ABSTRACT
Senna alata L. has been ethnobotanically used extensively in traditional medicines for the treatment of a variety of diseases such as skin problems, arthritis, HBP (high blood pressure), and laxative or purgative. The phytochemical, anti-nutritive and antioxidant activities of the leaf and root bark of Senna alata L. grown in Bida Niger State, Nigeria were determined using standard analytical methods. Phytochemical screening of the flower and seed of the plant revealed the presence of flavonoids, phenols, saponins, tannins, alkaloid and anthraquinone. Steroid and cardiac glycosides, were slightly present, while resins were absent. The metabolites present were quantitatively determined with alkaloid contents of 14.09±0.50 and 15.89±0.72, saponin 40.57±0.57 and 33.02±0.07, flavonoid 42.28±0.90 and 36.52±0.38, tannin 59.48±0.50 and 44.38±0.72, and phenol 7.84±0.49 and 9.91±0.68 mg/100 g for leaf and root bark respectively. These results confirm that the metabolites obtained from the two parts of this plant were within the range of toxicity levels according to World Health Organization safe limits. The results of anti-nutritional factors revealed oxalate contents of 7.84±0.74 and 9.91±0.62, cyanide content 13.04±0.09 and 21.69±0.11 and phytate content 15.07±0.58 and 12.44±0.31 mg/100 g for leaf and root bark respectively. The values of anti-nutritional factors obtained from this work show that they may not pose any effects based on their toxicity levels and as recommended by World Health Organization. The major components of the chemical compounds deduced from GC/MS for the two parts of this plant investigated revealed the presence of α-d-mannofuranoside (53.35%), oleic acid (12.30%), β-d-glucopyranoside (12.59%), β-d-mannofuranoside (22.41%), n-hexadecenoic acid (5.73%), 1,2,3- propanetriol (21.54%), α-d-glucopyranoside (16.41%) and oleic acid (14.65%). Thus, if properly domesticated and produced in commercial quantities, this plant will serve as a source of bioactive agents for pharmaceuticals.
Key words: Antioxidant, phytochemical, anti-nutritional, leaf, root bark and Senna alata.
Plants have been used for medicinal purposes over the years, which have provided mankind with a source of essentials of life such as food, medicine and raw materials for clothing and shelter (Midawa et al., 2010).
According to World Health Organization (2002), traditional medicine refers to health approaches, practices, beliefs and knowledge incorporating animals, plants, and mineral based medicines, spiritual therapies, manual techniques and exercises, applied singularly or in combination to treat, prevent and diagnose illnesses. Over 80% of world population uses traditional medicine to cure various diseases (WHO, 2002). The major ingredients are obtained from medicinal plants. It has been discovered that majority of modern medicine are plant-derived therapeutic agents. This could be attributed to the fact that many plants contain a variety of phytochemicals, which have found very important applications in the field of human medicine. Natural products play a dominant role in the development of novel drug- leads for the treatment and prevention of disease (Newman et al., 2003). Reactive oxygen species (ROS) such as superoxide radical, hydroxyl radical, singlet oxygen, and hydrogen peroxide are produced in the body during normal metabolism or on exposure to exogenous factors. These reactive species can initiate deterioration of biomolecules such as proteins, lipids, carbohydrates and nucleic acids and are implicated in several diseases such as ageing, atherosclerosis, inflammatory injury, cancer, cardiovascular disease, neurological disorders etc. Oxidative stress results, when the balance between the generation of ROS and antioxidant defense system of the body is disturbed. Cells have innate defense system which protects against the adverse effects caused by these ROS and includes enzymatic and non-enzymatic defense. However, during pathophysiological conditions, there is an extra need for antioxidants from exogenous sources. Synthetic antioxidants have been suspected to cause or promote negative health effects. Hence, there is a need for development of safer antioxidants particularly from natural sources. Many studies have demonstrated the efficacy of plant derived products as antioxidants against various diseases induced by these free radicals (Koleva et al., 2000). It has been shown that the antioxidant nature of plants is mainly attributed to phenolic compounds, such as flavonoids and phenolic acids (Pietta, 2000). Senna alata L. has been ethnobotanically used extensively in traditional medicines for the treatment of a variety of diseases such as skin problems, arthritis, high blood pressure (HBP) and laxative using its organs. Therefore, there is need to investigate these plant organs for the bioactive compounds to be used for therapeutic purposes.
Collection, identification and preparation of plant material
Leafy plant of S. alata was obtained from Edokota forest along Bida-Zungeru road, Bida, Niger State, Nigeria. The identity was confirmed by plant taxonomist from the National Institute of Pharmaceutical Research Development, Idu-Abuja where a voucher specimen was deposited with Herbarium No.1369. The samples (leaf and root bark) collected from the experimental sites were washed with distilled water to remove impurities and dried at room temperature. These were then grind into uniform powder manually. It was then sieved, weighed, bottled, labelled and used for laboratory analysis.
Extraction of the plant extracts
Powdered flower and seed of S. alata were extracted with 70% aqueous methanol at room temperature. The extract solution of each sample was filtered, and the solvent was evaporated under reduced pressure at 35°C (Figure 1).
Qualitative phytochemical screening of the samples
Phytochemical screening procedures carried out were adopted from Mann (2014), where tannins, saponins, steroids, alkaloids, cardiac glycoside, terpenoids and flavonoids were determined.
Quantitative phytochemical analysis of the samples
Standard analytical methods were used for the quantitative phytochemical analysis of these samples (Edeoga et al., 2005). Tannins and saponins were determined using standard method of Onwuka (2005), while flavonoids and alkaloids were determined using standard method as described by Harborne (1989) and the total phenolic content was estimated using the modified Folin-Ciocalteu photometric method by Schuler (1990).
Anti-nutritional properties of the samples
Oxalate and cyanide contents were determined using the method of Day and Underwood (1986). Phytate content was determined by the method described by Wheeler and Ferrel (1971).
Antioxidants activities of the samples
The ferric reducing antioxidant power (FRAP) assay was done according to Benzie and Strain (1996) with some modifications while total phenolics of various fractions of plant were determined by reported method of Valentao et al. (2002).
GC/MS analysis of the samples
GC-MS analysis was carried out on a Shimadzu (Kyoto, Japan) GC-MS model QP 2010 at National Research Institute for Chemical Technology, Zaria, according to the EN 14103 standard method (Adams, 2007; Orishadipe et al., 2010). The GC column oven temperature (70°C), injecting temperature (250°C), flow control mode (linear velocity), total flow (40.8 ml/min) column flow (1.80 ml/min), pressure (116.9 kpa), linear velocity (49.2 cm/s) and purge flow (3.0 ml/min) were employed for this analysis. A sample volume of 8.0 µl was injected using split mode (split ratio of 20:0). The peak area, that is, the % amount of every component was calculated by comparing its average peak area to the total areas. Software was used to handle mass spectra and chromatogram.
Identification of components from the samples
Interpretation of mass spectrum GC-MS was conducted by comparing the database peaks of National Institute of Standard and Technology (NIST) library with those reported in literature, the mass spectra of the peaks with literature data (Stein et al., 2002). The spectrum of the unknown component was compared with the spectrum of the known components stored in the NIST library. Component relative percentages were calculated based on GC peak areas without using correction factors. The name, molecular weight and structure of the components of the test materials were ascertained.
Statistical analysis
All the experiments were conducted in triplicate unless stated otherwise and statistical analysis of the data was performed by analysis of variance (ANOVA), using SPSS 11.0 for Windows software. A probability value of difference p ≤ 0.05 was considered to denote a statistically significance. All data were expressed as mean values ± standard deviation (SD).
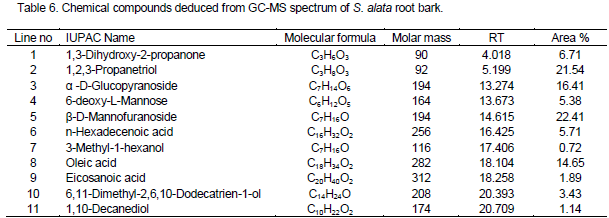
The results of qualitative analysis of the crude methanolic extract of two parts of S. alata shown in Table 1 revealed the presence of tannins, flavonoids, terpenoids, saponins, alkaloid, glycosides, anthraquinone, which are the basis of therapeutic potentials of medicinal plants. Similar results were reported for Senna obtusifolia by Essiett and Bassey (2013) where the presence of saponins, tannins, alkaloids, terpenoids, anthraquinone and a trace of steroids were reported. The presence of tannins as reported in this work may be the cause of lowering of available protein by antagonistic competition and can therefore elicit protein deficiency syndrome “Kwashiokor” (Maynard, 1997). Saponin may be responsible for its anti-yeast, anti-fungal, antidote, antimicrobial and anti-inflammatory activities. It is also believed that the role of saponin is to protect plant against attack by potential pathogens (Sparg et al., 2004). Flavonoids which are also known as vitamin p or plan modifier, elicit a wide range of therapeutic activities as antihypertensive, antirheumatism as well as antimicrobial as identified with flavonoids (Veerachari and Bopaiah, 2011). Essiett et al. (2010) reported that many plants containing flavonoids have diuretic and antioxidant properties. The leaf and root bark of this plant can equally be used accordingly; glycosides were detected in the extracts and this class of compound has been found useful in the treatment of asthma (Trease and Evans, 1989; Evans, 2002). Steroids were also found and their pharmaceutical importance might hinge on their relationship with such compounds as sex hormones (Bell, 2007). Glycosides were detected in the leaf and root bark of S. alata. Glycoside has been used for over two centuries as stimulant in cases of cardiac failure and diseases (Taiwo et al., 2009). This perhaps justifies the already locally established function of the plant in the treatment and management of hypertension (Duke, 1985). Alkaloids have been found to have microbiocidal effect and their antidiarrheal effect is probably due to their action on small intestine. In addition, they effect antihypertensive antifungal, anti-inflammatory, and anti fibrogenic effect (Awoyinka et al., 2007). However, the results of this work are similar to the findings of McDevitt et al. (1996) who reported the presence of alkaloid in Cnidoscolusa conitifolius. Some alkaloids are useful against HIV infection as well as intestinal infection associated with AIDS (Scalbert, 1991).
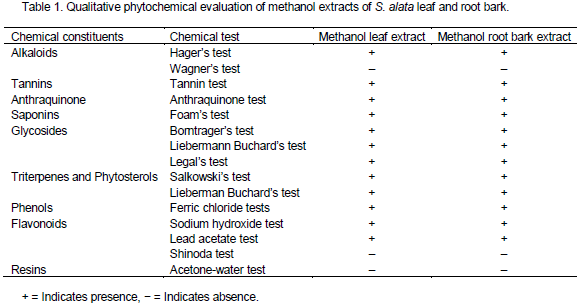
The results of quantitative analysis of the parts of S. alata as presented in Table 2 showed saponin contents of 40.57±0.57 and 33.02±0.07 mg/100 g for leaf and root bark respectively. It was observed that saponin concentrations were higher in leaf than root bark. These results were high compared to 12.1 mg/100 g of M. utilis reported by Siddhuraju and Becker (2005). Saponins are naturally occurring surface – active glycosides. They are mainly produced by plants, but also by lower marine animals and some bacteria (Riguera, 1997). The results of quantitative analysis of alkaloid content obtained from this plant organ were 14.09±0.50 and 15.89±0.72 mg/100 g for leaf and root bark respectively. The alkaloid contents were higher in root bark than leaf. This is similar to the values reported for S. alata flower (8.50±0.01 mg/100 g) by Abdulwaliyu et al. (2013). Alkaloids are more or less toxic substances which act primarily on the central nervous system (Hegnuauer, 1963). The tannin contents analyzed in this work were 59.48±0.50 and 44.38±0.72 mg/100 g for leaf and root bark respectively. The concentration was high with S. alata leaf while root bark had the least. The contents of tannin obtained in these were similar to 46.08 mg/100 g of M. utilis reported by Siddhuraju and Becker (2005). The values of flavonoids analyzed from the two samples were 42.28±0.90 and 36.52±0.38 mg/100 g for leaf and root bark respectively. The flavonoid contents were higher in S. alata leaf than root bark. The phenol contents analyzed from this work were 7.84±0.49 and 9.91±0.68 mg/100g for leaf and root bark respectively. The concentration of the root bark was found to be high while leaf had the least value. These values were high compared to 2.00±0.21 mg/100 g for S. alata leaf reported by Abdulwaliyu et al. (2013).
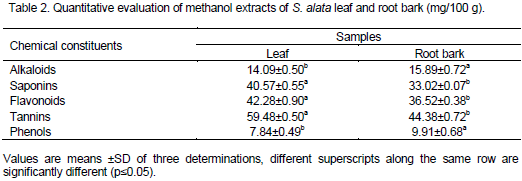
Anti-nutritional factors affect the availability of nutrients required by the body and interfere with metabolic process so that growth and development of the body is negatively influenced (Richard et al., 2006). The results of anti-nutritional factors obtained for this work were presented in Table 3. The phytate content in the samples analyzed was 12.44±0.31 and 15.07±0.58 mg/100 g for root bark and leaf respectively. The content of phytate was higher in S. alata leaf. Phytate helps in adequate iron bioavailability. The result obtained in this study was high when compared to the 3.55 mg/100 g of S. alata leaf reported by Abdulwaliyu et al. (2013). The contents of oxalates obtained from this work were 7.84±0.74 and 9.91±0.62 mg/100 g for leaf and root bark respectively. The concentration of oxalate was higher in S. alata root bark than the leaf. Similar values were obtained for the S. alata leaf (8.03±0.06 mg/100 g) reported by Abdulwaliyu et al. (2013). The presence of oxalate in food causes irritation in the mouth and interferes with absorption of divalent minerals particularly calcium by forming insoluble salts (Ola and Oboh, 2000). The cyanide content in the samples analyzed ranged from 13.04±0.09 and 21.69±0.11 mg/100 g for root bark and leaf respectively. The contents of cyanide were higher with S. alata leaf. These values are low when compared to the toxic level of 26.05±0.45 mg/100 g, reported for S. alata leaf by Abdulwaliyu et al. (2013).
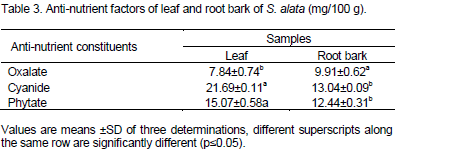
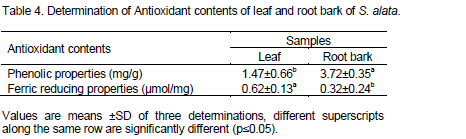
Table 4 shows the antioxidants properties obtained from this study using phenolic and ferric reducing properties. The antioxidant values of leaf and root bark were 1.47±0.66 and 3.72±0.35 mg/g respectively for phenolic properties with root bark exhibiting higher value. These values were similar to 3.58 mg/g reported for S. hirsute by Essiett and Bassey (2013). The ferric reducing properties obtained from this work were 0.62±0.13 and 0.32±0.24 µmol/mg for leaf and root bark respectively. From these results, high ferric reducing properties was recorded for leaf (0.62±0.13 µmol/mg) over root bark (0.32±0.24 µmol/mg). These values were higher when compared to 0.17±0.04 µmol/mg reported for Pueraria mirifica by Buran and Supak (2007).
Tables 5 to 6 show the analytical parameters for GC-MS for the two organs of S. alata. It was observed that the organ of this plant contains all important fatty acid needed in the body for proper functioning. The fatty acids recorded in this work are major source of energy. Most diets contain a great deal of fatty acids which were seen in this plant in form of triacylglycerol. The result of GC-MS spectrum of S. alata leaf, as presented in Table 5, revealed the presence of many major components, namely α-d-mannofuranoside, oleic acid, β-d-glucopyranoside, glycolaldehyde dimer and. The percentage concentration for each of the organic compounds was 53.35, 12.30, 12.59, 8.98 and 4.56% respectively (Table 5). The content of S. alata leaf was similar to Cassia alata reported by Isiaka et al. (2010).

The GC-MS spectra of S. alata root bark gave eleven compounds (Table 6). The major components were β-d-mannofuranoside, 1,2,3- propanetriol, α-d-glucopyranoside, oleic acid, 1,3-dihydroxy-2-propanone, n-hexadecenoic acid and 6-deoxy-L-mannose. The percentage concentration for each of the organic compounds were 22.41, 21.54, 16.41, 14.65, 6.71, 5.71 and 5.38% respectively. The contents of S. alata root bark were observed to be high when compared to Senna podocarpa used for medicinal purposes in Nigeria reported by Adebayo et al. (2014).
There is need for more research on the activity of the extracts in this plant against a wider range of bacteria and fungi and on the toxicology and further purification of the extracts for isolation of the pure active constituents. However, the two parts of the plant studied can contribute to human medication. It can be concluded that the plant contains various phytochemical constituents such as tannins, flavonoids, terpenoids, saponins, alkaloids, glycosides, steroids, phenol and anthraquinone. The presences of these secondary metabolites can inhibit the growth of micro-organisms and also have potentials of being developed for pharmaceuticals.
The authors have not declared any conflict of interest.
REFERENCES
|
Abdulwaliyu I, Arekemase SO, Bala S, Ibraheem AS, Dakare AM, Sangodare R, Gero M (2013). Nutritional Properties of Senna alata Linn Leaf and Flower. Int. J. Mod. Biol. Med. 4(1):1-11. |
|
|
|
Adams RP (2007). Identification of Essential Oil Components by Gas chromatography/Quadrupole Mass spectroscopy. Allured Publishing Corporation, Carol Stream, Illinois, USA. |
|
|
|
Adebayo MA, Lawal OA, Sikiru AA, Ogunwande IA, Avoseh ON (2014). Chemical Constituentsand Antimicrobial Activity of Essential Oil of Senna podocarpa (Guill. etPerr.) Lock. Am. J. Plt. scis. 5:2448-2453. |
|
|
|
Awoyinka AO, Balogun IO, Ogunnowo AA (2007). Phytochemical screening and in-vitro bioactivity of Cnidoscolus aconitiifolus (Euphorbiaceae), J. Med. Pla. Res. 1(3):63-65. |
|
|
Benzie IEF, Strain JJ (1996). The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 239:70-76.
Crossref |
|
|
Bell E (2007). Vitamin D3 promotes immune function on the skin. http//www.signalinggateway.org/upd ate/updates/
Crossref |
|
|
|
Buran P, Supak P (2007). Antioxidant Capacities of Pueraria mirifica,Steviare baudiana Bertoni, Curcuma longa Linn., Andrographis paniculata(Burm.f.) Nees.And Cassia alata Linn.for the Development of Dietary Supplement.Kase. J. Nat. Sci. 41:548–554. |
|
|
|
Day RA, Underwood AL (1986). Qualitative Analysis. 5th Ed. New Delhi, India: Prentice Hall Publications. 701. |
|
|
|
Duke JA (1985). Handbook of Medicinal Herbs. CRC press Inc. Boca Raton, Fla, pp. 72-80. |
|
|
Edeoga HO, Okwu DE, Mbaebei BO (2005). Phytochemical constituents of some Nigerian Medicinal plants. Afr. J. Biot. 4(7):685-688.
Crossref |
|
|
|
Essiett UA, Bala DN, Agbakahi JA (2010). Pharmacognosticstudies of the leaves and stem of Diodiascandens SW in Nigeria. Archives of Appl. Sci. Res. 2(5):124-198. |
|
|
|
Essiett UA, Bassey IE (2013). Comparative Phytochemical Screening and Nutritional Potentials of the Flowers (petals) of Senna alata (L) roxb, Senna hirsute (L) Irwin and barneby, and Senna obtusifolia (L.)Irwin and barneby (Fabaceae). J. Appl. Pharm. Sci. 3(08):097-101. |
|
|
|
Evans WC (2002). Pharmacognosy, (15th edition), London W.B. Saunders Company Ltd., pp. 191-393. |
|
|
|
Harborne JB (1989). Biosynthensis and function of antinutritional factors in plants. Aspects Appl. Biol. 19:21-25. |
|
|
Hegnuauer R (1963). Chemical Plant Taxonomy. Academic Press Inc., New York, P. 389.
Crossref |
|
|
|
Isiaka AO, Guido F, Pier LC, Omikorede O, Ridwan AA, Akinwunmi AA, Yusuff OK (2010). Aromatic plants growing in Nigeria: Essential oil constituents of Cassia alata (Linn.) Roxb. and Helianthus annuus L. Record of Natural Products. 4(4):211-217. |
|
|
Koleva II, Niederlander HAG, Van Beek TA (2000). An online HPLC method for detection of radical scavenging compounds in complex mixtures. Anal. Chem. 72:2323-2328.
Crossref |
|
|
|
Maynard LA (1997). Animal nutrition. McGraw Hill book company Ltd. New York, P. 47. |
|
|
|
McDevitt JT, Schneider DM, Katiyar SK, Edlind FS (1996). Berberina: a candidate for the treatment of diarrhea in AIDS patients abstract 175, In program and Abstracts of the 36th Interscience on ference on Antimicrobial Agents and Chemotherapy, American Society for Microbiology, Washington, D. C. |
|
|
|
Midawa SM, Ali BD, Mshelia BZ, Johnson J (2010). Cutaneous wound healing activity of the ethanolic extracts of the leaf of Senna alata L. J. Biol. Sci. Bio. 26(5):343–356. |
|
|
|
Ola FL, Oboh G (2000). Anti-nutritional factors, in nutritional quality of plant foods. J. Technol. 4:1-3. |
|
|
|
Onwuka GI (2005). Food analysis and instrumentation theory and practice. Naphthali print, Nigeria. pp. 63-98. |
|
|
|
Orishadipe AT, Okogun JI, Mishelia E (2010). Gas chromatography - mass spectrometry analysis of the hexane extract of Calliandra portoricensis and its antimicrobial activity. Afr. J. Pure Appl. Chem. 4(7):131-134. |
|
|
|
Richard W, Katie E, Ferrell T (2006). Squirrels: the animal answer guide. Baltimore: Johns Hopkins University Press. seeds. Pakistan J. Nut. 6:40-43. |
|
|
|
Riguera R (1997). Isolating bioactive compounds from marine organisms. J. Mar. Biotech. 5:187-193. |
|
|
Schuler P (1990). Natural antioxidants exploited commercially. Food Antioxidants, Elsevier Appl. Food Sci. Ser. pp. 99-170.
Crossref |
|
|
Scalbert A (1991). Antimicrobial properties of tannis. Phytochemistry 30:3875-3882.
Crossref |
|
|
Siddhuraju P, Becker K (2005). Nutritional and antinutritional composition, in vitro amino acid availability, starch digestibility and predicted glycemic index of differently processed mucuna beans (Mucuna pruriens var. utilis): An underutilized legume. Food Chem. 91:275-286.
Crossref |
|
|
|
Stein S, Mirokhin D, Tchekhovskoi D, Mallard G (2002). The NIST Mass Spectral Search Program for the NIST/EPA/NIH Mass Spectra Library. Standard Reference Data Program of the National Institute of Standards and Technology. Gaithers-burg, MD, US; 2002. |
|
|
Sparg SG, Light ME, Stadan JV (2004). Biological activities and distribution of plant saponins. J. Ethnoph. 94:219-243.
Crossref |
|
|
|
Taiwo A, Abidemi C, Oyedepo J, Adebayo B, Oluwadare I, Agboto D (2009). Nutrient content and antinutritional factor in Shea butter (Butryospermum parkii) leaves. Afr. J. Biotech. 8(21):5888-5890. |
|
|
|
Trease MT Evans SE (1989).The phytochemical analysis and anti-bacterial screening of extract of some common vegetables. Chem. Sci. Nig. 26:57-58. |
|
|
Valentao P, Fernandes E, Carvalho F, Andrade PB, Seabra, RM. Bastos ML (2002). Antioxidant properties of cardoon (Cynara cardunculus L.) infusion against superoxide radical, hydroxyl radical, and hypochlorous acid. J. Agric. Food Chem. 50:4989-4993.
Crossref |
|
|
|
Veerachari U, Bopaiah AK (2011). Preliminary phytochemical evaluation of the leaf extract of five Cassia species. J. Chem. Pharm. Res. 3(5):574-583. |
|
|
|
Wheeler EI, Ferrel RE (1971). Methods for phytic acid determination in wheat and wheat fractions. J. Cer. Chem. 48:312-320. |
|
|
|
World Health Organization (2002). General Guidelines for methodologies on research and evaluation of Traditional medicines. Geneva, World Health Organization. 8:235-239. |