Malaria is a leading cause of morbidity and mortality in many developing tropical countries, particularly in children and pregnant women. With growing concerns about the development of resistance to current antimalarial drugs, herbal alternatives may provide significant and sustainable treatment options in affected regions. This review aimed at providing an updated overview of available data on Herbal Medicinal Products (HMPs) with reproducible evidence-based antimalarial efficacies. In addition, it identified the antiplasmodial phyto-constituents and discussed the potential of these products to modulate activities of drug metabolizing enzymes and drug transport systems. This is particularly important because the co-morbidity of malaria with other diseases, often prevalent in malaria endemic regions, is marked by a concurrent use of herbal antimalarial products with conventional drugs. An extensive literature search was undertaken and the information obtained were critically analysed. Enormous work has been reported on investigations of the antiplasmodial activity of herbs, and more than 1000 plants have been studied using in vitro and animal malaria infection models. However, only a few of these HMPs have been subjected to randomized clinical trials. Herb-drug interactions are of great concern since phytochemical compounds in HMPs are subject to the same pharmacokinetic processes that determine the fate of synthetic drugs in the human body. Thus, specific effects of these clinically effective antimalarial HMPs on drug metabolizing enzymes were reported with a view to documenting information for optimization of their therapeutic utility. Beneficial and adverse clinically significant interactions were also identified for close therapeutic attention.
Malaria is an infectious disease caused by plasmodium parasites and transmitted by female anopheline mosquitoes. It is one of the severe public health problems which remains a leading cause of morbidity and mortality in many developing countries, where young children and pregnant women are the groups most affected (WHO, 2012). In the malaria transmission regions of the world, the estimated number of malaria cases and mortalities vary between the regions (WHO, 2016). An estimated 212 million cases of malaria occurred worldwide in 2015. Most of the cases were in the African (90%), followed by the South-East Asia (7%) and the Eastern Mediterranean region (2%). Also, in 2015, it was estimated that 429,000 deaths from malaria occurred globally with most of the deaths estimated to have occurred in the African Region (92%), followed by the South-East Asia Region (6%) and the Eastern Mediterranean Region (2%) (WHO, 2016). P. falciparum which causes the most severe malaria is predominantly found in Africa while P. vivax malaria, which also causes life-threatening symptoms, is the most prevalent species in Latin America, South-East Asia, the Eastern Mediterranean and the Western Pacific.
On the other hand, P. malariae and P. ovale are less prevalent, and cause less severe disease in humans (WHO, 2011). Malaria is associated with enormous socio-economic burden. The economic impact of malaria is estimated to cost Africa $12 billion every year (Gallup and Sachs, 2001). This figure factors in the costs of health care, work absenteeism, days lost in education, decreased productivity due to brain damage from cerebral malaria, and loss of investment and tourism. A 2011 ‘Roll Back Malaria’ report found that in sub-Saharan Africa, 72% of companies reported a negative malaria impact, with 39% perceiving these impacts to be serious (Roll back Malaria, 2011). Malaria can strain national economies, impacting some nations' gross domestic product by as much as an estimated 5 to 6% (World Economic Forum, 2006).Malaria is preventable and treatable, but a delay in or ineffective treatment, can result in rapid increase in parasite burden which has a case fatality rate of 10 to 20% even among those receiving treatment (WHO, 2010). Treatment modality depends on the plasmodium species involved as well as the severity of the disease.
There are different classes of antimalarial drugs and the treatment guidelines have been updated over the years from monotherapy with aminoquinolines and other drug combinations to the current artemisinin-based combination therapy (ACT), for the treatment of uncomplicated malaria caused by P. falciparum parasite (WHO, 2015). Antimalarial drugs traditionally have five classes when the mode of action is employed for classification, while there are eight classes when the chemical structures are considered (White, 1996). What could be a new class of antimalarial drugs is hinged on rare approaches adopted by new scientific, thinking as contained in the work of Wang et al. (2016), where research target the heat shock protein 90 (Hsp90), a universal ‘molecular chaperone’ performing vital functions. Both in the parasite and in human cells, which were carried out with huge success and promises. Hsp90 plays a central role in the pathogen's life cycle and survival, as well as its resistance to treatments. This protein, which is also present in human cells form slightly different from that found in the parasite, functions as a 'molecular chaperone', by assisting other proteins. Therefore, one molecular target that should receive more attention in development of antimalarial drugs is the molecular chaperone Hsp90.
The widespread and indiscriminate use of currently available synthetic antimalarial drugs has resulted in development of drug resistance, which now constitutes a threat to the effectiveness of malaria treatment. In fact, resistance development has been reported to result in treatment failures in significant number of cases (Tripathi, 2006). Malaria parasites have consistently been able to develop resistance to each new class of drugs. However, the extent of resistance varies among the different classes of antimalarial drugs in different regions as well as countries. For example, resistance to quinine, the oldest antimalarial drug, was reported first in Brazil and later in Southeast Asia (Pukrittayakamee et al., 1994) while resistance to the antifolates (Pyrimethamine and sulphadoxine) appeared rapidly in the Asia Pacific regions in the late1970s and in South America (Hurwitz et al., 1981). Resistance to these drugs has currently been reported in other malaria transmission regions (Cui et al., 2016). Similarly, in the Cambodia–Thailand border region of Southeast Asia, declining efficacy of the artesunate/mefloquine combination has been noted.
Thus, while there are several documented cases of resistance to older generations of antimalarial drugs in several countries, there are reports of the emergence of parasites resistant to artemisinins in Southeast Asia along with altered sensitivities to artemisinin partner drugs (Cui et al., 2015). Herbal medicines have been used to treat malaria for thousands of years and they are source of some classes of conventional antimalarial drugs including artemisinin and quinine derivatives. Herbal products enjoy increasing popularity as an essential part of the trend, towards complementary and alternative medicine (CAM) practices worldwide. In Africa, up to 80% of the population uses traditional medicine for primary health care (WHO, 2003) and, on average, fifth patients use herbal remedies for malaria in endemic countries (Willcox and Bodeker, 2004). High prevalence use of Herbal Medicinal Products (HMPs) has also been reported in developed countries (Martin et al., 2002; Nordeng et al., 2011; Hunt et al., 2012). The resurgence of public interest in herbal medicines has been attributed to several factors including the various claims on the efficacy or effectiveness of plant medicines and the perception that herbal products are safer because they are natural in origin.
Others have included the dissatisfaction with the results from orthodox pharmaceuticals, the belief that herbal medicines might be effective in the treatment of certain diseases where conventional therapies which have proven to be ineffective, high cost and side effects of most modern drugs (Bandaranayake, 2006; Lynch and Berry,2007; Ekor, 2014). In addition, the increased use of herbal products has been linked with the desire to improve general health, as well as cultural and personal beliefs (Ernst and White, 2000). With the problems of increasing levels of drug resistance, accessibility and affordability of effective synthentic antimalarial drugs in poverty ravaged regions, herbal medicines had become an important and sustainable source of treatment options (Willcox and Bodeker, 2004). Also, medicinal plants can serve as a rich reservoir from which new antimalarial drugs can be developed (Ginsburg and Deharo, 2011). Consequent upon the global increase in the use of HMPs over the last two decades (Shaw et al., 2012), interactions between herbal remedies and conventional drugs have been receiving increased attention (Izzo, 2005; Williamson, 2005). The need for evaluation of herb-drug interactions has been reinforced by reports from various studies which indicate that concomitant intake of herbal and conventional prescription drugs can vary from as low as 5% to as much as 40% in various patient groups and in different countries (Kaufman et al., 2002; Singh and Levine, 2007; Nordeng et al., 2011; Zhang et al., 2011; Dju et al., 2013).
The high incidence of co-morbidity of malaria with other infections and non-communicable diseases (Gwer et al., 2007; Etiaba et al., 2015) have resulted in marked prevalence of concurrent use of herbal or synthetic antimalarial products and conventional drugs. We have demonstrated in several of our studies that concurrent administrations of synthetic antimalarial drugs with other conventional drugs or herbal products have, in some cases, been associated with adverse effects (Soyinka et al., 2013; Igbinoba et al., 2015A, 2015B, 2016). Herbal medicines are mixtures of phytochemicals that are subject to the same pharmacokinetic processes, including xenobiotics metabolism and transport systems that determine the fate of synthetic drugs in the human body (Hermann and von Richter, 2012; Izzo, 2012). Hence, with such combinations of compounds in herbs, the possibility of herb-drug interactions is theoretically evidently higher compared to drug-drug interaction, when the herb is co-administered with conventional drugs which usually contain single chemical entities.
A wide variety of plants belonging to several families have been identified through ethnobotanical and ethno-pharmacological studies as antimalarial medicinal plants (Bahekar and Kale, 2013). A study reported that 1277 plant species from 160 families from different parts of the world have been used to treat malaria. However, only a few of these herbs have undergone clinical trials (Willcox and Bodeker, 2004; Willcox, 2011). A recent systematic review documented 752 medicinal plants belonging to 254 genera that are in traditional use for malaria treatment, and reported that about 80% of the plants experimented were reported to be inactive (Lemma et al., 2017). This article was aimed at providing an updated overview of the data regarding herbal products with documented and reproducible evidence-based antimalarial efficacies. The report also identified the antiplasmodial phytochemical constituents of the HMPs as well as reviewed information on the potentials of these HMPs to modulate the activities of drug metabolizing enzymes and drug transport systems. Beneficial and adverse clinically significant interactions are identified as close therapeutic attention.
Herbal products with clinical evidence of antimalarial efficacy
Herbal medicinal products used by communities provide a sort of an anecdotal evidence of their efficacy by the community and is further strengthened if the same active ingredient has been documented to be used by several communities (Wells, 2011). Herbal preparations or remedies have been used by generations for the treatment of fevers including malaria. There are numerous evidences based on laboratory research (In vitro or animals) supporting their traditional use as having antimalarial activities but with little or no clinical evidence or well conducted clinical research evaluating the effectiveness of most of the medicinal plants (Willcox, 1999).This review is focused on identifying and updating information on herbal medicinal products (HMPs) that have undergone clinical trial studies and have been found to possess good antiplasmodial efficacy when used as single herbal formulation in the treatment of malaria infections in various countries.
Cryptolepis sanguinolenta
Cryptolepis sanguinolenta (Lindl.) Schlechter (Apocynaceae) root and root bark decoction has been used by traditional medical practitioners to treat a number of diseases including malaria (Willcox et al., 2011A; Osafo et al., 2017). Preliminary in vitro and in vivo investigations of aqueous extract of C. sanguinolenta root as an anti-malarial indicated the antiplasmodial activity of the extract against falciparum malaria (Wright et al., 1996; Cimanga et al., 1997). Interestingly, Paulo et al. 1995) identified that the root extracts demonstrated more activity against Plasmodium falciparum than the leaf extracts. The active antiplasmodial components found in the root are known to be the indoquinoline alkaloids, which have been reported to have both in vitro and in vivo activity against P. falciparum, including chloroquine-resistant strains (Kirby et al., 1995).
A major indoloquinoline alkaloid in C. sanguinolenta root, cryptolepine, administered orally to Plasmodium berghei-infected mice in doses of 50 mg/kg/day for four days reduced parasitaemia by 80% though the mice were not cured of malaria (Wright et al., 1996). Bugyei et al. (2010) investigated the clinical efficacy of the tea-bag formulation (PHYTO-LARIA®) of C. sanguinolenta root powder for the treatment of acute uncomplicated falciparum malaria in a Ghanaian population. After a five-day treatment period with one 2.5 g of the root powder tea-bag three times administered daily to 44 patients with acute uncomplicated malaria, over half of the patients were cleared of their P. falciparum parasitaemia within 72 h. It had an overall cure rate 93.5 % by day 7, suggesting its high efficacy in the treatment of acute uncomplicated malaria.
The study by Bugyei et al. (2010) appears to be the only antimalarial clinical trial report of C. sanguinolenta when used as a single herb formulation. A post treatment rise in serum alkaline phosphatase was observed in malaria patients, suggesting a need for close observation on repeated use of this formulation of C. sanguinolenta (Bugyei et al., 2010). More recently, a combination of cryptolepine with artemisinin derivatives were investigated in a rodent model. The combination was found to be safe in addition to, showing synergistic anti-malarial activity in vivo and in vitro (Forkuo et al., 2016). Since more clinical trials are conducted on the antimalarial efficacy and safety of this herb, it is possible that more evidences will accumulate, supporting the selection of cryptolepine as a prospective lead compound for development of a new anti-malarial drug.
Cochlospermum planchonii
The leaves and rhizomes of two Cochlospermum species (C. planchonii and Cochlospermum tinctorium) have been acclaimed to be commonly used by traditional healers to treat malaria in Burkina Faso and other West African countries (Benoit-Vical et al., 2003). They have also been extensively tested for their in vitro and in vivo antimalarial activity (Benoit-Vical et al., 1999; Benoit-Vical et al., 2001; Yerbanga et al., 2012; Dakuyo et al., 2015). Lamien-Meda et al. (2015) observed moderate antiplasmodial activity with all tested extract of C. planchonii. The efficacy of decoction of C. planchonii root (named N'Dribala) in the treatment of patients with uncomplicated P. falciparum infection in Banfora and Burkina Faso was compared to that of patients who received standard regimen of chloroquine (Benoit-Vical et al., 2003). In this study, a decoction of C. Planchonii root was prepared by boiling 50 g dried root powder in 1500 mL water for 10 min. Upon cooling, the decoction was administered at a dose of 200 mL thrice daily for 5 days to 46 patients while 21 patients received standard chloroquine regimen (10 mg/kg first two days, and 5 mg/kg for remaining three days). After day five of treatment, 52% of those who received the decoction were cured with no detectable parasitaemia and more than 90% of patients were asymptomatic (Benoit-Vical et al., 2003). Side effects noted during the study included headache, anorexia, abdominal pain, vomiting, arthralgia-myalgia (Benoit-Vical et al., 2003) some of which may be connected with the malaria infection in such individuals. More clinical studies are required to evaluate and validate reproducibility of the efficacy of this herbal product, which might result in their being considered as alternative option for treatment of non-severe malaria.
Argemone mexicana
Argemone mexicana is a weed that has a long history of use in traditional medicine for treating a variety of diseases and particularly useful as an antimalarial in several African countries, including Benin, Mali and Sudan (Willcox et al., 2007; Graz et al., 2015; Haidara et al., 2016). It is considered as one of the most effective traditional medicines for the treatment of uncomplicated falciparum malaria in Mali (Simoes-Pires et al., 2014). A number of studies have confirmed its in vitro efficacy against P. falciparum (Daillo et al., 2006; Bapna et al., 2015). The first clinical study conducted to determine A. mexicana’s safety and antimalarial efficacy in human patients was focused on young children (most vulnerable group) in Mali (Willcox et al., 2007). The study was a prospective, dose-escalating, quasi-experimental clinical trial conducted in collaboration with a traditional healer, who formulated the herb. A decoction of A. mexicana (called “AM” in Mali) was administered to 80 patients with uncomplicated malaria in three dosing regimens: once daily for 3 days (Group A; n = 23); twice daily for 7 days (Group B; n = 40); and four times daily for the first 4 days followed by twice daily for 3 days (Group C; n = 17). The clinical response at Day 14 for Groups A, B and C were 35, 73 and 65%, respectively.
Though a dose response correlation was noted, very few patients had complete parasite clearance at day 14 (Willcox et al., 2007). In a latter study in humans, 301 patients with presumed uncomplicated malaria were randomly assigned to receive AM (a decoction of A. Mexicana) or artesunate-amodiaquine (artemisinin combination therapy (ACT)) as first-line treatment. The proportion of patients with parasitaemia at day 28 was 63 to 76% in the AM group, and 21 to 49% in the ACT group. Gametocytes were present at day 28 in 13% of the AM group compared with 3% of the ACT group (Graz et al., 2010). It was apparent that the herbal product did not produce total parasite clearance in the majority of patients (Willcox et al., 2007; Graz et al., 2010). No major adverse effects have been associated with the use of A. Mexicana in humans. Side effects that have been reported include vomiting, abdominal pain, nausea, pruritus, appetite loss and dizziness (Willcox, 1999). These findings leave no doubt that better conducted clinical research is needed in future to establish and confirm the dosing regimen for the optimal antimalarial efficacy of A. Mexicana.
Vernonia amygdalina
Due to ready availability, accessibility, and affordability of the leaves of Vernonia amygdalina in many remote areas in Africa that do not have ready access to modern medicines, it has gained wide use in treating malaria. Examples of such places include regions in Kenya, Uganda, Ethiopia, Nigeria, Ghana etc (Asase et al., 2005; Odugbemi et al., 2006; Tabuti, 2008; Idowu et al., 2010; Araya et al., 2015; Mukungu et al., 2016). Results from the in vitro and in vivo studies have indicated that the ethanolic extract of the plant leaf has significant activity against P. berghei (Abay et al., 2015; Omoregie and Pal, 2016). Solvents (organic) extracts of the leaves and roots also showed strong activity against P. falciparum in vitro (Zofou et al., 2011). A clinical study examined the efficacy and safety of an infusion of fresh V. amygdalina leaves for the treatment of uncomplicated malaria in patients aged 12 years and over.
Each of the 31 patients were given 1 L of freshly made infusion (containing 25 g of freshly chopped V. amgydalina leaves per litre of boiled water) daily. It was taken as 250 mL, 4 times daily for 7 days. Adequate clinical response was observed at day 14 in 67% cases, complete parasite clearance in only 32% of those with adequate clinical response while recrudescence occurred in 71% (Challand and Willcox, 2009). Side effects such as nocturia, insomnia, cough, unpleasant test, diarrhea, abdominal pain, and irregular heart beat which may have cause other than the medicinal infusion have been reported in a study (Challand and Willcox, 2009). The investigators however suggested that further studies are needed to determine whether the efficacy can be improved by increasing the dose, changing the preparation, or adding other antimalarial plants.
Nauclea pobeguinii
Nauclea pobeguinii along with other Nauclea species are used as ethnomedicine throughout sub-Saharan Africa and several populations, especially in remote areas, consider these species as a major source of remedy for malaria (Diarra et al., 2015; Haudecoeur et al., 2017). Also, N. pobeguinii plant part is used as an aqueous decoction for the treatment of uncomplicated malaria in traditional medicine of several Central African countries (Mesia et al., 2012A). The aqueous and 80% ethanolic extract from N. pobeguinii stem bark, and its isolated constituents were inactive or only moderately active in vitro against P. falciparum (chloroquine-sensitive Ghana-strain). However, in an in vivo study, 300 mg/kg daily oral dosing of 80% ethanolic extract (containing 5.6% strictosamide, putative active constituent), resulted in 86% reduction of parasitaemia in 4-day P. berghei mouse model, and 75% reduction in P. yoelii N67 model (Mesia et al., 2010). Mesai et al. (2011) also reported acceptable safety and tolerability of 80% ethanolic quantified extract from N. pobeguinii stem containing 5.6% strictosamide in 15 healthy male volunteers.
Subsequently, the same team of researchers conducted phase II clinical trial (open cohort study) to assess the efficacy of the same 80% ethanolic quantified extract from N. pobeguinii stem (containing 5.6% strictosamide), in eleven small group of adult patients diagnosed with uncomplicated P. falciparum malaria. In the clinical trial, N. pobeguinii stem, denoted as PR 259 CT1, was administered at a dosage regimen of two 500 mg capsules taken thrice daily for three days, followed by outpatient treatment of one 500 mg capsule thrice daily for subsequent four days. This regimen was proven to be effective as, 11 patients enrolled in the study were completely cleared of malaria parasitaemia and fever as early as day 3 and had sustained parasite clearance except for one patient, who experienced a recurrence of parasitaemia at days 7 until 14. Also, no malaria symptom was observed on day 14 in all subjects (Mesia et al., 2012A). A follow up study from same group of researchers (Mesai et al., 2012B) called Phase IIB study, was a single blind prospective trial in 65 patients proven P. falciparum malaria in order to evaluate the effectiveness and safety of this herbal drug (coded PR 259 CT1) using artesunate-amodiaquine combination (Coarsucam®) as a positive control.
The same treatment regimen for herb was followed as in the earlier reported study (Mesai et al., 2012A). The positive control group received two tablets containing 100 mg artesunate and 270 mg amodiaquine (fixed-dose) once daily for three consecutive days. Antimalarial responses were evaluated according to the WHO (2003) guideline for 14-day test, which showed a significantly decreased parasitaemia in patients treated with PR 259 CT1 and artesunate-amodiaquine with adequate clinical parasitological responses (APCR) at day 14 of 87.9 and 96.9%, respectively. N. pobeguinii extract was however found to be better tolerated. Quantified extract of N. pobeguinii stem back has been reported to be well tolerated with only mild and self-resolving adverse effects including fatigue and headache (Mesia et al., 2012A). These findings indicate that N. pobeguinii extract can be considered as a promising herbal antimalarial medicinal product for larger scale, use for the treatment of uncomplicated falciparum malaria (Mesia et al., 2012B).
Punica granatum
Punica granatum (Pomegranate) is a popular recognized antimalarial plant since ancient times within the eastern sea border of India where malaria is endemic. Pomegranate fruit is known to have innumerable health benefits and its seeds and peels are intensively used in traditional medicine as a natural therapy (Rahmani et al., 2017). Sun-dried rind of the immature fruit of P. granatum is used as herbal formulation (OMARIA) in Orissa, India, for the treatment and prophylaxis of malaria (Dell'Agli et al., 2009; Bhattacharya, 2011). In vitro studies have established the antiplasmodial activity of P. granatum extract and some of its isolated constituents, gallagic acid and punicalagins, also exhibited antiplasmodial activity against P. falciparum in vitro (Reddy et al., 2007). Another study which assessed the antiplasmodial activity of the methanolic extract, and a tannin enriched fraction of the antimalarial plant observed that P. granatum methanolic extract inhibited parasite growth of chloroquine-susceptible (D10) and -resistant (W2) strains of P. falciparum. However, curative efficacy estimation of the P. granatum extracts indicated that its methanolic extract and the fraction enriched with tannins did not show any curative effect in the rodent malaria model (Dell'Agli et al., 2009).
Recent study used mice treated with pomegranate to evaluate the protective role of pomegranate peel extract against Plasmodium chabaudi-induced spleen tissue damage and results revealed a significantly reduced parasitaemia compared to untreated control (Mubaraki et al., 2016). Omaria, a popular capsule formulation consisting of 700 mg of dried and powdered fruits of P. granatum was used in a clinical trial to determine the malaria therapeutic and prophylactic dose of the product. Therapeutic dose was assessed by administration of one capsule thrice daily for 3 to 4 days and a total of 531 cases were treated. In all cases, 100% parasite clearance was recorded within 72 h of Omaria administration. For prophylactic dose, two sets of doses were employed. First regimen entailed one capsule per week for 4 weeks with a one-month gap followed by administration of one capsule per month for the subsequent 4 months.
Second regimen in another set of subjects received 1 capsule/day for two consecutive days per week within a period of 2 months. Interestingly, of 236 individuals recruited for the prophylactic dose determination, only 10 had clinical symptoms of malaria within the study year (Bhattacharya, 2011). Omaria containing 500 mg/cap of P. granatum powder and administered 1-capsule thrice daily at an approximate interval of 8 h, for 3 consecutive days, which effectively cured malaria and seems to be effective against all stages of malarial parasite (Bhattacharya et al., 2013). Omaria was observed to be physiologically compartible and appears not to have clearly documented side effects (Bhattacharya, 2011). These findings are stronger evidence that support P. granatum extract as a potent antimalarial product that may warrant further studies to optimize.
Other single herbs with antimalarial clinical trials
Clinical trial results of some antimalarial herbs used in Ayurvedic medicine, for instance Nyctanthesarbor-tristis, Swertia Chirata Powder, Caesalpenia crista and Gomphostema nevium, have been inconclusive as randomized clinical trials or more studies will be needed to confirm their antimalarial efficacy. 120 patients with malaria treated with a paste of 5 fresh leaves of N. tristis, thrice a day for between 7 to 10 days experienced relief from symptoms with complete parasitic clearance in 76.7% within 7 days (Karnik et al., 2008). The air-dried powder of S. chirata (formulated into tablet containing 500 mg) administered to 18 patients with cases of vivax malaria at a dose of 50 mg/kg/day in two divided doses for 5 days, resulted in parasite clearance within 6 days in 66%, while 33% had a significant reduction in parasite load (Panda et al., 2004).
G. nevium is a plant whose leaves are being used traditionally by ethnic tribes of Assam to cure malaria. The dried and powdered leaves of the trial drug G. nevium was given in the dose of 6 g with one cup of water, once daily, after meals for a period of 3 days to 100 patients with uncomplicated cases of malaria infection caused by P. vivax. At day four, about 52% of those who completed the study tested negative to malaria by RDT diagnosis (Das and Sarma, 2017). More studies are, however, required to improve on the clinical efficacy of these herbal products through optimization of their dosage regimen.
Multiherbal antimalaria preparation
“Malarial-5” is the name given to a multiherbal antimalarial preparation consisting of Cassia occidentalis L. (Caesalpinaceae) leaves decoction (62%), Lippia chevalieri (Verbenaceae) leaves decoction (32%) and Spilanthes oleraceae L. (Compositae) flowerheads decoction (6%). It was developed in Mali as an important antimalarial phytomedicine for clinical use in the country. There are evidences that each of the component herbs has antiplasmodial activity (Willcox, 2011). In preclinical studies, “Malarial-5” demonstrated mild in vitro activity (IC50 = 470 to 600 μg/mL) against P. falciparum, while in mice (infected with P. berghei) the herbal product given orally at 200 mg/kg daily for five days also showed antimalarial effect in vivo (Gasquet et al., 1993). Three clinical studies evaluated the safety and efficacy of the product. The first randomized controlled trial compared the efficacy of the herbal product to chloroquine (Koita, 1990) in 53 patients of which 36 were randomised to “Malarial-5” and 17 to chloroquine. 75% of the “Malarial-5” group, and 59% of the chloroquine group were successfully followed up to 21 days and parasite clearance was better in the chloroquine group, though there was still a marked reduction in parasitaemia in the “Malarial-5” group. Fever clearance between the two groups was comparable (Koita, 1990).
In a comparable study (except for inclusion criteria of higher fever and parasitaemia) conducted after the first report by Koita (1990), reduction in parasitaemia was slower for patients on “Malarial-5” which may be explained by the higher initial parasitaemia in this later study (Willcox, 2011). It was felt that the amount of S. oleraceae (4%) present in the formulation of “Malarial-5” in this second study was insufficient for a truly effective schizonticidal activity, and was therefore increased to 6% in the third trial which was an observational cohort study of 30 patients with uncomplicated malaria (Doumbia, 1997). Parasitaemia declined to about 11% of the initial level and symptoms improved. The side effects of “Malarial-5” were minimal and the product was better tolerated than chloroquine (Koita, 1990). From the foregoing, it is evident that plants used in traditional medicine should not only be considered as potential useful sources of new lead compounds, but should be researched with the aim of developing safe and efficacious herbal medicinal products that may lead to valuable therapeutic agents either in its crude form or isolates (Xu et al., 2011).
Modulation of drug metabolizing enzymes and p-glycoprotein by herbal antimalarial products
The prevalent use of HMPs, and the significant incidence of concurrent intake of herbal medicine and conventional drugs, raises concern for herb-drug interactions which are mainly mediated through drug metabolizing enzymes and drug transporters (Zhou et al., 2003; Bardia et al., 2007; de Lima et al., 2012; Hermann and von Richter, 2012). Cytochrome P450s (CYP450s), a superfamily of haemoproteins responsible for the phase I metabolism of various xenobiotics and some endogenous substances (Cho and Yoon, 2015), are known to metabolize about 80 to 90% of clinically used drugs (Guengerich, 2006; Lynch and Price, 2007). CYP450 enzymes belonging to families 1, 2, and 3 are principally involved in xenobiotic metabolism, and the isoforms found to be very important in human drug metabolism include CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 (Guengerich, 2006). The most significant CYP isoenzymes in terms of quantity are CYP2D6 and CYP3A4. The CYP450 subfamily, CYP3A, is the most abundant CYP in the human liver and small intestine (Shimada et al., 1994) and the subfamily is involved in the metabolism of approximately 50% of clinically used drugs (Seripa et al., 2010).
On the other hand, P-glycoprotein (P-gp), also known as multidrug resistance protein, is an ATP-dependent efflux pump with broad substrate specificity which plays an important role in the intestinal absorption, distribution to the central nervous system, and biliary/urinary excretion of drugs (Zhou et al., 2004; Cho and Yoon, 2015). Majority of herb-drug interactions are as result of the herbal modulation of CYP450 and/or P-gp resulting in their inhibition or induction which could lead to therapeutic failure. However, such interactions can also have beneficial impact when applied as a useful strategy to improve the efficacy of drugs that are substrates of CYP450 and/or P-gp (Marchetti et al., 2007; Oga et al., 2016). This review deliberately de-emphasized herbal preparations that were developed centuries ago, nd from which their active antiplasmodial compounds have already been isolated and are now in use as conventional antimalarial drugs. These include Cinchona bark and Artemisia annua preparations which had long undergone several clinical trials (Honigsbaum and Willcox, 2004; Willcox, 2009).
The best-known alkaloids out of 30 that have been reported in C. bark are quinine, its D-isomer quinidine, and cinchonine it’s L-isomer, cinchonidine. They have been reported to show different degrees of activities against plasmodium parasites (Karle et al., 1999). A. annua contains a wide variety of compounds such as monoterpenes, sesquiterpenes, steroids, flavonoids, and coumarins (Bhakuni et al., 2002). Some of these compounds are reported to have some antimalarial activity, but the most active is the sesquiterpene lactone artemisinin, from which other derivatives are produced and used for Artemisinin-based combination Therapy (ACT). Obtaining information on the phyto-constituents of herbal extracts is an important step in elucidating the possible mechanisms for herb-drug interaction (HDI) potential. Some classes of compounds are known to have high potential to modulate activities of drug metabolizing enzymes. For example, some flavonoids and polyphenols are known to inhibit CYP450 (Kimura et al., 2010; Vijayakumar et al., 2014). Thus, extracts containing such compounds will be deemed to have a high potential for HDI.
Modulation by Cryptolepis sanguinolenta
Phytochemical analyses of various fractions of C. sanguinolenta have shown the presence of a variety of secondary metabolites which include alkaloids, tannins and flavones. Extracts from the root bark of various Cryptolepis species especially C. sanguinolenta have yielded several alkaloids which have been demonstrated to possess strong antiplasmodial activity (Barku et al., 2012). The major alkaloid has been identified as Cryptolepine, an indole-quinoline, and other minor alkaloids with significant antiplasmodial activities which include11-hydroxycryptolepine, isocryptolepine and neocryptolepine (Ablordeppey et al., 1990; Cimanga et al., 1997). The chemical structures of these compounds are presented in Figure 1. Among natural products, indole alkaloids represent a prominent class of compounds that contribute considerably to the therapeutic options in malaria treatment (Frederich et al., 2008). Very few studies have been carried out on the modulation of drug metabolizing enzyme activity by the extracts of C. sanguinolenta. In a study to determine the effect of extracts of C. sanguinolenta on the activities of CYP2E1, CYP2B1 and CYP1A in rats, the herbal product was found to induce the activity of only CYP1A isozyme (Ocloo et al., 2012). There are no reports on whether or not C. sanguinolenta extract can modulate the activities of other major human CYP450 isoforms such as CYP2C9, CYP2C19, CYP2D6, and CYP3A4.
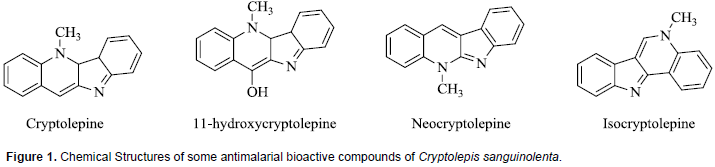
Co-administration of this herbal product with artesunate was, however, associated with an increase in the elimination rate constant and clearance, and decrease in bioavailability of dihydroartemisinin, the most potent metabolite of artesunate (Sakyiamah et al., 2011). Artesunate is known to be primarily metabolized via esterase-mediated hydrolysis and CYP2A6 enzyme to the active metabolite dihydroartemisinin (Li et al., 2003), which is subsequently metabolized via uridine diphosphate glucuronosyltransferases as well as CYP 1A8/9 and 2B7 (Ilett et al., 2002). Thus, it can be inferred from this interaction study with artesunate that C. sanguinolenta induced the metabolism of dihydroartemisinin which resulted in decreased plasma levels of this artesunate metabolite. It was suggested that the effect of this herbal extract could cause sub-therapeutic blood levels of dihydroartemisinin or artesunate. This has been demonstrated in a study which showed that concurrent administration of aqueous extract of C. anguinolenta with artesunate resulted in reduction of the effectiveness of artesunate (Ocloo et al., 2014).
The effect of aqueous extract of C. sanguinolenta on the pharmacokinetics of another conventional antimalarial drug, chloroquine, was studied in male Sprague-Dawley rats. Results of the study indicated that concurrent use of C. sanguinolenta resulted in significant reduction of plasma concentrations of chloroquine (Sakyiamah et al., 2012). This interaction may likely be due to the induction of CYP1A and other isozymes since chloroquine metabolism is mediated by CYP1A1/2, 2C8, 2C19, 2D6 and 3A4/5 (Kyoung-Ah et al., 2003). Cryptolepine, the major antimalarial phytochemical in extract of C. sanguinolenta has been reported to be a substrate for aldehyde oxidase which oxidizes the compound to cryptolepine-11-one (Godfrey et al., 2012). This implies that there is a possibility of pharmacokinetic interaction between C. sanguinolenta extract and drugs that are substrates of aldehyde oxidase. In vitro pharmacokinetic assays of cryptolepine in rat and human plasma demonstrated that the compound has high passive permeability and low human P-glycoprotein efflux potential (Donkor, 2016). Therefore, C. sanguinolenta extract is not likely to interact with concurrently administered prescription drug via modulation of P-gp activity.
Modulation by Cochlospermum planchonii
The leaves and roots (rhizomes) of both Cochlospermum species; C. planchonii and C tinctorium, are very rich sources of phytochemicals and minerals which justify the various therapeutic uses attributed to them (Anaga and Oparah, 2009; Lamien-Meda et al., 2015). These phytochemicals include saponins, phenolics, alkaloids, steroids, flavonoids, phlobatannins, triterpenes and anthraquinones (Nafiu et al., 2011; Olugbemiro et al., 2013). In many West African countries, the decoction of the rhizomes of both Cochlospermum species is indifferently used for malaria treatment (Benoit-Vical et al., 2003). Compared to the closely related species (C. tinctorium) only few scientific studies have been done on the phytochemical composition of C. planchonii. In both species, Triacylbenzenes A, B, C, D called cochlospermines along with carotenoids (cochloxanthine and dihydrocochloxanthine), as well as tetradecanone and 1-hydroxy-3-tetradecan-3-one have been isolated as the antimalarial bioactive compounds (Addae-Mensah et al., 1985; Diallo and Vanhaelen, 1987; Benoit-Vical et al., 2001; Ballin et al., 2002; Nergard et al., 2005; Tvete Inngjerdingen et al., 2013). The rhizomes of C. tinctorium have also been reported to contain additional antimalarial compounds, alphitolic acid and 3-O-E-p-coumaroylalphitolic acid (Ballin et al., 2002). The chemical structures of some of these compounds are presented in Figure 2.
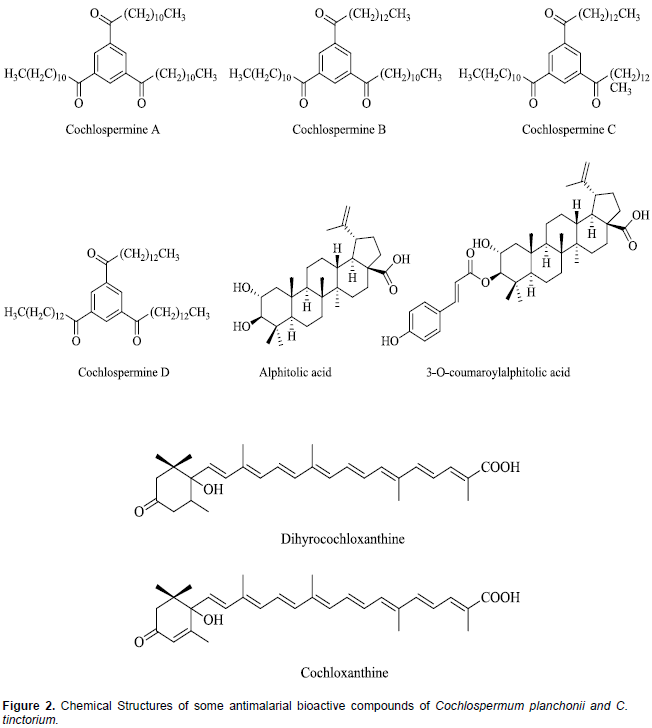
There is a major variability of carotenoid content and antiplasmodial activity of both C. planchonii and C. tinctorium (Lamien-Meda et al., 2015). An extensive search of the literature revealed very limited studies done on the ability of extracts C. planchonii or C. tinctorium to modulate CYP450 activities. Using microsomes from rat liver, it was demonstrated that the aqueous extract of C. planchonii rhizome contains an effective inhibitor of two CYP450 monoxygenase enzymes, aniline hydroxylase and aminopyrine-N-demethylase (Aliyu et al., 1995a). The same authors further identified the inhibitor in the extract as zinc formate, and reported that C. planchonii rhizomes contain unusually high levels of manganese and zinc. Also, synthetic and plant-derived zinc formate was equally effective as inhibitors of CYP450 enzymes (Aliyu et al., 1995b). This raises the theoretical possibility of pharmacokinetic interaction between the herbal products of these plants and concomitantly administered conventional drugs. However, no recent study has been undertaken to authenticate these findings and identify the particular CYP450 isozymes inhibited by the plant extract. Similarly, the pharmacokinetic interactions between the herbal products of these plants and other drugs that may be concurrently administered have not been investigated.
Modulation by Argemone mexicana
Chemical investigations of A. mexicana have revealed the presence of a wide variety of compounds including alkaloids, amino acids, phenolics and fatty acids, and this may explain why plant is extensively used in traditional medicine for the treatment of numerous diseases (Sharanappa and Vidyasagar, 2014). Antiplasmodial bioactive compounds isolated from extracts of A. mexicana which are all alkaloids include berberine, protopine, allocryptopine, and sanguinarine. Whereas all are active in vitro, the absorption of berberine is poor in animal models (Willcox et al., 2011B; Simoes-Pires et al., 2014). The chemical structures of some of these compounds are shown in Figure 3. It is pertinent to note that the compound sanguinarine is considered toxic, the primary cause of outbreaks of human poisoning known as ‘epidemic dropsy syndrome’ (Singh et al., 2000).
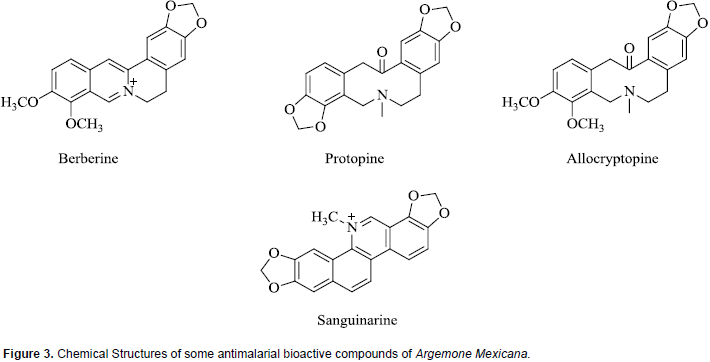
This herbal product and some of the identified pure bioactive compounds have been shown to modulate CYP450 activities. An in vivo study on effect of argemone oil on hepatic CYP450 in albino rats showed that, oil caused a significant inhibition with differential effects on CYP450 isozymes exemplified by inhibition of activities of aminopyrine-N-demethylase, aryl hydrocarbon hydroxylase and ethoxycoumarin-O-deethylase (Upreti et al., 1991). Using human hepatic microsomes and recombinant CYP1A1 or CYP1A2, it was reported that sanguinarine, a bioactive antiplasmodial compound, produced inhibition of these isozymes and also inhibited activity of NADPH: CYP450 reductase, an enzyme required for CYP450 activity (Vrba et al., 2004). Another study which investigated the interaction of sanguinarine with rat hepatic CYP450 indicated that, the alkaloid has higher affinity and binding towards the CYP1A family (Reddy and Das, 2008).
Catalytic and immunochemical activities of CYP450 isoforms were investigated in rats pre-treated with different CYP inducers administered with sanguinarine. The results suggested that these compound inhibit the activities of CYP1A1, 1A2, 2D1, 2E1, 3A1, and Phase II enzymes especially glutathione-S-transferase (Eruvaram et al., 2009). Other antiplasmodial alkaloids herbal product, allocryptopine, was also shown to exhibit time-dependent inhibition of CYP3A4, CYP2C9, and CYP2C19, while protopine and allocryptopine showed a reversible inhibition of CYP2D6 enzyme. The expression of P-gp was unaffected by these compounds (Manda et al., 2016). Contrary to the observations with pure bioactive phytochemicals, a study by Fasinu et al. (2016) revealed that, A. Mexicana extracts showed more than 2-fold induction of the pregnane-X receptor (PXR) activity. Pregnane-X receptor upregulates the expression of some drug metabolizing enzymes. Further studies are, no doubt, required to clarify these discordant observations so as to have a proper guidance on the safe use of this HMP, concurrently with other drugs.
Some constituents of A Mexicana such as berberine have been shown to be a substrate of P-gp (Pan et al., 2002; Preeti et al., 2015). Theoretically, a P-gp substrate can compete with another substrate resulting in herb- or drug-drug interaction. For example, in an in vitro investigation of active moieties found in several medicinal herbs for their P-gp stimulation/inhibition profiles, berberine produced an inhibition at high concentration, suggesting a possible interaction of this phytoconstituent at the level of P-gp (Najar et al., 2010). Such interaction was demonstrated in a study where the in vivo effects of berberine on the pharmacokinetics of carbamazepine (a substrate of CYP3A), digoxin (a substrate of P-gp) and cyclosporine A (a dual substrate of CYP3A and P-gp) were evaluated in rats. Berberine produced a dose-dependent increased bioavailability of digoxin and cyclosporine A by inhibition of intestinal P-gp. No significant changes in CYP3A activity by berberine were observed (Qiu et al., 2009).
Similarly, an in vivo study on pharmacokinetic interactions between ketoconazole (an inhibitor of CYP3A and P-gp) and berberine showed that after oral co-administration of both compounds, the area under the curve (AUC) and the maximum concentration (Cmax) for both ketoconazole and berberine markedly increased (Zhou et al., 2012). It is evident from all these studies with the pure compounds from A. Mexicana extracts that, the herbal product has the potential for pharmacokinetic interaction with concurrently administered conventional drugs via modulation of CYP450 and P-gp activities. But, it should be noted that the concentrations of the bioactive compounds in the herbal extract may not be high enough to produce the effects observed with the pure compounds. Hence, practically relevant information must be generated by conducting the studies using the herbal extract. However, very limited studies have been reported evaluating the effect of this herbal product itself on the pharmacokinetic disposition of co-administered drugs.
Modulation by Vernonia amygdalina
A wide array of phytochemicals has been shown to be present in V. amygdalina and these include stigmastane-type saponins, steroidal saponins, sesquiterpene lactones, flavonoids, alkaloids, cardiac glycosides, anthraquinones, and glycosides (Tona et al., 2004; Erasto et al., 2006; Luo et al., 2011; Ijeh and Ejike, 2011; Odeh and Usman, 2014). The broad variety of compounds may explain the basis for the numerous documented medicinal uses of this plant (Audu et al., 2012; Egharevba et al., 2014). Sesquiterpene lactones such as vernolepin, vernolin, vernolide, venodalol, vernodalin and hydroxyvernodalin, as well as a steroid glycoside, Vernonioside B1, isolated from V. amygdalina leaves have been reported to be responsible for the antiplasmodial activities of the plant (Ijeh and Ejike, 2011; Kraft et al., 2003). The chemical structures of these antiplasmodial bioactive compounds are presented in Figure 4.
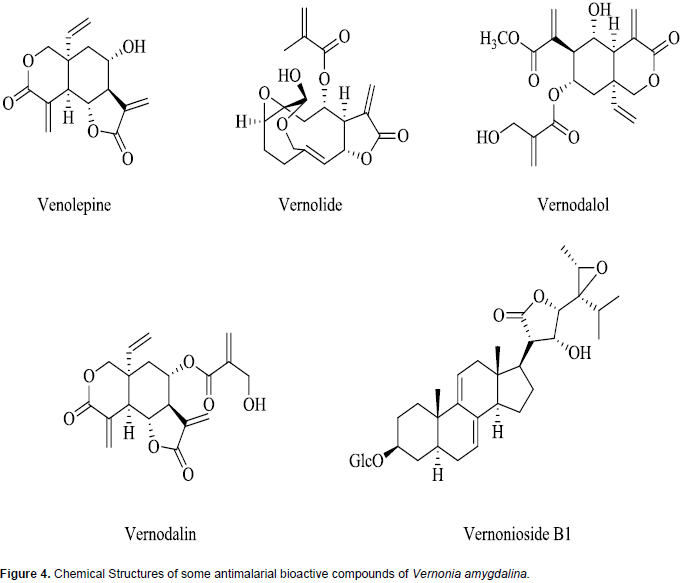
To investigate the effect of V. amygdalina extract on drug metabolizing enzymes, semi-quantitative measurements of CYPP450 and microsomal epoxide hydrolase gene expression levels were obtained in response to treatment of MCF-7 breast cancer cells with the herbal extract. The results of Western blot and RT-PCR analyses showed a dose and time-dependent induction of phase 1 (CYP3A4) and phase 2 (microsomal epoxide hydrolase) enzyme gene expression (Howard et al., 2003). The findings from this study suggest that the herbal extract has the potential to affect the disposition of some concurrently administered conventional drugs. This possibility has been verified and demonstrated in an investigation that evaluates the effect of the leaf extract of V. amygdalina on the pharmacokinetics of chloroquine in rat. It was shown that, the herbal extract significantly reduced the Cmax, AUC and elimination half-life of chloroquine (Igboasoiyi et al., 2008).
Similar results were obtained in a study that investigated the effects of this herbal product on the pharmacokinetics of dihydroartemisinin. Plasma levels of dihydroartemisinin were found to be significantly reduced when the drug was co-administered with V. amygdalina (Eseyin et al., 2012). These findings indicate that this herbal product may reduce the effectiveness of chloroquine or dihydroartemisinin if they are co-administered. Contrary to the reduced plasma drug levels reported for chloroquine and dihydroartemisinin, concurrent administration of V. amygdalina with antidiabetic drug, metformin, investigated in rabbits, resulted in significantly increased drug exposure. Following V. amygdalina co-administration, metformin showed significantly higher Cmax, and AUC than the control group, but no significant difference was seen in the elimination half-life (Owolabi et al., 2014). Similarly, in a study done with an animal model, concomitant use of V. amygdalina with nifedipine resulted in significant increase in the plasma level of the drug. The AUC, Cmax and elimination half-life were significantly increased (Owolabi et al., 2013).
The authors suggested that the effect of this herbal product on nifedipine was attributable to inhibition of CYP450. This is not consistent with an earlier study which reported that the herbal extract induces CYP3A4 gene expression (Howard et al., 2003). Nifedipine and metformin are known to be metabolized via hepatic CYP2C and CYP3A subfamilies (Choi and Lee, 2012). However, both nifedipine and metformin have been demonstrated to inhibit P-gp efflux activity (Choi et al., 2013; Abbasi et al., 2016). Hence, it is most probable that the herbal product interacted with nifedipine and metformin at P-gp level. This speculation is further strengthened by reports of effects of V. amygdalina on P- gp activity. Studies based on an in vitro (Caco-2 cell), ex vivo (using chamber) and in vivo rat model revealed that, extract of V. amygdalina significantly inhibit P-gp mediated digoxin transport.
The authors suggested that interactions with conventional P-gp substrate drugs are likely to occur on co-administration with this herbal product and this may result in altered therapeutic outcomes (Oga et al., 2012; Oga et al., 2013). Extracts of V. amygdalina have very high concentrations of flavonoids and there is abundant evidence that flavonoids can inhibit P-gp (Lohner et al., 2007; Bansal et al., 2009; Oga et al., 2013). However, it has been demonstrated that no correlation exists between herbs inhibitory potentials towards CYP3A4 and P-glycoprotein activities (Brent et al., 2008). Inhibition of drug transporters at the intestines reduces the efflux of drug back into the gut lumen with a resultant increase in the bioavailability of the co-administered drug that is, a substrate for the transporter. Also, inhibition of P-gp at the renal tubules is expected to result in increase in elimination half-life of a P-gp substrate. More studies are therefore required to clarify the mechanisms mediating these observed herb-drug interactions of V. amygdalina.
Modulation by Nauclea pobeguinii
The stem bark extract of N. pobeguinii that has undergone clinical trials are standardized to contain 5.6% strictosamide which is the putative active constituent. Phytochemical investigations have revealed that steroids, saponins, alkaloid and their glycosylated derivatives are the major class of compounds identified and these may in large part be responsible for the various biological activities of this herbal product, including antimalarial activity (Azas et al., 2002; Haudecoeur et al., 2017). The herbal extract of N. pobeguinii contains several indole-quinolizidine alkaloids and glycoalkaloids out of which the main antiplasmodial compound identified is strictosamide which is also the major compound in the extract (Haudecoeur et al., 2017). There is a series of minor constituents which reportedly have weak or moderate antiplasmodial activities and these include angustine, 3, 14-dihydro-angustine, angustoline, 19-O-acetyl-angustoline, 19-O-methylangustoline, nauclefine, and naucletine. In addition, two quinovic acid glycosides, namely, 3-O-fucosyl-quinovic acid, 3-ketoquinovic have been identified (Mesia at al., 2010; Xu et al., 2012). Chemical structures of strictosamide and some other antiplasmodial phytochemicals in N. pobeguinii are presented in Figure 5.
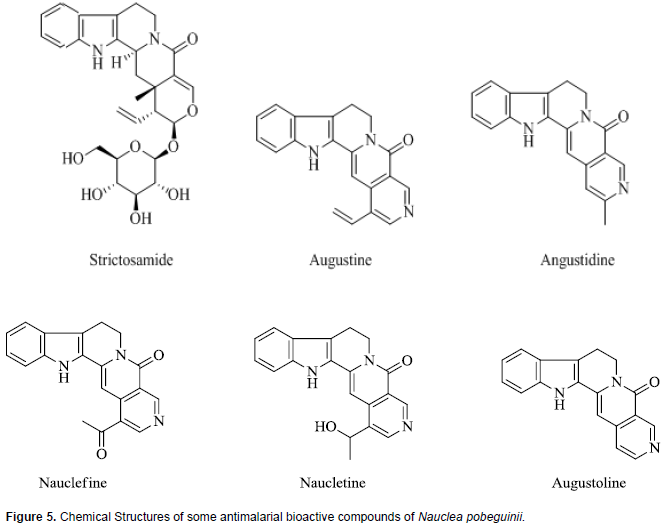
Excellent antiplasmodial activity has been reported for strictosamide (Abreu and Pereira, 2001), which is the most abundant compound in the crude extract (Mesia et al., 2010). However, some studies found strictosamide to be inactive in vitro (IC50 > 64 M) (Camacho et al., 2004; Mesia et al., 2010). It was suggested that the in vitro inactivity of strictosamide may be attributable to the glycosidic nature of the compound which limits its ability to be taken up by the parasite and reach an intracellular target. Thus, it has been hypothesized that since the extract is active in vivo, strictosamide is metabolically activated in the gastrointestinal tract by cleavage of the glucosidic moiety which enables its absorption (Mesia et al., 2010). An extensive literature search revealed paucity of studies done to evaluate the effect of this herbal product or its putative bioactive antimalarial, strictosamide, on drug metabolizing enzymes. A few related investigations found were on a closely related species, Nauclea latifolia. Phytochemical investigations of N. pobeguinii and N. latifolia indicate that they contain essentially the same range of compounds and are used interchangeably for malaria treatment (Haudecoeur et al., 2017).
The effect of NIPRD-AM1 (an aqueous root extracts of Nauclea latifolia) on CYP3A4 was evaluated in vitro in order to generate clinically significant data for its safe use (Adzu et al., 2013). Results showed that the extract significantly inhibited the activity of CYP3A4 with a very low IC50 value of 0.01 mg/mL comparable to that of ketoconazole (0.016 mg/mL), a known CYP3A4 inhibitor. Although an in vitro interaction may not necessarily reflect in vivo to the same magnitude, in all provides a useful indication for an appropriate guidance. This finding therefore suggests the need to exercise caution in concurrent administration of this herbal product with conventional drugs that are CYP3A4 substrates. However, studies are required to validate this modulation in vivo and also to determine whether the herbal product modulates the activities of other CYP450 isozymes. In alternative investigation, the effect of concurrent administration of NIPRD-AM1 on the pharmacokinetics of metronidazole was investigated in healthy volunteers. Results showed that there was no interaction between NIPRD-AM1 and metronidazole. The findings, therefore, indicate that metronidazole and NIPRD-AM1 can be used concurrently without any fear of adverse interaction (Obodozie et al., 2011).
Since metronidazole is known to be mainly metabolized by CYP2A6 (Pearce et al., 2013), it can be projected that this herbal product is not likely to affect the metabolism or disposition of conventional drugs that are CYP2A6 substrates. Another study examined the influence of NIPRD-AM1 on the pharmacokinetics of paracetamol in healthy human volunteers. The herbal product produced statistically significant reductions in AUC and Cmax, with no significant alterations in the elimination half-life of paracetamol when co-administered (Obodozie et al., 2004). Since the elimination kinetics of paracetamol was not affected, it is suggested that the significant reduction in the bioavailability of the drug is attributable to some pre-absorption process or interaction in the GIT that may have affected the quantity of paracetamol available for absorption. However, this has to be confirmed experimentally. There is need for further studies to evaluate the effect of N. pobeguinii extract on different CYP450 isoforms.
Modulation by Punica granatum
Phytochemical analysis of P. granatum (also called pomegranate) extract shows the presence of a wide range of phytochemicals constituents which include tannins, saponins, flavonoids, steroids, alkaloids coumarin, terpenoids, lignin and sugars (Sreekumar et al., 2014; Nalini and Anuradha, 2015). This diversity of phyto-constituents may be responsible for the reported use of this herbal product for several disease conditions in folklore medicine (Miguel et al., 2010; Shaygannia et al., 2016; Wu and Tian, 2017). The antiplasmodial bioactive compounds in fruit rind of the P. granatum which has shown clinical efficacy in treatment of malaria are ellagic acid and hydrophyllic ellagitannins and include punicalin, punicalagin, and punicafolin (Bhattacharya, 2011; Vishal et al., 2011; Wu and Tian, 2017). Chemical structures of some of these antiplasmodial phytochemicals are presented in Figure 6. The diversity of compounds in this herbal product has been reported to modulate drug metabolizing enzymes (Srinivas, 2013; Mallhi et al., 2015). It has been shown to inhibit different CYP450 isoforms. For example, the ability of the fruit juice of P. granatum to inhibit CYP3A4 was examined and established using human liver microsomes (Hidaka et al., 2005).
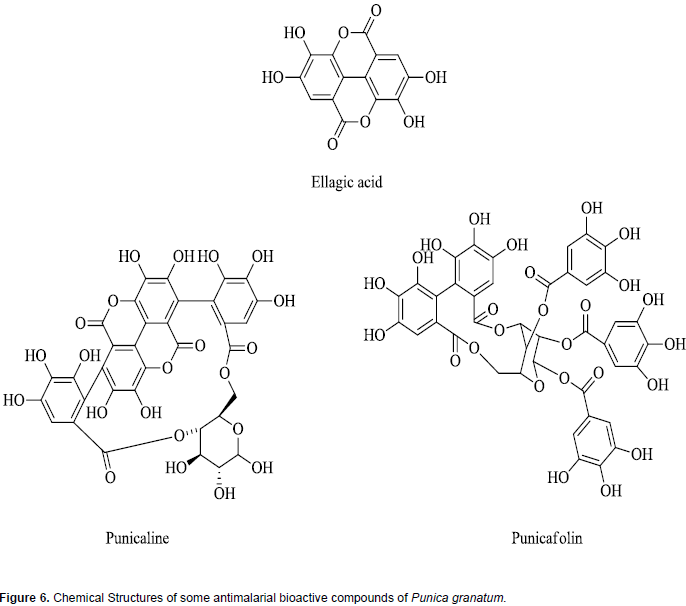
A similar significant inhibition of CYP3A4 activity by P. granatum fruit extract was further confirmed by another group using a luminescence assay (Ashour et al., 2017). P. granatum fruit extract has been analysed for its capacity to inhibit in vitro metabolism by human CYP3A4 and CYP2D6 with a radiometric assay technique. The extract showed significant inhibitory effects on both CYP450 isoforms (Usia et al., 2006). Similar inhibitory effect of P. granatum fruit juice was reported on midazolam 1'-hydroxylase activity, a marker of CYP3A, which was evaluated in pooled human liver microsomes. The inhibitory potency is; however, less than that of grapefruit juice (Kim et al., 2006). Hidaka et al. (2005) also investigated the in vivo interaction in rats between P. granatum fruit juice and carbamazepine. This result showed that concurrent oral administration resulted in 50% increase in the AUC of carbamazepine. Carbamazepine is a known CYP3A4 substrate (Kerr et al., 1994) and was concluded that the fruit juice inhibits the activity of enteric but not hepatic CYP3A, since the elimination half-life of carbamazepine and the metabolic ratio (AUC ratio of carbamazepine-10, 11-epoxide to carbamazepine) were not altered when the juice was administered parenterally.
This is similar to the well-known inhibitory effect of grapefruit juice on intestinal CYP450. This interaction can be positively exploited, to enhance the bioavailability of CYP3A4 substrates that have poor bioavailability resulting from extensive pre-systemic metabolism. A similar finding is reported for nitrendipine (CYP3A4 substrate) whose Cmax and AUC are significantly increased by co-administration of the fruit juice (Voruganti et al., 2012). The effect of P. granatum fruit juice consumption on the expression and activities of CYP1A1/2, CYP2E1, and CYP3A were assessed in mice. It was found that juice consumption decreased total hepatic CYP content as well as the expression of CYP
Literature is replete with information on varieties of plants from different parts of the world that have been tested in vitro and in vivo in animal models, documented to possess antiplasmodial activities. But very few of them have been taken through clinical trials. This is a very important missing step in a drug development process. Although some of these plant extracts have been in use for hundreds of years for traditional treatment of malaria, there is a clear need for their efficacies to be scientifically established through randomized clinical trials. With the increase use of herbal products in recent years, there is an urgent demand for further studies with regard to their safety and efficacy. Since CYP450 enzymes and P-glycoprotein play major roles in drug disposition, studying their interactions with herbal extracts is an important requirement in promoting safe use of these herbal products. The interaction of conventional drugs with herbal medicines is a significant safety concern because such can result in sub-therapeutic or toxic plasma levels of the concurrently administered drug. Malaria is associated with co-morbidities with other infections and non-communicable diseases which engenders co-administration of other conventional drugs with herbal antimalarial products.
Herbal medicines contain several active compounds that can serve as substrate for or induce/inhibit enzymes involved in the metabolism of other drugs. Theoretically, there is an increased risk of potential herb-drug interactions compared to conventional drugs that usually contain one chemical compound. Hence, as part of the process of standardization and development of herbal medicine for clinical use, it is important to identify drugs that may interact with the herbal medicines. It is evident from this review that the issue of antimalarial herb-drug interaction has not received the deserved attention. Only a few reports on herb-drug interactions were found, suggesting that very little attention is being given to the safety of herbal medicines. Therefore, much research is required in this area so as to improve on the safe use of these herbal products. Also, pharmacological studies are still needed on the mechanisms of action by which the plant extracts and the active compounds exert their pharmacological effects. With this knowledge, a putative bioactive constituent can serve as a lead compound for further investigation with regard to structure-activity relationship studies for optimization of the drug efficacy.
The authors have not declared any conflict of interests.
REFERENCES
|
Abay SM, Lucantoni L, Dahiya N, Dori G, Dembo EG, Esposito F, Lupidi G, Ogboi S, Ouédraogo RK, Sinisi A, Taglialatela-Scafati O, Yerbanga RS, Bramucci M, Quassinti L, Ouédraogo JB, Christophides G, Habluetzel A (2015). Plasmodium transmission blocking activities of Vernonia amygdalina extracts and isolated compounds. Malar. J. 14(1):288.
Crossref
|
|
|
|
Abbasi MM, Valizadeh H, Hamishehkar H, Zakeri-Milani P (2016). Inhibition of P-glycoprotein expression and function by anti-diabetic drugs gliclazide, metformin, and pioglitazone in vitro and in situ. Res. Pharm. Sci. 11(3):177-186.
|
|
|
|
|
Abena AA, Diatewa M, Gakossi G, Gbeassor M, Hondi-Assah TH, Ouamba JM (2003). Analgesic, antipyretic and anti-inflammatory effects of essential oil of Lippia multiflora. Fitoterapia 74(3):231-236.
Crossref
|
|
|
|
|
Ablordeppey SY, Hufford CD, Bourne RF, Dwuma-Badu D (1990). 1HNMR and 13C-NMR assignments of Cryptolepine, a 3:4 benzo-dcarboline derivative isolated from Cryptolepis sanguinolenta. Planta Med. 56(4):416-417.
Crossref
|
|
|
|
|
Abreu P, Pereira A (2001). New indole alkaloids from Sarcocephalus latifolius. Nat. Prod. Lett. 15(1):43-48.
Crossref
|
|
|
|
|
Addae-Mensah I, Waibel R, Achenbach H (1985). Constituents of West African Medicinal Plants, XVI. Novel Long-chain Triacylbenzenes from Cochlospermum planchonii. Liebigs Ann. Chem. 6:1284-1287
Crossref
|
|
|
|
|
Adukondalu D, Shravan KY, Vamshi VY, Shiva KR, Madhusudan RY (2010). Effect of pomegranate juice pre-treatment on the transport of carbamazepine across rat intestine. Daru J. Facult. Pharm. Tehran Univ. Med. Sci. 18(4):254-259.
|
|
|
|
|
Adzu B, Mustapha KB, Masimirembwa C, Obodozie O, Kirim RA, Gamaniel KS (2013). Simulation of metabolism-based herb-drug interaction: towards safe and efficacious use of NIPRD-AM1. Avicenna J. Phytomed. 3(3):201-204.
|
|
|
|
|
Aliyu R, Okoye ZS, Shier WT (1995a). The hepatoprotective cytochrome P-450 enzyme inhibitor isolated from the Nigerian medicinal plant Cochlospermum planchonii is a zinc salt. J. Ethnopharmacol. 48(2):89-97.
Crossref
|
|
|
|
|
Aliyu R, Okoye ZSC, Shier TW (1995b). Cochlospermum planchonii rhizome extract with hepatoprotective activity inhibits cytochrome P-450 monooxygenases. Phytother. Res. 9(8):600-602.
Crossref
|
|
|
|
|
Anaga AO, Oparah NQ (2009). Investigation of the methanol root extract of Cochlospermum planchonii for pharmacological activities in vitro and in vivo. Pharm. Biol. 47(11):1027-1034.
Crossref
|
|
|
|
|
Araya S, Abera B, Giday M (2015). Study of plants traditionally used in public and animal health management in Seharti Samre District, Southern Tigray, Ethiopia. J. Ethnobiol. Ethnomed. 11:22.
Crossref
|
|
|
|
|
Asase A, Oteng-Yeboah AA, Odamtten GT, Simmonds MSJ (2005). Ethnobotanical study of some Ghanaian anti-malarial plants. J. Ethnopharmacol. 99(2):273-279.
Crossref
|
|
|
|
|
Ashour ML, Youssef FS, Gad HA, Wink M (2017). Inhibition of Cytochrome P450 (CYP3A4) activity by axtracts from 57 plants used in Traditional Chinese Medicine (TCM). Pharmacogn. Mag. 13(50):300-308.
Crossref
|
|
|
|
|
Audu SA, Taiwo AE, Ojuolape AR (2012). A study review of documented phytochemistry of Vernonia amygdalina (family asteraceae) as the basis for pharmacologic activity of plant extract. J. Med. Plants Res. 2(7):1-8.
|
|
|
|
|
Azas N, Laurencin N, Delmas C, Di Giorgio C, Gasquet M, Laget M, Bahekar S, Kale R (2013). Herbal Plants Used for the Treatment of Malaria- A Literature Review. J. Pharmacogn. Phytochem. 1(6):141-146.
|
|
|
|
|
Ballin NZ, Traore M, Tinto H, Sittie A, Mølgaard P, Olsen CE, Kharazmi A, Christensen SB (2002). Antiplasmodial compounds from Cochlospermum tinctorium. J. Nat. Prod. 65(9):1325-1327.
Crossref
|
|
|
|
|
Bandaranayake WM (2006). Quality control, screening, toxicity, and regulation of herbal drugs. In: Modern Phytomedicine. Turning Medicinal Plants into Drugs; Ahmad I, Aqil F, Owais M (eds.), (Weinheim: Wiley-VCH GmbH & Co. KGaA). pp. 25-57. Available at:
Crossref
|
|
|
|
|
Bangou MJ, Almaraz-Abarca N, Méda NTR, Zeba B, Kiendrebeogo M, Millogo-Rasolodimby J Nacoulma OG (2012a). Polyphenolic composition of Lantana camara and Lippia chevalieri, and their antioxidant and antimicrobial activities. Int. J. Phytomed. 4:115-124.
|
|
|
|
|
Bangou MJ, Kiendrebeogo M, Compaore M, Coulibay AY, Meda NR., Abraca NA., Zeba B., Millogo-Rasolodimby J., Nacoulma OG (2011). Enxyme Inhibition effect and polyphenolic content of medicinal plant extract from Burkina Faso. J. Biol. Sci. 11(1):31-38.
Crossref
|
|
|
|
|
Bangou MJ, Norma AA, Meda RN, Yougbaré-Ziébrou M, MillogoRasolodimby J, Nacoulma OG (2012b). Lippia chevalieri Moldenke: A brief review of traditional uses, phytochemistry and pharmacology. Int. J. Drug Deliv. 4(3):289-296.
|
|
|
|
|
Bansal T, Jaggi M, Khar RK, Talegaonkar S (2009). Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J. Pharm. Pharm. Sci. 12(1):46-48.
Crossref
|
|
|
|
|
Bapna S, Choudhary PK, Ramaiya M, Chowdhary A (2015). Antiplasmodial activity af Argemone Mexicana: An in vivo and in vitro study. World J. Pharm. Res. 4(11):1653-1663.
|
|
|
|
|
Barbosa AF, de Carvalho MG, Smith RE, Sabaa-Srur AUO (2016). Spilanthol: occurrence, extraction, chemistry and biological activities. Rev. Bras. Farm. 26:128-133.
Crossref
|
|
|
|
|
Bardia A, Nisly NL, Zimmerman MB, Gryzlak BM, Wallace RB (2007). Use of herbs among adults based on evidence-based indications: Findings from the National Health Interview Survey. Mayo Clinic Proceed. 82(5):561-566.
Crossref
|
|
|
|
|
Barku VYA, Opoku-Boahen Y, Dzotsi EY (2012). Isolation and pharmacological activities of alkaloids from Cryptolepis sanguinolenta (Lindl) schlt. Int. Res. J. Biochem. Bioinform. 2(3):58-61.
|
|
|
|
|
Bassole IHN, Ouattara AS, Nebie R, Ouattara AT, Kabore ZI, and Traore SA (2003). Chemical composition and antibacterial activities of the essential oils of Lippia chevalieri and Lippia multiflora from Burkina Faso. Phytochemistry 62(2):209-212.
Crossref
|
|
|
|
|
Benoit-Vical F, Valentin A, Da B, Dakuyo Z, Descamps L, Mallié M (2003). N'Dribala (Cochlospermum planchonii) versus chloroquine for treatment of uncomplicated Plasmodium falciparum malaria. J. Ethnopharmacol. 89(1):111-114.
Crossref
|
|
|
|
|
Benoit-Vical F, Valentin A, Mallie M Bessiere J (2001). Antiplasmodial activity of Cochlospermum planchonii and C. tinctorium Tubercle essential oils. J. Essent. Oil Res. 13(1):65-67.
Crossref
|
|
|
|
|
Benoit-Vical F, Valentin A, Mallié M, Bastide JM, Bessière JM (1999). In vitro antimalarial activity and cytotoxicity of Cochlospermum tinctorium and C. planchonii leaf extracts and essential oils. Planta Med. 65(4):378-381.
Crossref
|
|
|
|
|
Bhakuni R, Jain D, Sharma R (2002). Phytochemistry of Artemisia annua and the development of artemisinin-derived antimalarial agents. In: Wright C, editor. Artemisia. London: Taylor & Francis.
Crossref
|
|
|
|
|
Bhattacharya D (2011). Fight malaria at home: Therapeutic and prophylaxis clinical data. Asian Pac. J. Trop. Dis. 1(2):142-149.
Crossref
|
|
|
|
|
Bhattacharya D, Bhuyan BM, Pradhan PK, Nayak DK (2013). Transmission blocking of year-round resistant malaria in Koraput (India) by OMARIA – A New Antimalarial Phytotherapy. Br. J. Pharm. Res. 3(1):54-77.
Crossref
|
|
|
|
|
Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G (2003). The conduct of in vitro and in vivo drugâ€drug interaction studies: A PhRMA perspective. J. Clin. Pharmacol. 43(5):443-469.
Crossref
|
|
|
|
|
Bugyei KA, Boye GL, Addy ME (2010). Clinical efficacy of a tea-bag formulation of Cryptolepis sanguinolenta root in the treatment of acute uncomplicated falciparum malaria. Ghana Med. J. 44(1):3-9.
|
|
|
|
|
Camacho MDR, Phillipson JD, Croft SL, Yardley V, Solis PN (2004). In vitro antiprotozoal and cytotoxic activities of some alkaloids, quinones, flavonoids, and coumarins. Planta Med. 70(1):70-72.
Crossref
|
|
|
|
|
Challand S, Willcox M (2009). A clinical trial of the traditional medicine Vernonia amygdalina in the treatment of uncomplicated malaria. J. Altern. Complement. Med. 15(11):1231-1237.
Crossref
|
|
|
|
|
Cho HJ, Yoon IS (2015). Pharmacokinetic Interactions of Herbs with Cytochrome P450 and P-Glycoprotein. J. Evid. Based Complement. Altern. Med. 2015:736431.
Crossref
|
|
|
|
|
Choi JS, Choi I, Choi DH (2013). Effects of nifedipine on the pharmacokinetics of repaglinide in rats: possible role of CYP3A4 and p-glycoprotein inhibition by nifedipine. Pharmacol. Rep. 65(5):1422-1430.
Crossref
|
|
|
|
|
Choi YH, Lee MG (2012). Pharmacokinetic and pharmacodynamic interaction between nifedipine and metformin in rats: competitive inhibition for metabolism of nifedipine and metformin by each other via CYP isozymes. Xenobiotica 42(5):483-495.
Crossref
|
|
|
|
|
Choudhary PK, Nagori BP (2014). Evaluation of in vitro antimalarial activity of Cassia occidentalis. World J. Pharm. Pharm. Sci. 3(2):2241-2248.
|
|
|
|
|
Christensen SB, Kharazmi A (2001). Antimalarial natural products. Isolation, characterisation and biological properties. In: Tringali C (Ed.), Bioactive Compounds from Natural Sources. Taylor & Francis, London and New York. pp. 379-432.
|
|
|
|
|
Cimanga K, De Bruyne T, Pieters L, Vlietinck AJ, Turger CA (1997). In vitro and in vivo antiplasmodial activity of cryptolepine and related alkaloids from Cryptolepis sanguinolenta. J. Nat. Prod. 60(7):688-691.
Crossref
|
|
|
|
|
Clarkson C, Maharaj VJ, Crouch NR, Grace OM, Pillay P, Matsabisa MG, Bhagwandin N, Smith PJ, Folb PI (2004). In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 92(2-3):177-191.
Crossref
|
|
|
|
|
Cui L, Mharakurwa S, Ndiaye D, Rathod PK, Rosenthal PJ (2015). Antimalarial Drug Resistance: Literature Review and Activities and Findings of the ICEMR Network. Am. J. Trop. Med. Hyg. 93(Suppl 3):57-68.
Crossref
|
|
|
|
|
Dakuyo Z, Lamien-Meda A, Da O, Kiendrebeogo M, Traoré-Coulibaly M, Novak J, Benoit-Vical F, Weisbord E, Willcox M (2015). SAYE: The story of an antimalarial phytomedicine from Burkina Faso.J. Altern. Complement. Med. 21(4):187-195.
Crossref
|
|
|
|
|
Das M, Samra BP (2017). Clinical trial of G. neivum as anti-malarial drug in Malaria. J. Sci. 2(4):9-12.
|
|
|
|
|
de Lima M, Vieira T, Huang SM (2012). Botanical-drug interactions: a scientific perspective. Planta Med. 78(13):1400-1415.
Crossref
|
|
|
|
|
Dell'Agli M, Galli GV, Corbett Y, Taramelli D, Lucantoni L, Habluetzel A, Maschi O, Caruso D, Giavarini F, Romeo S, Bhattacharya D, Bosisio E (2009). Antiplasmodial activity of Punica granatum L. fruit rind. J. Ethnopharmacol. 125(2):279-285.
Crossref
|
|
|
|
|
Deshpande HA, Bhalsing SR (2013). Recent advances in the phytochemistry of some medicinally important cassia species: a review. Int. J. Pharm. Med. Biol. Sci. 2(3):61-78.
|
|
|
|
|
Diallo B, Vanhaelen M (1987). Apocarotenoids from Cochlospermum tinctorium. Phytochemistry 26(5):1491-1492.
Crossref
|
|
|
|
|
Diallo D, Maiga A, Diakite C, Willcox M (2004). Malarial-5: Development of an antimalarial phytomedicine in Mali. In: Willcox M, Bodeker G, Rasoanaivo P (eds.), Traditional medicinal plants and malaria. Boca Raton: CRC Press; 2004.
|
|
|
|
|
Diarra N, Klooster CV, Jong J, de Togola A, Diallo D, Willcox M (2015). Ethnobotanical study of plants used against malaria in Selingue subdistrict, Mali. J. Ethnopharmacol. 166:352-360.
Crossref
|
|
|
|
|
Dju A, Nilsen OG, Steinsbekk A (2013). The co-use of conventional drugs and herbs among patients in Norwegian general practice: a cross-sectional study. BMC Complement. Altern. Med. 13:295.
Crossref
|
|
|
|
|
Donkor AF (2016). Efficacy, pharmacokinetics and safety evaluation of cryptolepine-artemisinin based combinations in the management of uncomplicated malaria," PhD Thesis, Kwame Nkrumah University of Science and Technology.
|
|
|
|
|
Doumbia S (1997). Etude des plantes antipaludiques au Mali. Bamako: Universite de Bamako, Faculte de Medecine, Pharmacie et Odonto-Stomatolo-gie, 78.
|
|
|
|
|
Dubey S, Maity S, Singh M, Saraf SA, Saha S (2013). Phytochemistry, Pharmacology and Toxicology of Spilanthes acmella: A review. Adv. Pharmacol. Sci. 2013:423750.
Crossref
|
|
|
|
|
Egharevba C, Osayemwenre E, Imieje V, Ahomafor J, Akunyuli C, Udu- Cosi AA, Theophilus O, James O, Ali I, Falodu A (2014). Significance of bitter leaf (Vernonia amagdalina) in tropical diseases and beyond: A Review. Malar. Chemoth. Cont. 3:120.
Crossref
|
|
|
|
|
Ekor M (2014). The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 4(177):1-9.
Crossref
|
|
|
|
|
Elango H, Ponnusankar S, Sundaram S (2015). Assessment of Pharmacodynamic and Pharmacokinetic Interaction of Aqueous Extract of Cassia auriculata L. and Metformin in Rats. Pharmacogn. Mag. 11(Suppl 3):S423-S426.
Crossref
|
|
|
|
|
Erasto P, Grierson DS, Afolayan AJ (2006). Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina. J. Ethnopharmacol. 106(1):117-120.
Crossref
|
|
|
|
|
Ernst E, White A (2000). The BBC survey of complementary medicine use in the UK. Complement. Ther. Med. 8(1):32.
Crossref
|
|
|
|
|
Eruvaram NR, Das M (2009). Phenotype of hepatic xenobiotic metabolizing enzymes and CYP450 isoforms of sanguinarine treated rats: effect of P450 inducers on its toxicity. Toxicol. Mechanisms Methods 19(8):510-517.
Crossref
|
|
|
|
|
Eseyin AO, Igboasoiyi CA, Igbo C, Igboasoiyi A, Ekarika J, Dooka B (2012). Effects of the leaf extract of Venonia amygdalina on the pharmacokinetics of dihydroartemisinin in rat. Pharmacologia 3(12):713-718.
Crossref
|
|
|
|
|
Etiaba E, Onwujekwe O, Uzochukwu B, Uguru N, Okoronkwo I, Adjagba A (2015). What co-morbidities do people with malaria have and what are their patterns of health seeking in Nigeria? Niger. J. Clin. Pract. 18(1):22-26.
|
|
|
|
|
Faria A, Monteiro R, Azevedo I, Calhau C (2007). Pomegranate Juice Effects on Cytochrome P450s Expression: In Vivo Studies. J. Med. Food. 10(4):643-649
Crossref
|
|
|
|
|
Fasinu PS, Manda VK, Dale OR, Egiebor NO, Walker LA, Khan S (2016). Evaluation of the influence of selected herbal extracts on the activity of p-glycoprotein and pregnane X receptor activation. Planta Med. 82(05):PB13.
Crossref
|
|
|
|
|
Forkuo AD, Ansah C, Boadu KM, Boampong JN, Ameyaw EO, Gyan BA, Arku AT, Ofori MF (2016). Synergistic anti-malarial action of cryptolepine and artemisinins. Malar. J. 15:89.
Crossref
|
|