ABSTRACT
This study was carried out to investigate the hypoglycemic effects of ethanol extract and its chloroform and ethyl acetate fractions of Rhaphidophora glauca (Wall.) Schottleaves in normal and glucose induced hyperglycemic mice (in vivo). Area under curve (AUC) was also calculated during oral glucose tolerance test (OGTT). Ethanol extract and its fractions of R. glauca leaves at 400 and 800 mg/kg doses significantly (P < 0.05-0.001) reduced fasting glucose level in normal mice as compared to standard drug glibenclamide (5 mg/kg). Ethanol extract of R. glauca (EERG) at 800 mg/kg dose showed the highest hypoglycemic effect among the extract and fractions and it decreased 25.13% of the blood glucose level after 2 h of administration in normal mice, where glibenclamide decreased to 49.30%. In oral glucose tolerance test, at 400 and 800 mg/kg dose of extract and fractions significantly reduced blood glucose level (P < 0.05) at 30 min, but at 60 and 90 min, blood glucose level reduction is not all properly significant as compared to the control. At 120 min, both doses of extract and fractions significantly (P < 0.01) reduced blood glucose level. Whereas glibenclamide (5 mg/kg) significantly reduced glucose level at every hour after administration. EERG at 800 mg/kg dose showed the highest hypoglycemic effect among the extract and fractions and it decreased to 13.28% of blood glucose level after 2 h of administration in glucose induced mice, where glibenclamide decreased to 41.18%. AUC during OGTT of extract and fractions are at the range of 12.713 to 13.188 h.mmol/L., and 14.573 and 9.835 h.mmol/L for control and glibenclamide, respectively. These findings suggest that the plant may be a potential source for the development of new oral hypoglycemic agent.
Key words: Rhaphidophora glauca, hypoglycemic activity, area under curve (AUC), ethanol extract, fractions, glucose induced.
Several medicinal plants have been reported to be useful in treating diabetes globally and have been used empirically in antidiabetic and antihyperglycemic cures. Antihyperglycemic activity of the plants is principally due to their ability to reinstate the function of pancreatic tissues by causing an increase in insulin production or inhibit the intestinal absorption of glucose or to the facilitation of metabolites in insulin reliant processes. More than 400 plant species having hypoglycemic activity have been accessible in literature, though, searching for new antihyperglycemic drugs from natural plants is still striking because they contain substances which make obvious alternative and safe property on diabetes mellitus (Haddad et al., 2012; Patel et al., 2012a, b). Most of the plants contain glycosides, alkaloids, terpenoids, flavonoids, carotenoids, etc., that are habitually implicated as having hypoglycemic effect (Salihu et al., 2015).
Diabetes is a metabolic disorder of sugar, fat and protein, influencing a substantial number of populaces on the planet (Matsumoto et al., 2015). Diabetes mellitus is not a solitary disorder, rather it is a gathering of metabolic disorder described by ceaseless hyperglycemia, coming about because of deformities in insulin discharge, insulin activity, or both. Expanded thirst increased urinary yield, ketonemia and ketonuriaare, the basic side effects of diabetes mellitus which occur due to the abnormalities in carbohydrate, fat, and protein metabolism. At the point when ketones body is available in the blood and urine, it is called ketoacidosis; hence, legitimate treatment ought to be taken quickly, else it can prompts other diabetic complications (Low et al., 2015). Diabetes mellitus has brought about critical grimness and mortality because of microvascular (retinopathy, neuropathy, and nephropathy) and macrovascular (heart assault, stroke and fringe vascular sickness) complexities (Singh et al., 2015).
Diabetes is basically ascribed to the fast ascent in undesirable way of life, urbanization and maturing. Hyperglycemia which is the primary side effect of diabetes mellitus produces reactive oxygen species (ROS) which cause lipid peroxidation and layer harm. ROS assumes an imperative part in the improvement of auxiliary complications in diabetes mellitus such as cataract, neuropathy and nephropathy. Antioxidants protect β-cells from oxidation by inhibiting the peroxidation chain reaction and along these lines they assume an important part in the diabetes. Plants containing regular cancer prevention agents, for example, tannins, flavonoids, vitamin C and E can safeguard β-cell work and anticipate diabetes prompted ROS development. Polyphenols, which are ordered into numerous gatherings, for example, flavonoids, tannins and stilbenes, have been known as health-beneficial properties, which incorporate free radical searching, restraint of hydrolytic and oxidative proteins, antiinflammatory activity and hypoglycemic potentiality (Patel et al., 2011; Roy et al., 2015). Aldose reductase as a key catalyst, catalyze the diminishment of glucose to sorbitol and is related in the perpetual complications of diabetes, such as peripheral neuropathy and retinopathy. Utilization of aldose reductase inhibitors and α-glucosidase inhibitors has been reported for the treatment of diabetic complications (Jung et al., 2011).
Oral hypoglycemic medications can bring about different adverse reactions, including hypoglycemia, weight gain, fluid retention, cardiac failure, and gastrointestinal side effects (El-Refaei et al., 2014; Chiniwala and Jabbour, 2011; Kar et al., 2015). Specifically, hypoglycemia is connected with cardiovascular incidents and cognitive dysfunction, and it is known that hypoglycemic episodes can lead to falls and cracks (Geier et al., 2014). Elderly patients are more inclined to create hypoglycemia than more youthful patients when treated with different medications, and in addition quickly after release from healing center, if they have renal failure, and if their diet is poor and they are also less likely to detect the onset of hypoglycemia (Kamei et al., 2015; Penfornis et al., 2015). These distinctions make treatment of diabetes more troublesome in elderly patients, so that cautious training and drug selection are needed.
Rhaphidophora glauca (Wall.) Schott (family: Araceae), is an aroid liane native to the subtropical and warm temperate regions of the eastern Himalaya, which is also distributed in Nepal through North East India to Bangladesh and Myanmar and North Thailand to North Laos and Vietnam. Leaves of R. glauca have activities like antiarthritic, membrane stabilizing, α-amylase inhibitory and anthelmintic (Hossain et al., 2015; Kabir et al., 2015).
This study intends to explore the ethanol extract and its chloroform and ethyl acetate fractions of R. glauca for its hypoglycemic activity in normal and glucose induced hyperglycemic mice.
Plant collection and identification
Leaves of R. glauca were collected from Alutila, khagrachari, Chittagong, Bangladesh in the month of September 2014 at the last time of its flowering. It was authenticated by reputed plant taxonomist, Department of Botany, University of Chittagong, Chittagong-4331, Bangladesh. A specimen of the plant has been preserved in the national herbarium with the Accession No.30145.
Extraction and fractionations
Leaves were cleaned with fresh distilled water and dried for a period of 10 days under shade and then powdered with a mechanical grinder, passing through sieve #40, and stored in a tight container. The powdered of leaves (900 g) of R. glauca was soaked in 1.3 L ethanol for 7 days with occasional shaking and stirring and filtered through a cotton plug followed by Whatman filter paper number-1. The extract was then concentrated by using a rotary evaporator at reduced temperature and pressure. A portion (50 g) of the concentrated ethanol extract (EERG) was fractioned by the modified Kupchan partitioning method (Islam et al., 2010; Bulbul et al., 2011) into chloroform (CHFRG, 13 g) and ethyl acetate (EAFRG, 12 g).
Chemicals and reagents
All the chemicals and reagents were of analytical grade. Ethanol, chloroform and ethyl acetate were purchased from Merck, Germany. Normal saline solution was purchased from Beximco Infusion Ltd. Rapid ViewTM (Blood glucose monitoring system, Model: BIO-M1, BIOUSA Inc, California, USA) with strips were purchased from Andorkilla, Chittagong. Glucose was purchased from local scientific market, Chowkbazar, Chittagong. Glibenclamide was obtained from Square Pharmaceutical Ltd., Bangladesh.
Animals and experimental set-up
Swiss albino mice, weighing about 28 to 35 g, were collected from Jahangir Nagar University, Savar, Bangladesh. The animals were furnished with standard lab nourishment and refined water ad libitum and maintained at natural regular day-night cycle having proper ventilation in the room. All the experiments were conducted in an isolated and noiseless condition. Then, the study protocol was approved by the P&D Committee, Department of Pharmacy, International Islamic University Chittagong, Bangladesh. The animals were acclimatized to laboratory condition for 7 days prior to experimentation.
Acute toxicity study
For acute toxicity study, forty Swiss albino female mice were used. According to the method of Walum (1998), mice were divided into four groups of five animals each. Different doses (1000, 2000, 3000 and 4000 mg/kg) of ethanol extract and its chloroform and ethyl acetate fraction of R. glauca leaves were administered by stomach tube. Then, the animals were observed for general toxicity signs.
Experimental protocol for in vivo hypoglycemic activity
Hypoglycemic effect in normal mice
Mice were kept fasting overnight with free access to water. Group I was treated as control group, Group II was treated with glibenclamide (5 mg/kg body weight), Groups III to VIII were treated with ethanol extract and its chloroform and ethyl acetate fraction of R. glauca leaves at 400 and 800 mg/kg body weight, respectively. Before administration of the drug, extract and fractions solutions fasting blood glucose levels were estimated by glucose oxidase method (Barham and Trinder, 1972). Then blood glucose levels were again estimated after 2 h of administration of drug and extract solutions. Glucose levels were measured by Rapid ViewTM (Blood glucose monitoring system, Model: BIO-M1, BIOUSA Inc, California, USA). The maximum hypoglycemic effect of glibenclamide was found after 2 h of its administration. Percent decrease of blood glucose level after 2 h was measured using the following equation:
Hypoglycemic effect in glucose induced hyperglycemic mice (OGTT)
Oral glucose tolerance test (OGTT) was performed according to the standard method (Xia et al., 2013) with minor modification. Group I was treated as normal control group, Group II was treated with glibenclamide (5 mg/kg body weight), Groups III to VIII were treated with ethanol extract and its chloroform and ethyl acetate fraction of R. glauca leaves at 400 and 800 mg/kg body weight, respectively. Glucose solution (1 g/kg body weight) was administered at first. Then, drug and extract solutions were administered to the glucose fed. Serum glucose level of blood sample from tail vein was estimated using glucometer at 0, 30, 60, 90 and 120 min. Areas under the curves (AUC) for OGTT were calculated to evaluate glucose tolerance (Purves, 1992). Percent decrease of blood glucose level after 120 min was measured using the following equation:
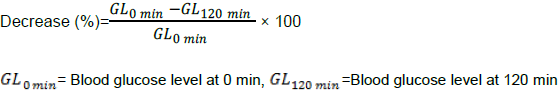
Statistical analysis
The results were expressed as the mean±standard error of mean (SEM). The results were statistically analyzed using repeated measures analysis of variance with Dunnett’s and Bonferroni multiple comparison when compared with control in OGTT. Paired t test was performed to show significant variation between before and after blood glucose level. P<0.05, P<0.01 and P<0.001 were considered as statistically significant. Statistical programs were used GRAPHPAD PRISM® (version 6.00; GraphPadSoftware Inc., San Diego, CA, USA).
Acute toxicity study
None of the animals showed behavioral, neurological or physical changes characterized by symptoms such as reduced motor activity, restlessness, convulsions, coma, diarrhea and lacrimation at the limit dose of 4000 mg/kg of ethanol extract and its chloroform and ethyl acetate fraction of R. glauca during the observation period. In addition, no mortality was observed at the test dose. Thus, the median lethal dose (LD50) of the plant extracts was found to be greater than 4000 mg/kg.
Hypoglycemic effect in normal mice
Both doses of ethanol extract, fractions and glibenclamide significantly reduced fasting blood glucose level. Glibenclamide showed significant reduction at level of P<0.01. Dose of 400 and 800 mg/kg leaves ethanol extract of R. glauca and its fractions showed significant reduction at the level of P<0.05 and P<0.001, respectively. These results suggest that hypoglycemic activity of 800 mg/kg dose and glibenclamide has similar significant level. All results are presented in Table 1 and percentage of decrease of blood glucose level in normal mice after 2 h with different treatment are shown in Figure 1. EERG at the highest dose of 800 mg/kg decreased blood glucose level (25.13%) than other treatments (accept standard Glibenclamide).
Hypoglycemic effect in glucose induced hyperglycemic mice (OGTT)
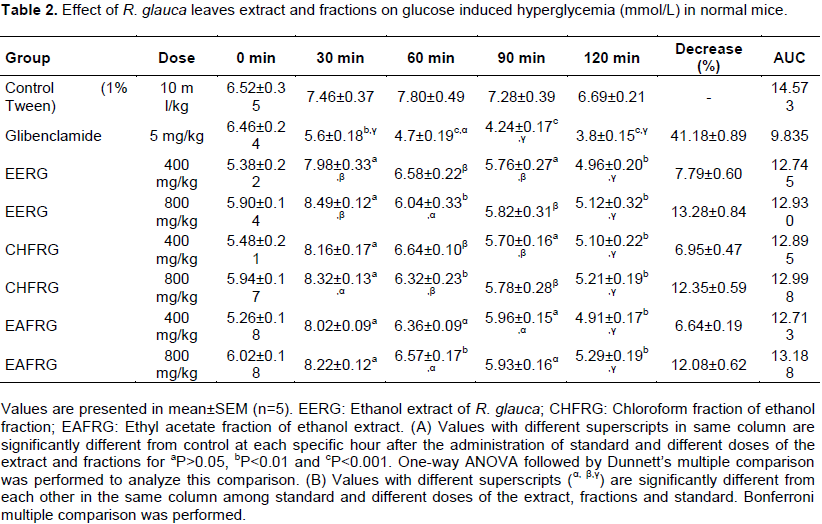
Investigational induction of hyperglycemia resulted in increased glucose level in blood (comparing the result of control of 0 and 1 h) (Table 2). Both dose of leaves extract and its fractions did not manifest any significant reduction in 30 min after administration. Most significant reduction (P<0.05) was observed for 800 mg/kg dose of ethanol extract of R. glauca and its fractions at 120 min. At 120 min, this dose also showed significant reduction (P<0.05). Standard glibenclamide (5 mg/kg) showed significant reduction in 30, 60, 90 and 120 min. These findings suggest that evidently 800 mg/kg dose is more potent than 400 mg/kg dose. Time interaction with each specific hour in this experiment was also found significant (P<0.05 and 0.001). All results are presented in Table 2 and results of AUC of OGTT are also presented in Table 2. Percentage of decrease of blood glucose level in glucose induced mice after 2 h with different treatment are shown in Figure 2. EERG at the highest dose of 800 mg/kg decreased blood glucose level (13.28%) than other treatments (accept standard Glibenclamide).
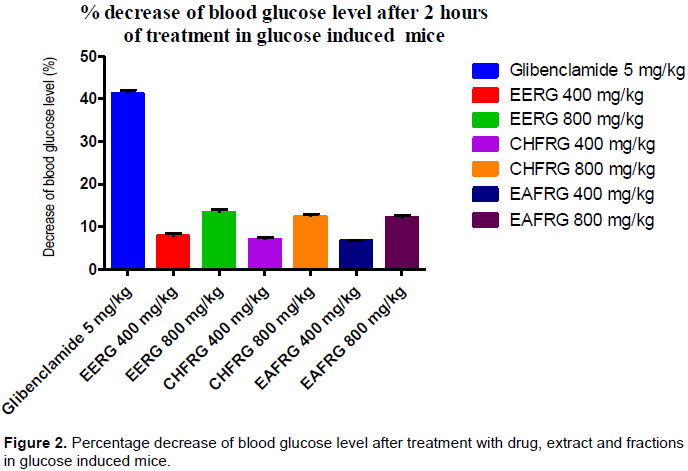
In this study, ethanol extract and its chloroform and ethyl acetate fractions of leaves of R. glauca exerted significant hypoglycemic activity in both fasting glucose level reduction in normal mice and oral glucose tolerance test in glucose induced hyperglycemic mice. Decrease of blood glucose level after 2 h of treatment is very well significant when compared with the control.
There are numerous pharmaceutical items which are accessible in current medicinal treatment have a long history of utilization as home grown cures, including ibuprofen, opium, digitalis and quinine. An expansive number of world's populace who live in creating nations cannot take the advantages of the present day pharmaceuticals as those are extremely expensive. More or less 25% of modern drugs used in the United States have been derived from plant origins (WHO, 2008). So, research on phytomedicine has got great momentum in recent years to find out noble pharmaceuticals.
Diabetes is a metabolic disease connected with host of difficulties, for example, intense and endless inconveniences. The intense intricacies may be activated by metabolic disorders including ketoacidosis and non-ketotic coma and infections, but these indications can be moderately very much controlled. Then again, the endless entanglements have a tendency to exacerbate as the diabetes advances. Chronic complications associated with diabetes include macroangiopathies, such as coronary artery disease and cerebrovascular disease, and microangiopathies, such as neuropathy, orthostatic hypotension, retinopathy and nephropathy (Chang et al., 2006; Takayuki et al., 2006; Gogoi et al., 2014; Toma et al., 2015).
In the normal mice, ethanol extract and all fractions reduced fasting blood glucose level in normal mice after the treatment. EERG at 800 mg/kg dose decrease 25.13% glucose level among extract and fractions, where standard drug glibenclamide decreased 49.30%. This is evident that this extract did not supply glucose in the blood due to its administration.
The OGTT is generally considered as more susceptible for the screening of impaired glycemia, because it detects changes in post-prandial glycemia that tend to precede changes in fasting glucose. All the current diagnostic criteria for diabetes depend on a threshold value imposed on a continuous distribution of blood glucose levels. Yet, the correct glycemic threshold that discriminates ‘normal’ from diabetic is not obvious. Though screening for undiagnosed type 2 diabetes remains a contentious issue, there is clear evidence that once it is diagnosed, complications can be prevented in many patients (Vamos et al., 2012; Hemmingsen et al., 2015). OGTT measures the body's ability to use glucose, the body's main source of energy. OGTT can be used to diagnose pre diabetes and diabetes. The ethanol extract and its fractions of leaves of R. glauca showed significant ability to reduce the elevated glucose level in normal mice as compared to the standard drug glibenclamide, where EERG showed the highest hypoglycemic activity in both examined model.
The OGTT determines the shape of the glucose curve based on the measurements at 0, 30, 60, 90 and 120 min. From the glucose curve, AUC was determined using
Trapezoidal Rule. AUC during OGTT for screening hypoglycemic effect of extract and fractions are at the range of 12.713 to 13.188 h.mmol/L, and 14.573 and 9.835 h.mmol/L for control and glibenclamide, respectively.
These outcomes suggest that ethanol extract and its chloroform and ethyl acetate fractions of R. glauca leaves possess a hypoglycemic principle and can be useful for the treatment of diabetes. Further studies are warranted to isolate the active principle and to find out its accurate mechanism of action.
From the study, it was concluded that R. glauca may have hypoglycemic effect, but not sure about how this extracts and fractions can exert potent hypoglycemic activity. It is a logical inference that this plant may recover the metabolism of glucose and increase insulin secretion by stimulating beta cells. It is possible to propose that the bioactive compounds present in the leaves extract and its fractions may be responsible for versatile effects. Based on the literature search, this is the first study about hypoglycemic activity of R. glauca. That is why the exact mode of action is not determined yet. However, further co-ordinate and well-structured studies would be required to isolate the bioactive compounds and determine their underlying molecular mechanism of action on diabetes induced mice model. These findings suggest that the plant may be a potential source for the development of new oral hypoglycemic agent.
The authors have not declared any conflict of interests.
The authors are grateful to the authority of International Islamic University Chittagong, Bangladesh, for providing the facilities to conduct this research work. The authors are thankful to the Taxonomist and Professor, Dr. Shaikh Bokhtear Uddin, Department of Botany, University of Chittagong, for identifying the plant. The authors like to thank Mr. Md. Mominur Rahman, Assistant professor, IIUC for supervision of the experiment. The authors are also thankful to all members of GUSTO (A research group), for their kind help in the experiment.
REFERENCES
|
Bulbul IJ, Nahar L, Haque M (2011). Antibacterial, cytotoxic and antioxidant activity of chloroform, n-hexane and ethyl acetate extract of plant Coccinia. Agric. Biol. J. N. Am. 2:713-719.
Crossref
|
|
|
|
Barham D, Trinder P (1972). An improved color reagent for the determination of blood glucose by oxidase system. Analyst J.97:142-145.
Crossref
|
|
|
|
|
Chiniwala N, Jabbour S (2011). Management of diabetes mellitus in the elderly.Curr. Opin. Endocrinol. Diabetes Obes.18:148-52.
Crossref
|
|
|
|
|
Chang H, Song Z, Zuo X (2006). Antihyperglycemic Activity of Herb Extracts on Streptozotocin-Induced Diabetic Rats. Biosci. Biotechnol. Biochem. 70:2556-2559.
Crossref
|
|
|
|
|
El-Refaei MF, Abduljawad SH, Alghamdi AH (2014). Alternative medicine in diabetes - role of angiogenesis, oxidative stress, and chronic inflammation.The Review of Diabetic Studies: RDS. 11(3-4):231-244.
Crossref
|
|
|
|
|
Gogoi B, Kakoti BB, Borah S, Borah NS (2014).Antihyperglycemic and in vivo antioxidative activity evaluation of cinnamomumbejolghota (buch.-ham.) in streptozotocin induced diabetic rats: An ethnomedicinal plant in assam.Asian Pac. J. Trop. Med. 7S1, S427-34.
|
|
|
|
|
Geier AS, WellmannI, Wellmann J, Kajuter H, Heidinger O, Hempel G (2014). Patterns and determinants of new first-line antihyperglycaemic drug use in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 106(1):73-80.
Crossref
|
|
|
|
|
Haddad PS, Musallam L, Martineau LC, Harris C, Lavoie L, Arnason JT (2012). Comprehensive evidence-based assessment and prioritization of potential antidiabetic medicinal plants: A case study from Canadian eastern JamesBayCree traditional medicine. Evidence-Based Complementary and Alternative Medicine, ECAM. 10:1155/893426.
|
|
|
|
|
Hossain MM, Kabir MSH, Hasanat A, Kabir MI, Chowdhury TA, Kibria ASMG (2015). Investigation of in vitro anti-arthritic and membrane stabilizing activity of ethanol extracts of three Bangladeshi plants. The Pharma Innovation J. 4(1):76-80.
|
|
|
|
|
Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal TP, Wetterslev J (2015). WITHDRAWN: Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. The Cochrane Database of Systematic Reviews, 7, CD008143.
Crossref
|
|
|
|
|
Islam MK, Eti IZ, Chowdhury JA (2010). Phytochemical and antimicrobial analysis on the extracte of Oroxylum indicum Linn, Stem-Bark, IJPT 9:25-28.
|
|
|
|
|
Jung HA, Islam MD, Kwon YS, Jin SE, Son YK, Park JJ (2011).Extraction and identification of three major aldose reductase inhibitors from Artemisia montana. Food Chem. Toxicol. 49:376-384.
Crossref
|
|
|
|
|
Kar P, Jones KL, Horowitz M, Deane AM (2015). Management of critically ill patients with type 2 diabetes: The need for personalised therapy. World J. Diabetes. 6(5):693-706.
Crossref
|
|
|
|
|
Kamei S, Kaneto H, Irie S, Kinoshita T, Tanabe A, Hirukawa H (2015). Pseudoaldosteronism induced by yokukansan in an elderly Japanese type 2 diabetic patients with Alzheimer's disease. J. Diabetes Investig. 6(4):487-488.
Crossref
|
|
|
|
|
Kabir MSH, Hossain MM, Hasanat A, Rahman MM, Masum MAA, Hasan M, Kamal ATMM (2015). Anthelmintic and α-amylase inhibition effects of ethanol extract and its different fractions of Rhaphidophora glauca (Wall.) Schott leaves. IOSR J. Pharm. Biol. Sci. 10(3):99-104.
|
|
|
|
|
Low SK, Sum CF, Yeoh LY, Tavintharan S, Ng XW, Lee SB (2015). Prevalence of chronic kidney disease in adults with type 2 diabetes mellitus. Annals of the Academy of Medicine, Singapore 45(5):164-171.
|
|
|
|
|
Matsumoto Y, Ishii M, Hayashi Y, Miyazaki S, Sugita T, Sumiya E (2015). Diabetic silkworms for evaluation of therapeutically effective drugs against type II diabetes. Sci. Rep. 5:107-122.
Crossref
|
|
|
|
|
Patel D, Kumar R, Laloo D, Hemalatha S (2012a). Diabetes mellitus: An overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac. J. Trop. Biomed. 2(5): 411-420.
Crossref
|
|
|
|
|
Patel D, Prasad S, Kumar R, Hemalatha S (2012b). An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2(4):320-330.
Crossref
|
|
|
|
|
Patel DK, Kumar R, Prasad SK, Hemalatha S (2011). Pedalium murex Linn (Pedaliaceae) fruits: A comparative antioxidant activity of its different fractions. Asian Pac. J. Trop. Biomed.1:395-400.
Crossref
|
|
|
|
|
Penfornis A, Fiquet B, Blickle JF, Dejager S (2015). Potential glycemic overtreatment in patients >/=75 years with type 2 diabetes mellitus and renal disease: Experience from the observational OREDIA study. Diabetes, Metab. Syndr. Obes. 8:303-313.
|
|
|
|
|
Purves R (1992). Optimum numerical integration methods for estimation of area-under-the curve (AUC) and area-under-the-moment-curve (AUMC) J. Pharmacokine.t Biopharm. 20:211-226.
Crossref
|
|
|
|
|
Roy P, Abdulsalam FI, Pandey DK, Bhattacharjee A, Eruvaram NR, Malik T (2015). Evaluation of antioxidant, antibacterial, and antidiabetic potential of two traditional medicinal plants of india: Swertiacordata and swertiachirayita. Pharmacogn. Res. 7(l1):57-62.
|
|
|
|
|
Salihu ST, Bello L, Wara HS, Ali S (2015).An ethnobotanical survey of antidiabetic plants used by hausa-fulani tribes in sokoto, northwest Nigeria. J. Ethnopharmacol.172:91-98.
Crossref
|
|
|
|
|
Singh V, Jain M, Misra A, Khanna V, Prakash P, Malasoni R (2015). Curcuma oil ameliorates insulin resistance & associated thrombotic complications in hamster & rat. Indian J. Med. Res. 141(6):823-832.
Crossref
|
|
|
|
|
Takayuki H, Chisato M, Hitoshi A (2006). Antihyperglycemic Effect of Polyphenols from Acerola (Malpighia emarginata DC) Fruit. Biosci. Biotechnol. Biochem. 70:1813-1820.
Crossref
|
|
|
|
|
Toma A, Makonnen E, Mekonnen Y, Debella A, Adisakwattana S (2015). Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complement Altern. Med. 15(1):242.
Crossref
|
|
|
|
|
Vamos EP, Harris M, Millett C, Pape UJ, Khunti K, Curcin V (2012). Association of systolic and diastolic blood pressure and all cause mortality in people with newly diagnosed type 2 diabetes: Retrospective cohort study. BMJ (Clinical Research Ed.), 345, e5567.
Crossref
|
|
|
|
|
Walum E. (1998). Acute oral toxicity. Environ. Health Perspect. 106(Suppl 2):497-503.
Crossref
|
|
|
|
|
Xia F, Xu X, Zhai H, Meng Y, Zhang H, Du S (2013). Castration-induced testosterone deficiency increases fasting glucose associated with hepatic and extra-hepatic insulin resistance in adult male rats. Reprod. Biol. Endocrinol. 11(27):106-111
Crossref
|
|
|
|
|
WHO (2008). Traditional medicine.
|
|