ABSTRACT
Dyslipidaemia is characterised by enhanced production of reactive oxygen species and increased oxidative stress, which may affect liver, kidney and heart function. It is considered as a critical risk factor for cardiovascular and liver diseases. The aim of our study was to assess the effect of vanadium chloride on the oxidative stress state of liver, kidney and heart functions along with electrolyte balance during treatment of dyslipidemia using simvastatin in an animal model. Rats were assigned to 1 of 5 groups: group 1, control group; group 2, received high fat diet (HFD); group 3, received HFD and 30 mg/kg body weight (BW) simvastatin; group 4, received HFD and 15 mg/kg BW vanadium chloride and group 5, received HFD, simvastatin and vanadium chloride. Drugs were administered orally by gavage for the last week of the experimental period. HFD was found to elicit a significant decrease (P ≤ 0.05) in non-protein sulfhydryls and significant increases (P ≤ 0.05) in hepatic and cardiac malondialdehyde (MDA), serum creatine kinase, lactate dehydrogenase, creatinine, urea, uric acid, calcium, sodium and potassium. Oral administration of vanadium chloride did not synergize simvastatin to ameliorate the negative effects of HFD, instead it worsens the negative effect of the HFD. Vanadium chloride administration decreased the concentration of non-protein sulfhydryls and increased MDA concentration in liver and heart tissues. It also caused further increase in the serum concentration of all measured serum parameters. These data proved that the vanadium concentration used in this study is not safe or efficient in ameliorating the oxidative stress or in improving kidney or heart functions in dyslipidemic rats.
Key words: Vanadium, simvastatin, oxidative stress, kidney, rats.
Dyslipidemia is characterized by elevated plasma triglycerides, cholesterol and LDL-cholesterol and reduced HDL-cholesterol. It is a complex disorder that involves both systemic, as well as organ-specific mechanisms (Chakravarthy et al., 2016). These mechanisms result in abnormal lipids plasma levels from disturbances in the release and uptake of lipids by adipose tissues as well as removal of lipids from circulation (Tikhonenko et al., 2010). Dyslipidemia is one of the key risk factors for cardiovascular disease and there is a strong causal relationship between dyslipidaemia and cardiac diseases (Galema-Boers and Roeters van Lennep, 2015). Low levels of high density lipoprotein cholesterol (HDL-C) were shown to be a predictor for high risk of premature development of atherosclerosis (Lawlor et al., 2006). Excess free fatty acids in dyslipidemic subjects leads to increased fatty acids oxidation in mitochondria resulting in mitochondrial overproduction of reactive oxygen species and oxidative stress (Tangvarasittichai, 2015). Also, Yuan and Kitts (2003) reported that high cholesterol diets reduce hepatic glutathione (GSH) levels and decrease the activities of antioxidant enzymes. Dyslipidemia and oxidative stress are the two main mechanisms responsible for development of atherosclerosis and its complications, such as cardiovascular diseases (Katakami, 2018). The hallmark of atherogenesis is the accumulation of LDL and other lipids in the vascular wall (Hansson, 2005). The oxidative modification converts LDL into more atherogenic particles (Albertini et al., 2002). Also, excess fat accumulation in hepatocytes, in the form of lipid droplets, may be a factor in pathophysiology of liver diseases (Katsiki et al., 2016).
Statins have been used for the last few decades in the management of adults with dyslipidemias (Joyce et al., 2016). Statins are a group of lipid-lowering agents acting by inhibition of 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductase. This enzyme catalyses the reduction of hydroxymethylgluteryl CoA (HMG-CoA) to mevalonic acid during cholesterol biosynthesis (Abbasi et al., 2015). It has several pleiotropic actions including hypolipidemic effects, its immunomodulatory effects, and its protective effects against oxidative stress and inflammation (Moutzouri et al., 2012; Mulhauptet al., 2003). Along with its lipid-lowering activity, statins improve function of endothelial cells, increase stability of atherosclerotic plaques, decrease oxidative stress, and inhibit thrombogenic responses (Patel and Kothari, 2017). It is also beneficial in prevention of coronary heat diseases even in patients with normal blood cholesterol levels (Abbasi et al., 2015). These benefits made statins widely prescribed for reducing mortality in cases of coronary heart diseases (Patel and Kothari, 2017). Although statins are usually well tolerated, they have adverse effects on many tissues, especially skeletal muscles and liver (Camerino et al., 2016). The main elimination route for statins is through bile after metabolism by the liver. Consequently, hepatic dysfunction is a risk factor for statin-induced myopathy (Patel and Kothari, 2017).
Vanadium is a naturally occurring metal which exists in various oxidation states from -1 to +5 (Barceloux and Barceloux, 1999). Vanadium is an ultra-trace element essential for many animal species and it is present in animal cells at concentrations of 10 to 20 nmol (Brichard and Henquin, 1995). Vanadium deficiency is associated with reproduction impairment, changes in red blood cell formation and iron metabolism (Gummow, 2011). Compounds of vanadium are regarded to have insulin-mimicking or enhancing properties and are used as antidiabetic agents. Overall, vanadium therapy was shown to normalize blood glucose levels in diabetic-induced rats and was also shown to cure many hyperglycaemia-related disorders (Liu et al., 2013). Some other researchers have investigated the toxcicity of vanadium compounds depending on oxidation state, administration, route of exposure, dose and the sensitivity of the organism (Pessoa et al., 2015). Besides its potential pharmaceutics, vanadium is also used as a dietary supplement for enhancing athletic performance. Upon starting this study, we believed that vanadium might be considered an antioxidant which might reduce the side effects of statins on heart, liver and kidney tissues and their respective functions. The uncertainty toward vanadium safety is complicated by the existence of multiple oxidation states with the potential to interconvert via oxidation-reduction, both in the environment as well as following ingestion (Heidari et al., 2016). Here, we try to investigate the effect of oral administration of 15 mg/kg vanadium chloride alone, or in combination with 30 mg/kg simvastatin, on oxidative stress. We investigate kidney function and electrolyte balance in an animal model fed a high cholesterol diet.
Chemicals and diet
All chemicals used were of analytical grade unless specified. Vanadium (III) chloride (purity 97%) was purchased from Acros, Belgium. Cholesterol (purity ≥92.5%) powder was purchased from Sigma Aldrich, St. Louis, MO, USA. All other chemical or kits were purchased from local markets. The high-fat diet composed of crushed pellets mixed with cholesterol powder (1% w/w). The mixed pellets were reconstituted with water as a slurry and dried properly to avoid any fungal contamination
Animals and treatment
Thirty male rats (Albino strain) were obtained from the Experimental Animal Care Centre, College of Pharmacy, King Saud University, Riyadh, KSA. Rats weight were about 150-200 g. They were kept at constant temperature (22±2°C), humidity (55%) and 12 h light dark conditions during the experiment and water was allowed ad libitum. After 1 week of acclimatization, rats were randomly allocated into five groups of 6 rats each. The five groups are; Group 1: (control group): rats fed a diet of rat pellets for 45 days and received saline solution orally by gavage during the last 7 days of the experiment. Group 2: (HFD group): rats were fed a high fat diet for 45 days and received normal saline orally by gavage for one week at the end of experimental period. Group 3: (HFD+S group): rats in this group were fed a high-fat diet for 45 days and received simvastatin orally by gavage in a dose of 30 mg/kg body weight daily for last 7 days of the trial. Group 4: (HFD+V group): rats were fed a high-fat diet for 45 days and were administered vanadium chloride (15 mg/kg body weight) orally by gavage daily during the last week of the experimental period. Group 5: (HFD+SV group): these rats received high-fat diet for 45 days and in the last week of experiment, rats treated also with both simvastatin (30 mg/kg BW) and vanadium chloride (15 mg/kg BW) orally by gavage. The animal experiment was approved by Institutional Animal Ethics Committee of College of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
Samples
At the end of the experimental period, rats were fasted for 12 h and blood samples were collected from retro-orbital plexus under light ether anaesthesia in a plan tubes. Serums were separated by centrifugation at 2500 × g for 10 min and transferred to pre-labelled Eppendorf tubes for various biochemical parameters. Immediately, after blood withdrawal, the animals were sacrificed after using light anaesthesia. Liver and heart tissues were isolated, washed with chilled normal saline, and frozen at -80°C for subsequent determinations of malondialdehyde (MDA) and non-protein sulfhydryls (NP-SH) concentrations in these tissues.
Estimation of malondialdehyde (MDA) in hepatic and heart tissues
Malondialdehyde was determined according to the method reported by Utely et al. (1967). Parts of liver and heart tissues were taken and homogenized in 0.15 mol/L KCl (at 4°C) in a Potter-Elvehjem type C homogenizer to give a 10% w/v homogenate. Aliquots (1 mL) of homogenates were incubated at 37°C for 3 h in a metabolic shaker. Then 1 mL of 10% aqueous trichloroacetic acid (TCA) was added and mixed. The mixture was then centrifuged at 800 × g for 10 min. One millilitre of the supernatant was mixed with 1 mL of 0.67% 2-thiobarbituric acid solution and placed in a boiling water bath for 10 min. The mixture was cooled and diluted with 1 mL distilled water. The mixture was centrifuged, and the absorbance was determined at the wavelength of 532 nm at room temperature against a blank. The concentration of MDA was calculated using a standard calibration curve plotted with different concentrations of 1, 1, 3, 3’-tetraethoxypropane (TEP). The extent of lipid peroxidation was expressed, and MDA values were expressed as nanomoles of MDA per gram of protein using a molar extinction coefficient for MDA of 1.56x105 M−1 cm−1. The protein content was estimated according to the method of Lowry et al. (1951).
Estimation of non-protein sulfhydryl (NP-SH) in hepatic and heart tissue
Hepatic nonprotein sulfhydryls were measured according to the method of Sedlak and Lindsay (1968). Liver and heart tissues were homogenized in ice-cold normal saline containing 0.02 mmol/L ethylenediaminetetraacetic acid (EDTA) to give a 10% w/v homogenate. Aliquots of 5 mL of the homogenates were mixed in 15 mL test tubes with 4 mL of distilled water and 1 mL of 50% TCA. The tubes were shaken intermittently for 10 min and centrifuged at 3000 rpm/min. Two millilitres of supernatant were mixed with 4 mL of 0.4 mol/L Tris buffer at pH 8.9, then 0.1 mL of 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB) was added and the tubes were shaken. The absorbance was measured within 5 min of DTNB addition at 412 nm against a blank. The concentrations of non-protein sulfhydryl were expressed as nanomoles per gram of protein. The protein concentrations in these samples were determined using the method of Lowry et al. (1951).
Determination of serum creatinine, urea, and uric acid
Serum creatinine was measured by the Jaffe reaction method (Fabiny and Ertingshausen, 1971) using a CS604 kit (Crescent Diagnostics, Jeddah, Saudi Arabia). Urea was determined by urease method and uric acid was determined by uricase method described by Tabacco et al. (1979) and Fossati et al. (1980) respectively, using Roche kits (Roche Diagnostics GmbH).
Determination of serum calcium, sodium and potassium
Calcium concentration in the serum was determined by the method described by Gitelman (1967) using the CE500 kit (Crescent Diagnostics). Calcium ions react with o-cresolphthalein in an alkaline medium forming a purple coloured complex. The intensity of this colour is proportional to calcium concentrations in the sample. Serum sodium was determined by the Mg-Uranylacetate method described by Henry et al. (1974) using Sodium rapid kit (Human). Potassium concentration in serum was determined by local kit based on the method of Ronald et al. (1992).
Statistical analysis
Data were presented as mean ± SE. Analyses was carried out using Statistical Package (SPSS, Version 12.0). Data were analysed using one-way ANOVA to assess differences between groups. Means were statistically compared using Dunnett’s multiple comparison tests at a 0.05 significance level. Probability values p ≤ 0.05 were considered to be statistically significant.
Vanadium chloride increases malondialdehyde concentrations
Significant (P ≤ 0.05) increases in malondialdehyde levels were detected in liver and heart tissues of HFD-group compared to control one. Liver tissue shows a non-significant (P≥ 0.05) reduction in the concentrations of malondialdehyde in the HFD+V group compared with HFD-group while, further significant (P ≤ 0.05) elevation in malondialdehyde concentration were observed in heart tissue of the HFD+V group compared to the HFD-group. On the other hand, malondialdehyde concentrations were significantly (P ≤ 0.05) lower in groups HFD+S and HFD+SV compared to the HFD group. The presence of vanadium with simvastatin in the HFD+SV group did not reduce malondialdehyde concentrations. Malondialdehyde concentrations were observed to be greater than those found in the HFD+SV group (Figures 1 and 3).
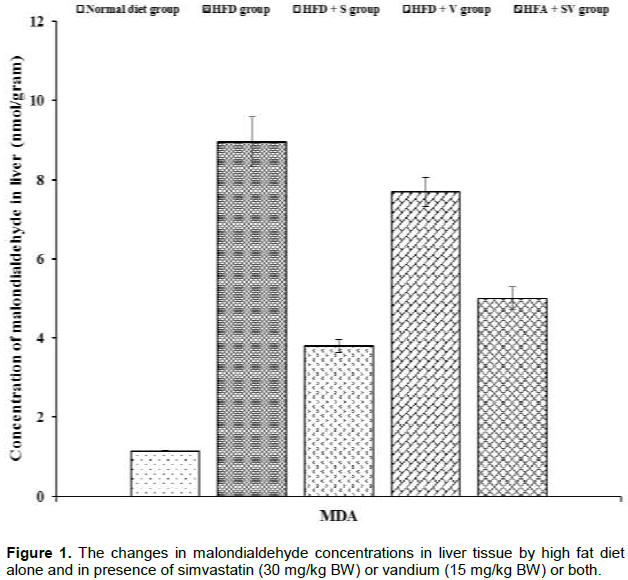
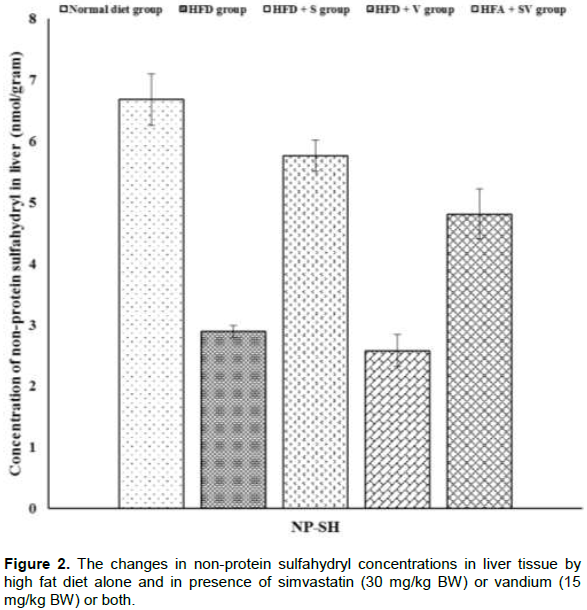
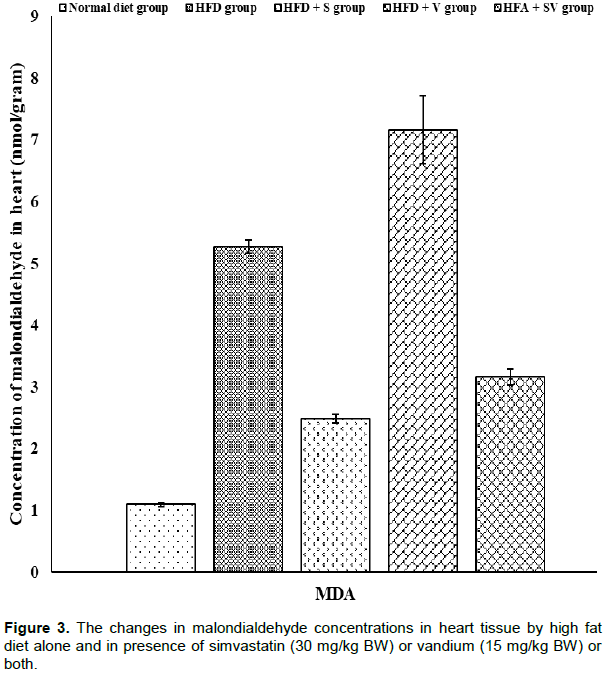
Vanadium chloride decreases non-protein sulfhydryl concentrations
The effect of vanadium chloride alone, or with simvastatin concentration, on non-protein sulfhydryl in liver and heart tissues is demonstrated in Figures 2 and 4. Significant (P ≤ 0.05) decreases the concentration of non-protein sulfhydryl were observed in liver and heart tissues of HFD-group compared to the control group. Liver and heart tissues show further non-significant (P≥ 0.05) reductions in the concentration of non-protein sulfhydryl in the HFD+V group when compared with the HFD-group, while non-protein sulfhydryl was significantly (P ≤ 0.05) increased in the HFD+S and HFD+SV groups when compared to the HFD group.
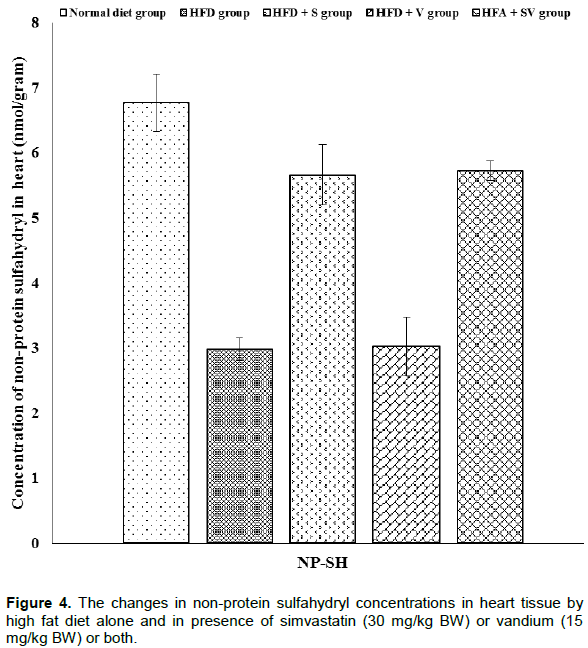
Vanadium chloride decreases lactate dehydrogenase and creatine kinase activities
The activities of lactate dehydrogenase and total creatine kinase in the studied groups are shown in Table 1. Significant (P ≤ 0.05) increases in the activities of lactate dehydrogenase (LDH) and total creatine kinase (CK) were observed in serum of HFD-group compared to control. Further significant (P ≤ 0.05) increases in the activities of both enzymes were observed in the group fed vanadium chloride with high fat diet (HFD+V) compared with the HFD-group. On the other hand, rats in the HFD+S group and in the (HFD+SV group both) show significant (P ≤ 0.05) reduction in the activities of LDH and CK compared to the HFD-group. However, rats in the HFD+SV group had significant increases in serum activities of both enzymes compared to HFD+S group.The values are expressed as mean ± SE. a,The mean values of HFD group are significantly different in comparison with control (P≤0.05). b,The mean values are significantly different in comparison with HFD group (P≤0.05).

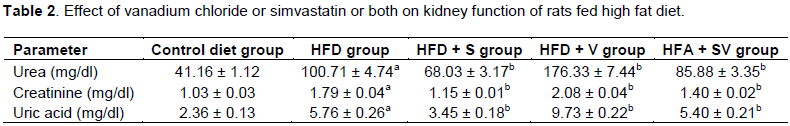
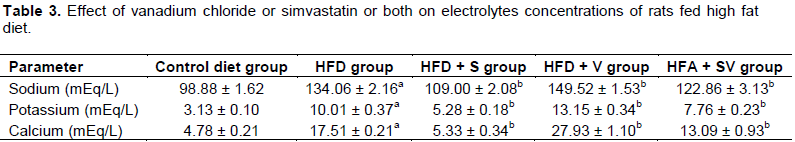
Vanadium chloride decreases kidney function and causes electrolytes imbalance
Significant increases (P ≤ 0.05) in serum concentration of urea, uric acid, creatinine, sodium, potassium and calcium were detected in the HFD-group compared to the control group. Vanadium chloride in HFD+V group caused a significant (P ≤ 0.05) increase in all these parameters compared with HFD-group. On the other hand, rats fed high fat diet and simvastatin alone (HFD+S group) or simvastatin and vanadium chloride (HFD+SV group) show significant (P ≤ 0.05) reduction in serum concentration of urea, uric acid, creatinine, Na+, K+ and Ca2+ when compared to the HFD- group. Vanadium chloride with simvastatin in the HFD+SV group significantly increase parameters of kidney function and electrolyte concentration when compared with group treated with simvastatin alone (HFD+S group) (Tables 2 and 3).The values are expressed as mean ± SE. a,The mean values of HFD group are significantly different in comparison with control (P≤0.05). b,The mean values are significantly different in comparison with HFD group (P≤0.05).The values are expressed as mean ± SE. a,The mean values of HFD group are significantly different in comparison with control (P≤0.05). b,The mean values are significantly different in comparison with HFD group (P≤0.05).
Endogenous antioxidants of non-protein sulfhydryls such as glutathione, vitamin C and vitamin E play an important role in cell protection against oxidative stress and lipid peroxidation (Birben et al., 2012). Oxidative stress may lead to neurotoxicity, cardiotoxicity, hepatotoxicity and nephrotoxicity in humans and animals (Chen et al., 2001). Our results in the present study shows that feeding a high fat diet significantly decreased the concentration of non-protein sulfhydryls. Interestingly, however, malondialdehyde (MDA) concentrations significantly increased in hepatic and cardiac tissues. These results agree with those of Zou et al. (2006) and Yuan and Kitts (2003) who reported that hepatic MDA concentrations increased and GSH levels and antioxidant enzymes activity declined after feeding a high fat diet. Increased oxidative stress in the HFD groups may be due to over production and accumulation of reactive oxygen species from impairment of mitochondrial oxidation and microsomal ω-oxidation of fatty acids (Kersten et al., 1999). The production of reactive oxygen species induces lipid peroxidation of polyunsaturated fatty acids and produces high reactive aldehydes, such as MDA and trans-4-hydroxyl-2-nonenal, which have long term adverse effects on liver and heart tissues. These aldehydes further damage cells by impairing nucleotides and protein synthesis and by interfering with glutathione’s antioxidant potential (Tessari et al., 2009).
High fat diet causes kidney and heart dysfunction and electrolyte imbalance. Kidney dysfunction was characterized by significant increases in serum levels of creatinine, uric acid and urea which indicate kidney’s inability to get rid of waste products. Electrolyte imbalance was characterized by the elevation of blood sodium, potassium and calcium concentrations. The highly elevated activity of LDH and creatine kinase, as a result of feeding a high cholesterol diet, indicates deterioration of cardiac cells. It has been reported that rodents consuming a high fat diet develop visceral adiposity, hyperglycaemia, dyslipidaemia, hyperinsulinemia and hepatic steatosis (Beyegue et al., 2012). These manifestations may be considered as a risk factor for heart and kidney dysfunctions. Oral administration of simvastatin improved dyslipidaemia (data not shown) and ameliorate the oxidative stress state of HFD-rats. This was indicated by the significant increase in the non-protein sulfhydryl concentration and the significant reduction in MDA concentration in liver and heart tissues of rats in the HFD group. Our results are consistent with those of Jahovic et al. (2006) who reported that simvastatin prevent lipid peroxidation, superoxide generation and cytokines production in trinitrobenzene sulfonic acid-induced colitis in rats. Also, simvastatin reduced oxidative stress which was induced by calcium (Parihar et al., 2012). Simvastatin may also reduce the production of reactive oxygen species by decreasing the influx through the respiratory complex I and II (Larsen et al., 2013).
Simvastatin may ameliorate nephrotoxic and cardiotoxic effects and electrolytes imbalance of high fat diet. This was indicated by significant reductions in serum levels of urea, creatinine, uric acid, sodium, potassium as well as calcium and reductions in the activity of LDH and CK. Simvastatin reduces coronary heart diseases with its pleiotropic effects, including antioxidant and anti-inflammatory properties (Shishehbor et al., 2003). Simvastatin also has anti-inflammatory properties that may reduce risk of cardiovascular diseases (Gilbert et al., 2017). Other studies show that simvastatin supresses the production of pro-inflammatory cytokines and exerts immunomodulatory effects (Komolafi et al., 2015) which may contribute to reduction in kidney and heart toxicity. Simvastatin was reported to preserve glomerular filtration rates and reduce proteinuria in patients with renal disease (Fried et al., 2001). Homeostasis of ions, such as Na+, K+, and Ca2+, is essential for living organisms (Yang et al., 2015). In the current study, vanadium did not improve dyslipidemic (data not shown) or oxidative stress effect of high fat diet in HFD-group. Instead, it worsens oxidative stress in heart and liver tissues of HFD-rats through the reduction of non-protein sulfhydryl and elevation of MDA levels in those tissues. Our generated results are in consistent with other study reported by Mahmoud et al. (2011). Another study has shown that metals such as vanadium, copper and iron can produce reactive oxygen species which leads to lipid peroxidation, DNA damage, depletion of sulfhydryl and altered calcium homeostasis (Stohs and Agchi, 1995). The mitochondria, as the main cellular source for ROS production, are an important target for vanadium accumulation (Soares et al., 2007).
The mechanism by which vanadium compounds promote mitochondrial ROS generation may be due to impaired activity of antioxidant enzymes and/or impaired respiration from vanadium interacting with mitochondrial’s inner membrane (Zhao et al., 2010). Also, increased LPO and MDA by vanadium exposure could be explained by vanadium’s ability to generate hydroxyl radicals via Fenton-like reaction (Shi et al., 1993). Contrary to what is hypothesised, the oral ingestion of vanadium chloride does not synergize simvastatin’s protection of heart or kidney tissues. Instead, it worsens the function and tissue integrity of these organs. This was indicated by further increases in serum activities of LDH and creatine kinase and serum concentration of urea, uric acid, creatinine, sodium, potassium and calcium. The elevation of blood urea, uric acid and creatinine indicates damage to the glomerulus and both proximal and distal tubules. Our results agree with the finding of Mahmoud et al. (2011). It has been reported that vanadium is a potentially toxic agent. Both nephro- and cardiotoxicity of vanadium are caused by its inhibition of many enzymes and induction of oxidative stress in heart and kidney (Mahmoud et al., 2011). Evidence has shown that the dissociated vanadium from metal oxide nanoparticles can alter electrolyte homeostasis in tissues via extensive mechanisms such as ion transporters, ion channels, redox reaction, and tubular reabsorption (Marchetti, 2014).
This study demonstrates that vanadium did not show beneficial effects on oxidative stress state and did not protect liver, kidney or heart tissues during the treatment of dyslipidemia with simvastatin in the experimental animals. Dissimilarly, vanadium chloride was found to cause an elevation in oxidative stress in heart and liver tissues and negatively affect kidney and heart functions, which provides evidence to avoid its use during treatment of dyslipidemia with statin. Other long-term study should be conducted in order to confirm these results.
The authors have not declared any conflict of interests.
Authors would like to thank the scientists and technicians at the Animal Research Centre at King Saud University and they are grateful to the Research Centre of the College of Applied Medical Sciences at Taibah University.
REFERENCES
|
Abbasi SH, Mohammadinejad P, Shahmansouri N, Salehiomran A, Akram AB, Zeinoddini A, Forghani S, Akhondzadeh S (2015). Simvastatin versus atorvastatin for improving mild to moderate depression in post-coronary artery bypass graft patients: A double-blind, placebo-controlled, randomized trial. J. Affect. Disord. 183:149-155.
Crossref
|
|
|
|
Albertini R, Moratti R, De Luca G (2002). Oxidation of low-density lipoprotein in atherosclerosis from basic biochemistry to clinical studies. Curr. Mol. Med. 2:579-592.
Crossref
|
|
|
|
Barceloux DG, Barceloux D (1999). Vanadium. J. Toxicol. Clin. Toxicol. 37(2):265-278.
Crossref
|
|
|
|
Beyegue CFN, Ngangoum RMC, Kuate D, Ngondi JL, Oben JE (2012). Effect of Guibourtia tessmannii extracts on blood lipids and oxidative stress markers in triton WR 1339 and high fat diet induced hyperlipidemic rats. Biol. Med 4(1):1-9.
Crossref
|
|
|
|
Birben E, Sahiner UM, Sackesen C, Erzurum S, and Kalayci O (2012). Oxidative stress and antioxidant defense. World Allergy Organ. J. 5:9-19.
Crossref
|
|
|
|
Brichard SM, Henquin JC (1995). The role of vanadium in the management of diabetes. Trends Pharmacol. Sci.16:265-270.
Crossref
|
|
|
|
Camerino GM, Bellis MD, Conte E, Liantonio A, Musaraj K, Cannone M, Fonzino A, Giustino A, Luca AD, Romano R, Camerino C, Laghezza A, Loiodice F, Desaphy JF, Camerino DC, Pierno S (2016). Statin-induced myotoxicity is exacerbated by aging: A biophysical and molecular biology study in rats treated with atorvastatin. Toxicol. Applied Pharmacol. 306:36-46.
Crossref
|
|
|
|
Chakravarthy H, Beli E, Navitskaya S, O'Reilly S, Wang Q, Kady N, Huang C, Grant MB, Busik JV (2016). Imbalances in mobilization and activation of pro-inflammatory and vascular reparative bone marrow-derived cells in diabetic retinopathy. PLoS One 11(1):e0146829.
Crossref
|
|
|
|
Chen F, Ding M, Castranova V, Shi X (2001). Carcinogenic metals and NF-kappa B activation. Mol. Cell Biochem. 222:159-171.
Crossref
|
|
|
|
Fabiny DL, Ertingshausen G (1971). Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clin. Chem. 17(8):696-700.
|
|
|
|
Fossati P, Prencipe L, Berti G (1980). Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4- aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem. 26(2):227-231.
|
|
|
|
Fried LF, Orchard TJ, Kasiske BL (2001). Effect of lipid reduction on the progression of renal disease: a metaanalysis. Kidney Int. 59:260-269.
Crossref
|
|
|
|
Galema-Boers JMK, Roeters van Lennep JE (2015). Dyslipidemia testing: Why, for whom and when. Maturitas 81:442-445.
Crossref
|
|
|
|
Gilbert R, Al-Janabi A, Tomlins-Netzer O, Lightman S (2017). Statin as anti-inflammatory agent: A potential therapeutic role in sight-threatening non-infectious uveitis. Proto Biomed. J. 2(2):33-39.
Crossref
|
|
|
|
Gitelman HJ (1976). An improved automated procedure for the determination of calcium in biological specimens. Anal. Biochem. 18(3):521-531.
Crossref
|
|
|
|
Gummow B (2011). Vanadium: environmental pollution and health effects. In: Nriagu, Jerome O, (ed.) Encyclopedia of Environmental Health. Elsevier, Amsterdam, the Netherlands, pp. 628-636.
Crossref
|
|
|
|
Hansson GK (2005). Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352:1685-1695.
Crossref
|
|
|
|
Heidari SR, Ganjkhanlou M, Zali A, Ghorbani GR, Dehghan-Banadaky M, Hayirli A (2016). Effects of vanadium supplementation on performance and metabolic parameters in periparturient dairy cows. Anim. Feed Sci. Technol. 216:138-145.
Crossref
|
|
|
|
Henry RF (1974). Clinical Chemistry Principles and Technics, 2nd Ed., Harper and Row, Hagerstein, M.D.
|
|
|
|
Jahovic N, Gedik N, Ercan F, Sirvanci S, Yüksel M, Sener G, Alican I (2006). Effects of statins on experimental colitis in normocholesterolemic rats. Scand. J. Gastroenterol. 41(8):954-62.
Crossref
|
|
|
|
Joyce L. Ross MSN (2016). Statins in the Management of Pediatric Dyslipidemia. J. Pediatr. Nurs. 31:723-735.
Crossref
|
|
|
|
Katakami N (2018). Mechanism of development of atherosclerosis and cardiovascular diseases in diabetes millitus. J. Atheroscler. Thromb. 25(1):27-39.
Crossref
|
|
|
|
Katsiki N, Mikhailidis DP, Mantzoros CS (2016). Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabol. Clin. Experiment. 65:1109-1123
Crossref
|
|
|
|
Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W (1999). Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 103(11):1489-1498.
Crossref
|
|
|
|
Komolaf AO, Ally MMTM, Van Tonder JJ, Greeff OBW (2015). The anti-inflammatory properties of simvastatin can benefit statin- naïve rheumatoid arthritis patients with associated risk of cardiovascular diseases. S. Afr. Fam. Pract. 57(1):28-30.
Crossref
|
|
|
|
Larsen S, Stride N, Hey-Mogensen M, Hansen CN, Bang LE, Bundgaard H (2013). Simvastatin effects on skeletal muscle: relation to decreased mitochondrial function and glucose intolerance. J. Am. Coll. Cardiol. 61:44-53.
Crossref
|
|
|
|
Lawlor DA, Smith GD, Ebrahim S (2006). Does the new international diabetes federation definition of the metabolic syndrome predict CHD any more strongly than older definitions? Findings from the British women's heart and health study. Diabetologia 49:41-48.
Crossref
|
|
|
|
Liu F, Xie M, Chen D, Li J, Ding W (2013). Effect of VIVO(dipic-Cl) (H2O)2 on Lipid Metabolism Disorders in the Liver of STZ-Induced Diabetic Rats. J. Diabetes Res. pp. 1-10.
|
|
|
|
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193(1):265-275.
|
|
|
|
Mahmoud KE, Shalahmetova T, Deraz S, Umbayev B (2011). Combined effect of vanadium and nickel on lipid peroxidation and selected parameters of antioxidant system in liver and kidney of male rat. Afri. J. Biotechnol. 10(79):18319-18325.
|
|
|
|
Marchetti C (2014). Interaction of metal ions with neurotransmitter receptors and potential role in neurodiseases. Biometals 27(6):1097-1113.
Crossref
|
|
|
|
Moutzouri E, Tellis CC, Rousouli K, Liberopoulos EN, Milionis HJ, Elisaf MS, Tselepis AD (2012). Effect of simvastatin or its combination with ezetimibe on Toll-like receptor expression and lipopolysaccharide e Induced cytokine production in monocytes of hypercholesterolemic patients. Atherosclerosis 225:381-387.
Crossref
|
|
|
|
Mulhaupt F, Matter CM, Kwak BR, Pelli G, Veillard NR, Burger F, Graber P, Luscher TF, Mach F (2003). Statins (HMG-CoA reductase inhibitors) reduce CD40 expression in human vascular cells. Cardiovasc. Res. 59:755-766.
Crossref
|
|
|
|
Parihar A, Parihar MS, Zenebe WJ, Ghafourifar P (2012). Statins lower calcium-induced oxidative stress in isolated mitochondria. Hum. Exp. Toxicol. 31:355-363.
Crossref
|
|
|
|
Patel M, Kothari C (2016). Critical review of statins: A bio-analytical perspective for therapeutic drug monitoring. Trends in Analyt. Chem. 86:206-221.
Crossref
|
|
|
|
Pessoa JC, Etcheverry S, Gambino D (2015). Vanadium compounds in medicine. Coordinat. Chem. Rev. 301:24-48.
Crossref
|
|
|
|
Ronald H, Katherine M, Sparks, Bernard E (1992). Colorimetric determination of potassium in plasma and serum by reflectance photometry with a dry-chemistry reagent. Clin. Chem. 38:1371-1372.
|
|
|
|
Sedlak J, Lindsay RH (1968). Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 25(1):192-205.
Crossref
|
|
|
|
Shi X, Dalal NS (1993). Vanadate-mediated hydroxyl radical generation from superoxide radicals in the presence of NADH: Harber- Weiss VS. Fenton mechanism. Arch. Biochem. Biophys. 207:336-341.
Crossref
|
|
|
|
Shishehbor MH, Brennan ML, Aviles RJ, Fu X, Pen MS, Sprecher DL, Hazen SL (2003). Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation 108:426-431.
Crossref
|
|
|
|
Soares SS, Gutiérrez-Merino C, Aureliano M (2007). Mitochondria as a target for decavanadate toxicity in Sparus aurata heart. Aquat. Toxicol. 83:1-9.
Crossref
|
|
|
|
Stohs SJ, Agchi D (1995). oxidative mechanisms in the toxicity of metal ions. Free Rad. Biol. Med. 18:321-336.
Crossref
|
|
|
|
Tabacco A, Meiattini F, Moda E, Tarli P (1979). Simplified enzymic/colorimetric serum urea nitrogen determination. Clin. Chem. 25:336-337.
|
|
|
|
Tangvarasittichai S (2015). Oxidative stress, insulin risistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 6(3):456-480.
Crossref
|
|
|
|
Tessari P, Coracina A, Cosma A, Tiengo A (2009). Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutri. Metabol. Cardiovasc. Dis. 19:291-302.
Crossref
|
|
|
|
Tikhonenko M, Lydic TA, Wang Y, Chen W, Opreanu M, Sochacki A, McSorey KM, Renis RL, Kern T, Jump DB, Reid GE, Busik JV (2010). Remodelling of retinal fatty acids in an animal model of diabetes: A decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes 59(1):219-227.
Crossref
|
|
|
|
Utley HG, Bernheim F, Hochstein P (1967) Effect of sulfhydryl reagents on peroxidation in microsomes. Arch. Biochem. Biophys. 118:29-32.
Crossref
|
|
|
|
Yang KC, Kyle JW, Makielski JC, Dudley (2015). Mechanisms of sudden cardiac death: oxidants and metabolism. Circ. Res. 116(12):1937-1955.
Crossref
|
|
|
|
Yuan YV, Kitts DD (2003). Dietary (n-3) fat and cholesterol alter tissue antioxidant enzymes and susceptibility to oxidation in SHR and WKY rats. J. Nutrit. 33(3):679-688.
Crossref
|
|
|
|
Zhao Y, Lihua Y, Liu H, Xia Q, Zhang Y, Yang X, Wang K (2010). Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. J. Inorg. Biochem. 104:371-378.
Crossref
|
|
|
|
Zou Y, Li J, Lu u, Wang J, Ge J, Huang Y, Zhang L, Wang Y (2006). High-fat emulsion-induced rat model of nonalcoholic steatohepatitis. Life Sciences79:1100-1107.
Crossref
|