 H NMR spectra of this compound revealed the presence of two isolated olefinic spin systems without any further coupling partners: on the one hand a vinyl group, indicated by its characteristic ABX-system at α 5.05 (d, J = 10.8 Hz), α 5.23 (d, J = 17.6 Hz) and α 6.37 (dd, J = 17.6 , 10.8 Hz), and on the other hand an olefinic methylene group with broad singuletts at α 4.99 and α 5.00 ppm, respectively. Besides a triplett at α 2.18 ppm, a bulk of aliphatic methylene and methine protons in the region between 1.60 to 1.00 ppm and signals of different methyl groups at around 0.90 ppm no more signals were found in the 1H NMR spectra. In combination with the hsqc experiment the j-modulated 13C nmr spectra showed signals of 4 olefinic carbons – one quaternary, one methine, and two methylenes – 4 methyl, 3 methine and 9 methylene carbons. In addition to the molecular mass peak at m/z = 278 these results indicated that compound is a noncyclic, nonoxidized diterpene with a molecular formula of C20H38. Extensive analysis of heteronuclear 2D NMR led to the elucidation of that structure which turned out to be neophytadiene, a widespread component of essential oils from different plant sources. Whereas Burkhardt et al. published only NMR data of the olefinic part of the molecule, we present here to our best knowledge for the first time the fully assigned nmr resonances (Table 2).
Neophytadiene, is a fatty acid-related compound which plays an important part in competitive inhibition of cyclooxygenase or lipoxygenase in an inflammation reduction, resulting in decreased production of prostaglandins and leukotriene (Pillai and Nair, 2013).
DPPH is a free radical, stable at room temperature, which produces a violet solution in methanol. It is reduced in the presence of an antioxidant molecule, giving rise to uncoloured methanol solutions. The use of DPPH provides an easy and rapid way to evaluate antioxidants. According to the results obtained from data in Tables 1 and 2 as well as those of statistical analysis, we can say that extracts from T. minuta as well as its essential oil possess strong antioxidant properties with an LC50 of 1.49 g/l-1 after 30 min as compared to other antioxidants reported in Parejo et al. (2005) and Xiao et al. (2010). A well-known antioxidant, ascorbic acid, was used as positive control. DPPH scavenging patterns for T. minuta versus time, along with IC50 values, are presented in Appendix 1 A and B.
We declare no competing interests in this research.
The authors wish to acknowledge the Robert Mc. Namara Fellowship Scheme of the World Bank for the sponsorship. The University of Natural Resources and Applied life Sciences, Vienna for hosting the researcher and providing techinical guidance. Special thanks go to Prof. Stefan Bohmdorfer ,Prof Thomas Rosenau and Dr. Markus Bacher for their techinical support. The authors express their gratitude to CARNEGIE Corporation of New York for the financial support through The Science Initative Group's RISE-AFNNET, Makerere University.
REFERENCES
Andreotti R, Garcia MV, Cunha RC, Barros JC (2013). Protective action of Tagetes minuta (Asteracea) essential oil in the control of Rhipicephalus microplus (Canestrini, 1887) (Acari: Ixodidae) in a cattle pen trial. Veterinary parasitol. 197:341-345.
Crossref |
|
|
Argolo ACC, Sant'Ana AEG, Pletsch M, Coelho LCBB (2004). Antioxidant activity of leaf extracts from Bauhinia monandra. Bioresour. Technol. 95:229-233.
Crossref |
|
|
|
Brand-Williams W, Cuvelier ME, Berset C (1995). Use of fre radical method to evaluate Antioxidant Activity. Lebensm. Wiss. U-Technol. 28:25-30. |
|
|
Burits M, Asres K, Bucar F (2001). The antioxidant activity of the essential oils of Artemisia afra, Artemisia abyssinica and Juniperus Procera. Phyotherapy Res. 15:103-108.
Crossref |
|
|
Cerruti RRH, Koon-Hui W, Antoon P, Robert M (2010). Using marigold (Tagetes spp.) as a cover crop to protect crops from plant-parasitic nematodes. Appl. Soil Ecol. 46:307-320.
Crossref |
|
|
Conti B, Canale A, Bertoli A, Gozzini F, Pistelli L (2010). Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 107:1455-1461.
Crossref |
|
|
Daizy RB, Arora K, Singh HP, Kohli RK (2007). Potential utilization of dried powder of Tagetes minuta as a natural herbicide for managing rice weeds. Crop Protection. 26:566-571.
Crossref |
|
|
Dharmagadda VSS, Naik SN, Mittal PK, Vasudevan P (2005). Larvicidal activity of Tagetes patula essential oil against three mosquito species. Bioresour. Technol. 96:1235-1240.
Crossref |
|
|
Gakuya DW, Itonga SM, Mbaria JM, Muthee JK, Musau JK (2013). Ethnobotanical survey of biopesticides and other medicinal plants traditionally used in meru central district of Kenya. J. Ethnophamacol. 145:547-553.
Crossref |
|
|
|
Gua L, Wua T, Wanga Z (2009). TLC bioautography-guided isolation of antioxidants from fruit of Perilla frutescens var. Acuta LWT - Food Sci. Technol. 42:131-136. |
|
|
Hamil FA, Apio S, Mubiru NK, Mosango M, Bukenya-Ziraba R, Maganyi OW, Soejarto DD (2000). Traditional Herbal drugs of southern Uganda. J. Ethnophamacol. 70:281-300.
Crossref |
|
|
Helle W, Anne SB, Malterud KE (2004). Antioxidant activity in extracts from coriander. Food Chem. 88:293-297.
Crossref |
|
|
Kamatenesi-Mugisha M, Oryem-Origa H (2007). Medicinal plants used to induce labour during childbirth in western Uganda. J. Ethnophamacol. 109:1-9.
Crossref |
|
|
Kulisica T, Radonic A, Katalinic V, Milosa M (2004). Analytical, Nutritional and Clinical Methods Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 85:633-640.
Crossref |
|
|
Kyarimpa CM, Böhmdorfer S, Wasswa J, Kiremire BT, Ndiege IO, Kabasa JD (2014). Essential oil and composition of Tagetes minuta from Uganda. Larvicidal activity on Anopheles gambiae. Industrial Crops Prod. 62:400-404.
Crossref |
|
|
Harris PWG, Prince SA, Olsen RJA, Oketch-Rabah RAOH, Madiega PA, Andersen A, Molgaard P (2002). Medicinal plants used by Luo mothers and Children in Bondo district, Kenya. J. Ethnophamacol. 83:39-54.
Crossref |
|
|
Parejo I, Bastida J, Viladomat F, Codina C (2005). Acylated quercetagetin glycosides with antioxidant activity from Tagetes maxima. Phytochemisty 66:2356-2362.
Crossref |
|
|
Paul S, Kasenene JM (2007). Medicinal plant diversity and uses in the Sango bay area, Southern Uganda. J. Ethnophamacol. 113:521-540.
Crossref |
|
|
|
Pillai LS, Nair BR (2013). GC-MS Analysis of Chloroform Extract of Cleome Burmanni W. and A. (Cleomaceae). Int. J. Phamaceutical Sci. Res. P. 4. |
|
|
Polatoglu K, Demirci B, Demirci F, Goren N, Baser KHC (2012). Biological activity and essential oil composition of two new Tanacetum chiliophyllum (Fisch. & Mey.) Schultz Bip. Var. Chiliophyllum chemotypes from Turkey. Industrial crops and products 39:97-105.
Crossref |
|
|
Tabuti JRS, Lye KA, Dhillion SS (2003). Traditional herbal drugs of Bulamogi, Uganda: Plants, use and administration. J. Ethnopharmacol. 88:19-44.
Crossref |
|
|
Vasudevan P, Kashyap S, Sharma S (1997a). TAGETES: A multipurpose plant. Bioresour. Technol. 62:29-35.
Crossref |
|
|
Vasudevan P, Kashyap S, Sharma S (1997b). Tagetes: A multipurpose plant. Bioresource Technol. 62:29-35.
Crossref |
|
|
Xiao G, Li G, Chen L, Zhang Z, Yin JJ, Wu T, Cheng Z, Wei F, Wang Z (2010). Isolation of antioxidants from Psoralea corylifolia fruits using high speed counter-current chromatography guided by thin layer chromatography-antioxidant autographic assay. J. Chromatography A. 1217:5470-5476.
Crossref |
APPENDIX