Full Length Research Paper
ABSTRACT
Heavy metals are one of the major pollutants in water and are very toxic. However, chitosan – a waste biomass of agricultural products possess the ability to chelate heavy metals in water. In this study, the application of snail (Helix pomatia) shell based chitosan for remediating heavy metals contaminated Osun River (OSR), Odo-ebu stream (OBS) and Aso well water (AWW), all in Osogbo community, Nigeria; and the binding efficiency of chitosan for heavy metals in these water sources were evaluated. The result shows the prevalence of Iron (Fe), Cadmium (Cd), Zinc (Zn), Copper (Cu), Chromium (Cr), and Lead (Pb) ions in the three water samples with OBS and OSR having higher metal concentrations in the order: Pb > Cd > Fe > Zn > Cr > Cu compared with AWW which follows the order: Fe > Zn > Pb > Cr > Cd > Cu. The concentration range of heavy metals in OBS and OSR were (0.0011 -2.831) mg/L and (0.0012 -1.687) mg/L respectively, while for AWW it was (0.0004-0.0237) mg/L and below the Standard Organization of Nigeria (SON) 2007 and World Health Organization (WHO) 2004; maximum permissible level of metal ions in drinking water. The prepared chitosan is a selective candidate for remediating four heavy metals ions from these water samples, with the maximum sorption capacities for the metal ions in the sequence: Pb > Cd > Fe > Cr. The sorption behaviour followed a pseudo-second-order model, while the equilibrium data correlated well with the Langmuir isotherm models with the RL values in the range 0 < RL < 1.
Key words: Heavy metals, Helix pomatia, chitosan, dumpsite, equilibrium studies.
INTRODUCTION
Heavy metals are of great concern due to their contrasting toxicities, long-term mobilities, bioavailabilities and their non-degradable nature in the environment. They enter the environment through a variety of anthropogenic and industrial activities, poor waste disposal mechanism; and pose serious threats to plants and human health (Kanamadi et al., 2003; Jude and Augustin, 2007). Heavy metals such as copper, zinc, nickel, chromium, cadmium and iron present in most Nigerian rivers have been found in concentrations well above acceptable and permissible levels (Eniola et al., 2010). Although, degradation of water quality has been claimed to be most severe in the four states that contain 80% of the nations industries that is, Lagos, Rivers, Kano and Kaduna state but this has extended to other regions. Thus, the importance of environmental quality of Osogbo (an urban settlement) in Osun state (south-western Nigeria) has attracted a great deal of interest due to the increasing population of the inhabitant, increasing industrialization, poor land use system, agricultural activities, industrialization and anthropogenic impact which has made the water and land being polluted (Olajire and Imeokparia, 2001). This is of great concern due to the great threat on health and economic development (Mustapha, 2008).
Bioremediation, the use of waste-derived materials or microbes to detoxify and degrade environmental contaminants provide safe and economic alternatives for the removal of toxic metal ions from wastewater or any aquatic environment and soil (Volesky, 2001). It also converts wastes into useful materials for soil and water decontamination. Chitosan, a poly-b (1-4)-2-amino-2-deoxy-D-glucosed derived from chitin - a natural biopolymer found in the outer shell of crustaceans such as crabs, shrimps and prawns has been found to have sorption capacity for several metal ions (Adewuyi et al., 2009; Yildiz et al., 2010). The metal sorption capacity of chitosan varies with crystallinity, affinity for water, deacetylation degree and amino group content. Kinetic studies have demonstrated that the rate of metallic ion sorption onto chitosan differs depending on the raw material (snail, shrimp, crab or lobster shells), preparation method, chemical modification, and chitosan particle shape (Martha, 2008).
This study presents the kinetic and uptake performance of the binding potential and selectivity of prepared biopolymers- chitosan for heavy metal ions (Cr, Pb, Cd, Fe) in the Osun River, Odo Ebu and Aso Hotel well water which serve as the major sources of water for the Isale-Osun community. The importance of this study lies on the direct evaluation of the metal sorption capacity of chitosan from real contaminated water samples from Isale-Osun community.
MATERIALS AND METHODS
Chitosan preparation
Helix pomatia (snail) shell procurement, deproteination, demineralization and N-deacetylation of the chitin were carried out using the methods of Adewuyi et al. (2009) and Taboada et al. (2003). All the chemicals used were of analytical grade products of British Drug House (BDH), Poole, England. De-ionized-distilled water was also used throughout the whole processes. The resulting chitosan was collected, washed and oven dried at 60°C for 4 h. The degree of deacetylation of the prepared chitosan was carried out using literature method of Tolaimate et al. (2002). Ultra Violet-Visible spectra analysis (using JENWAY spectrophotometer) of the prepared chitosan sample, covering the wavelength range of 190 to 800 nm with quartz cells (with a thickness of 0.2 cm) before and after treatment with the different water samples to determine its wavelength of adsorption.
Sampling and metal analysis
Surfaces water samples from Aso Hotel well water, Ebu stream (Odo Ebu) and Osun River water, all in the vicinity of dumpsite located at Isale Osun axis of Asubiaro area (Lat 07,44° N Long 04.74°E) in Osogbo, Osun state were collected (Figure 1). The pH and temperature of these water samples was determined using Jenway 3505 pH- portable meter. The water samples were digested using the method of Sallau et al. (2011). 50 cm3 of each sample was treated with 5 cm3 of conc. HNO3 and heated on a hot plate with gradual addition of conc. HNO3 as necessary until the solution boils. It was then evaporated to about 20 cm3; 5 cm3 of conc. HNO3 was finally added, covered and allowed to cool and then filtered. The pH of these solutions was initially fixed at a pH higher than that for the chitosan solubilization threshold (pH around 6.2). The filtrate was poured into a 50cm3 standard volumetric flask and made up to the mark with distilled water. Metal analyses of the digested samples were determined with Solaar AAS series 711047v1.22 atomic absorption spectrometer. Detection limits were estimated from digested blank (deionized water) which was run during the analysis. Triplicate digestions and analyses were run and average values were reported. Same procedure was repeated after the samples had been treated with chitosan.
Equilibrium studies
Equilibrium studies were carried out using the method of Adeogun et al. (2010). Adsorption was performed in a set of 100 ml flasks wherein the water samples (100 ml of each sample) were placed. Equal mass of 0.2 g of the prepared chitosan of particle size 40 mesh was added to the samples and kept on an isothermal shaker (orbital shaker) at 25 ± 1°C for 48 h for equilibrium to be reached between the solid-solution mixture. Similar procedure was followed for another set of 100 mls flasks containing the water samples without adsorbate (to be used as a control). The pH was adjusted to 4.8 - 6.0 by adding few drops of diluted HCl or NaOH (0.1 moldm-3). The flasks were then removed from the shaker and the final concentrations of heavy metals in the solutions were determined by Atomic Absorption Spectroscopy (AAS). Each experiment was duplicated under identical conditions. The amount of adsorption at equilibrium,  (mg/g), was calculated by:
(mg/g), was calculated by:
Where: Co and Ce (mg/ml) are the concentrations of metal ion at initial and equilibrium stage respectively, V is the volume of the solution (ml), and W is the mass of dry adsorbent used (g).
Adsorption kinetics
The kinetic experiments were identical to those of equilibrium tests except that the aqueous samples were taken at different time intervals from the solid-solution mixture, and the concentrations of heavy metals in the solutions were similarly measured (Adeogun et al., 2010). The amount of adsorption qt (mg/g), at time t, was calculated by:
Co and Ct (mg/ml) are the concentrations of the metal ions in the samples at initial and any time t, respectively, V is the volume of the solution (ml) and W is the mass of dry adsorbent used (g). An ideal sorbent for metal decontamination should not only have a large sorbate capacity but also a fast sorption rate (Crini and Badot, 2008). According to Sud et al. (2008), predicting the rate at which sorption takes place and the binding mechanism are essential to determine the efficiency of a sorption process. These may be controlled by: (i) solute transfer from the bulk solution to the boundary film that surrounds the sorbent’s surface, (ii) solute transport from the boundary film to the sorbent’s surface, (iii) solute transfer from the sorbent’s surface to the active intraparticle sites, and (iv) interaction(s) between solute and binding sites of the sorbent (intra-particle diffusion). The pseudo- first order and pseudo-second order kinetic and intra-particle diffusion (chemical binding reaction) kinetic models were employed to determine the rate constant and the controlling mechanism of the sorption process. These were done to further confirm the best fit kinetic model (s) for the sorption process. Although, several metal sorption studies have shown that most sorption kinetics usually followed the pseudo-second-order kinetic model. The pseudo-first-order and intra-particle diffusion equations were generally acclaimed to be applicable over the initial stage of the adsorption process (Mohan et al., 2006; Pan et al., 2009). The linear forms of pseudo- first order, pseudo- second order the intra-particle diffusion equations are as expressed in Equations 3, 4 and 5 respectively (Lagergren, 1898; Kamari, 2011; Ho and McKay, 2000).
Where:  and qt (mg/g) are the amount of metal ions sorbed (mg/g) at equilibrium and at time t (min) respectively while k1 (g/mg/min), k2 (g/mg/min) and kid (mg/g/min0.5) are the rate constants of the pseudo-first –order, pseudo-second-order and intra-particle diffusion equations for the bio-sorption processes respectively (Kamari et al., 2011).
and qt (mg/g) are the amount of metal ions sorbed (mg/g) at equilibrium and at time t (min) respectively while k1 (g/mg/min), k2 (g/mg/min) and kid (mg/g/min0.5) are the rate constants of the pseudo-first –order, pseudo-second-order and intra-particle diffusion equations for the bio-sorption processes respectively (Kamari et al., 2011).
Adsorption isotherm
Adsorption isotherm study was carried out using the Langmuir adsorption isotherm (Langmuir, 1916). Langmuir isotherm assumes monolayer adsorption onto a surface containing a finite number of adsorption sites of uniform strategies of adsorption with no transmigration of adsorbate in the plane of surface (Fytianos et al., 2003). The energy term in Langmuir equation varies as a function of the surface coverage (Fytianos et al., 2003). The applicability of the isotherm equation was judged by the correlation coefficients, R2. The linear form of Langmuir’s isotherm model is given by the equation:
Ce is the equilibrium concentration of the adsorbate (heavy metal ion) (mg/L); 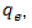 the amount of adsorbate adsorbed per unit mass of adsorbent (mg/g);
the amount of adsorbate adsorbed per unit mass of adsorbent (mg/g); 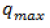 and b are Langmuir constants which are related to monolayer adsorption capacity and affinity of adsorbent towards adsorbate respectively. The important characteristic of the Langmuir isotherm is expressed in terms of a dimensionless equilibrium constant separation parameter (RL) (Saifuddin and Kumaran, 2005), which is defined as:
and b are Langmuir constants which are related to monolayer adsorption capacity and affinity of adsorbent towards adsorbate respectively. The important characteristic of the Langmuir isotherm is expressed in terms of a dimensionless equilibrium constant separation parameter (RL) (Saifuddin and Kumaran, 2005), which is defined as:
Where: b = the Langmuir constant, Co = the highest metal ion concentration (mg/L).
The value of RL indicates the shape or type of the isotherm to be either unfavourable (RL > 1), linear (RL = 1), favourable (0 < RL < 1) or irreversible (RL = 0) (Saifuddin and Kumaran, 2005).
RESULTS AND DISCUSSION
Chitosan yield and characterisation
Yield of chitosan
The yield after each process is as presented in Table 1. The snail shell yielded approximately 20% of chitosan. This implies that snail shells (Helix pomatia) can be considered as a better source of chitosan compared to freshwater crab (Potamon potamios) whose yield is about 4.65% (Bolat et al., 2010), however, the yield can be improved if loss of mass due to washing can be reduced to the minimum possible. A contributing factor to this significant amount of yield is the non-edibility of the snail shell itself.
Characterisation of the prepared chitosan
The degree of deacetylation (%DD) of this snail based as calculated using potentiometric titration following Tolaimate et al. (2002) was found to be 65%. The electronic absorption spectra of pure and used chitosan sample depicting the effect of chelation on the spectra properties of chitosan is as shown in Figure 2. The prepared chitosan absorbs at a wavelength of about 210 nm which is close to the characteristic bands (208 nm) reported for highly deacetylated chitosan (Tolaimate et al., 2002). There is shift in the wavelength (λmax) of absorption of the treated chitosan with each water sample (215, 235 and 236 nm for Aso well, Ebu stream and Osun river water treated chitosan respectively). This bathochromic shift indicated the binding of metal ions with chitosan which is one of its potential applications.
Evaluation of heavy metals in the sample
The concentration of heavy metals in the prepared chitosan-treated and untreated water samples as reported in Tables 2 and 3 indicated the prevalence of six heavy metals (Fe, Cd, Zn, Cu, Cr, and Pb) in all the three samples with Odo-ebu stream (OBS) and Osun River (OSR), having higher metal concentrations compared with AWW water. The pH and temperatures are also functions of these metal concentration accumulation. The level of contamination by heavy metal in Ebu stream and Osun River follow the order: Pb > Cd > Fe > Zn > Cr > Cu while for Aso well water, it was Fe > Zn > Pb > Cr > Cd > Cu. OSR and OBS samples whose sources are not too far from dumpsite contain high concentrations of Pb ions, followed by Cd, thus making both samples the most contaminated of the three water samples. Other metal ions concentration in OBS and OSR samples are also very high when compared with AWW sample. Although, sample from AWW can be said to be unpolluted since the concentrations of most of the analyzed heavy metals are below the allowable standards of SON and WHO limits for drinking water (Table 4), but it may not be declared as being totally safe for drinking due to the risk of bioaccumulation in the human body (Majolagbe et al., 2013; Sekabira, 2010). The high concentrations of these metals can be attributed to leaching from dumpsite which is about 50 m to Ebun stream, and 100 m to Osun River. The high Pb concentration could be coming from exhaust from heavy vehicles (trucks) that ply the routes and burning of refuse at the dumpsite. The activity of inhabitant e.g. washing of clothes and vehicles along the bank of Osun River could also be responsible for high concentration of the other metals. Some of these heavy metals have been reported to cause vomiting, dizziness, mortality, morbidity, pulmonary disorder and haematological disorder and cancer (Majolagbe et al., 2013).
Effect of contact time and adsorption capacity of chitosan
The time profiles of metal ions sorption by chitosan carried out on the three water samples at 25°C are presented in Figure 3. The amount of metal ions sorbed increased with contact time before plateauing, beyond which no more ions were removed from the samples (the concentrations of heavy metal ions left in the sample after treatment with chitosan are presented in Table 3). At this point, equilibrium has been reached between the amount of the heavy metal ions desorbed from the chitosan and heavy metal ions sorbed onto it. The selectivity of chitosan for the heavy metals ions (Pb, Cd, Cr, Fe) vary while Cu and Zn were below the detection limit. This can be explained on the basis of these metal ions ionic radii, hydration energies and their concentration in solution (Rhazi et al., 2002). The time required to attain this state of equilibrium is termed the equilibrium time, and the amount of heavy metal ions adsorbed at the equilibrium time reflects the maximum adsorption capacity of the adsorbent under those operating conditions. It was found that 96.04% of Pb, 99.83% of Cd, 94.47% of Cr and 77.6% of Fe were bound to chitosan within 15 min. The initial high amount of metal ions sorbed indicates instantaneous sorption, which can be attributed to the availability of binding sites on the sorbents. However, as these sites progressively react, the sorption of metal ions slowed before attaining equilibrium. The ability of chitosan to bind a large amount of metal ions within 15 min suggests that they are effective sorbents. Furthermore, the rapid kinetics has significant practical importance as it will facilitate the application to smaller reactor volumes ensuring efficiency and economy (Kamari et al., 2011).
The maximum adsorption capacities of each heavy metal based on the samples are presented in the Table 1. This further shows the selectivity of chitosan for adsorbing particular heavy metals ions when more than one heavy metal ions is present in solution. Chitosan showed the highest maximum adsorption capacity for Pb amongst the four heavy metal ions detected after metals sorption in OBS and OSR water samples. The selectivity of the snail shell based chitosan in these samples for the metal ions follow the sequence: Pb > Cd > Fe > Cr.
Adsorption kinetics
The kinetic parameters and their values obtained for the heavy metals sorption processes of the prepared chitosan on the three water samples are presented in Figures 4(a – c), 5(a - c) and Table 5.
The experimental equilibrium sorption capacities (qe experimental) determined from the contact time study were in good agreement with the theoretical equilibrium sorption capacities (qe theoretical) calculated from the pseudo-second-order kinetic model than for the pseudo-first-order. Moreover, experimental sorption data correlated well to the pseudo-second-order kinetic model (R2 values being greater than 0.95 in most cases except for Cd metal sorption and range from 0.984 to 0.999). Lagergren’s pseudo-first order and Intra-particle diffusion equations did not fit well for those metals they can be plotted, the R2 values are relatively small and in some cases, having negative values. This had earlier been reported by Kamari et al. (2011) and Mohan et al. (2006) who concluded that pseudo-first-order equations did not fit well to the whole range of contact time for heavy metal sorption processes of chitosan but were only applicable over the initial stages of the processes of adsorption The results suggest that the binding of metal ions studied onto chitosans was best described by the pseudo-second order kinetic model and that the chemical binding reaction was the rate-limiting step as discussed by Ho and McKay (2000).
Adsorption isotherm
The adsorption isotherm indicates how the adsorbed molecules distribute themselves between the liquid phase and the solid phase when the adsorption process reaches an equilibrium state. The analysis of equilibrium adsorption data by fitting them to an isotherm models is an important step to show that the Langmuir model can be used for design purpose (Haghseresht and Lu, 1998). When 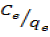 was plotted against Ce, a straight line with slope,
was plotted against Ce, a straight line with slope,  was obtained, and are presented in Figure 6(a – c). The Langmuir constants ‘b’ and
was obtained, and are presented in Figure 6(a – c). The Langmuir constants ‘b’ and  were calculated from this isotherm and their values are presented in Tables 6. The large values of b signify that the chitosan has a very high affinity for the heavy metals coupled with a high binding energy. The conformation of the experimental data,
were calculated from this isotherm and their values are presented in Tables 6. The large values of b signify that the chitosan has a very high affinity for the heavy metals coupled with a high binding energy. The conformation of the experimental data,  to that obtained using Langmuir isotherm model indicated the homogeneous nature of snail shell based chitosan surface, i.e., each metal ion molecule/chitosan biosorption has equal adsorption activation energy. The linear plot obtained further buttress the monolayer form of adsorption. Also from Figure 4(a and b), values of RL were found to be less than 1 but greater than zero in all cases. This confirmed that the biosorbent prepared from the snail shell is favourable for biosorption of Pb, Cd, and Cr ions under the conditions used in this study.
to that obtained using Langmuir isotherm model indicated the homogeneous nature of snail shell based chitosan surface, i.e., each metal ion molecule/chitosan biosorption has equal adsorption activation energy. The linear plot obtained further buttress the monolayer form of adsorption. Also from Figure 4(a and b), values of RL were found to be less than 1 but greater than zero in all cases. This confirmed that the biosorbent prepared from the snail shell is favourable for biosorption of Pb, Cd, and Cr ions under the conditions used in this study.
CONCLUSION
The findings from this study have shown that the Ebu stream and Osun river water samples were highly polluted with heavy metals and chitosan has great affinity for these metal ions in the analyzed samples in the following order: Pb > Cd > Fe > Cr. The adsorption kinetics follows pseudo-second order and the experimental equilibrium sorption capacities determined from the contact time study were in good agreement with the theoretical equilibrium sorption capacities calculated from the kinetic model. The Langmuir model fitted the isotherm equilibrium data with R2 ≥ 0.985. The values of maximum sorption capacities also correlated well with those determined theoretically and experimentally. RL values obtained also confirmed that the prepared biosorbent is favorable for biosorption of heavy metals. Similar results were reported by Kamari et al. (2011) for crab shell based chitosan and Adeogun et al. (2010) using adsorbent obtained from plumb shell.
CONFLICT OF INTEREST
The authors have not declared any conflict of interest.
REFERENCES
|
Adeogun AI, Bello OS, Adeoye MD (2010). Biosorption of lead ions on biosorbent prepared from plumb shells (Spondias mombin): Kinetics and equilibrium studies. Pak. J. Sci. Ind. Res. 53(5):246-251.
|
|
|
|
|
|
Adewuyi S, Adeoye MD, Sanyaolu NO (2009). Equillibruim removal efficiency of Nickel metal ion by chitosan. Proc. of the 33rd ann. Inter. Conf. Chem. Soc. Nig. (CSN), Abeokuta, Nigeria. pp. 403-405.
|
|
|
|
|
|
Bolat YÅž, Bilgin A, Günlü L, Izci SB, Koca SÇ, Koca HU (2010). Chitin Chitosan yield of freshwater crab (Potamon potamios, Olivier 1804) shell. Pak. Vet. J. 30(4):227-231.
|
|
|
|
|
|
|
|
|
|
|
|
Eniola EB, Chukwu LO, Olaide BS (2010). Hydro-chemistry, macro-invertebrate fauna and fish production of acdja fishing sites in a Tropical Lagoonal Ecosystem. J. Am. Sci. 6(1):22-28.
|
|
|
|
|
|
|
|
|
|
|
|
Haghseresht F, Lu GQ (1998). Adsorption characteristics of phenolic compounds onto coal-reject-derived adsorbents. Ener. Fuels. 12:1100-1107.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kamari A, Pulford ID, Hargreaves JSJ (2011). Binding of heavy metal contaminants onto chitosans: An evaluation for remediation of metal contaminated soil and water. J. Env. Mgt. 92:2675-2682. Crossref |
|
|
|
|
|
Kanamadi RD, Ahalya N, Ramachandra TV (2003). Review Paper: Biosorption of Heavy Metals. Res. J. Chem. Environ. 7(4):71-79.
|
|
|
|
|
|
Lagergren S (1898). Zur theorie der sogenannten adsorption gelöster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar 24:1-39.
|
|
|
|
|
|
|
|
|
|
|
|
Majolagbe TA, Azeez L, Salawu HO, Adeoye MD, Lawal AT, Agbaogun BKO, Tijani KO (2013). Assessment of heavy metal pollution in soils and wells around municipal dumpsites in Osogbo metropolis. Environ. Sci.-An Ind. J. 8(2):56-61.
|
|
|
|
|
|
Martha B (2008). Adsorption of Metallic Ions onto Chitosan: Equilibrium and Kinetic Studies Royal Institute of Technology. Department of Chemical Engineering and Technology. Division of Transport Phenomena, Stockholm, Sweden. Licentiate Thesis pp. 3-4.
|
|
|
|
|
|
Mohan D, Pittman Jr CU, Steele PH (2006). Single, binary and multi-component adsorption of copper and cadmium from aqueous solutions on Kraft lignin-a biosorbent. J. Colloid. Interf. Sci. 297:489-504. Crossref |
|
|
|
|
|
Mustapha MK (2008). Assessment of the water Quality of Oyun Reservoir, Offa, Nigeria, Using Selected Physico-Chemical Parameters. Tur. J. Fish. Aq. Sci. 8:309-319.
|
|
|
|
|
|
Olajire AA, Imeokparia FE (2001). Water quality assessment of osun river: studies on inorganic nutrients. Environ. Monit. Assess. 69:17-28. Crossref |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Saifuddin MN, Kumaran P (2005). Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal. Elec. J. Biotech. 8(1):22-25.
|
|
|
|
|
|
Sallau AA, Ekanem EO, Abubakar AB (2011). The 34th Annual International Conference of the Chemical Society of Nigeria (CSN) at Unilorin, EN001- EN007 Sattelite map of osogbo.
|
|
|
|
|
|
|
|
|
|
|
|
Standard Organization of Nigeria (2007). Nigerian Standard for Drinking Water Quality. pp.1-30.
|
|
|
|
|
|
Sud D, Mahajan G, Kaur MP (2008). Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions - A review. Bioresour. Technol. 99:6017-6027. Crossref |
|
|
|
|
|
Taboada S, Carbrera G, Cardenas G (2003). Retention capacity of chitosan for copper and Mercury ions. J. Chilian Chem. Soc. 48(1):10.
|
|
|
|
|
|
Tolaimate A, Rhazi M, Desbrieres J, Rinaudo M, Vottero P, Alagui A, El-Meray M (2002). Influence of the nature of the metal ions on the complexation with chitosan. Application to the treatment of liquid waste. Eur. Polymer J. 38:1523-1530. Volesky B (2001). Detoxification of metal-bearing effluents: biosorption for the next century. Hydromet. 59:203-216.
|
|
|
|
|
|
World Health Organization (WHO) (2004). The Guidelines for Drinking Water Quality, Third edition.
|
|
|
|
|
|
Yildiz B, Åžengül B, Ali G, Levent I, Seval BK, Soner Ç, Habil UK (2010). Chitin-Chitosan Yield of Freshwater Crab (Potamon potamios, Olivier 1804) Shell. Pak. Vet. J. ISSN: 0253-8318 (PRINT) 2074-7764,
|
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0