ABSTRACT
Prevalence of animal diseases is one of the major livestock production constraints in Kenya with high impacts on livelihoods due to related economic losses affecting food security in the country. The use of synthetic drugs for disease management has challenges. This makes the use of medicinal plants for treatment a rational alternative. Ascarids, Toxocara canis and Ancylostoma caninum are among the most frequently observed helminth parasites in dogs in Kenya. The two parasites are also known to cause helminthiasis in human beings. This study was designed to evaluate the in vitro efficacy of ethanol and aqueous extracts from bulbs of A. sativum and A. cepa and from leaves of J. curcas against T. canis and A. caninum parasites. Six (6) extracts from three (3) plants: A. cepa, A. sativum and J. curcas were selected for in vitro anthelmintic screening by measuring ability to inhibit hatching and development of eggs and survival of larvae in vitro. The ethanol extracts of A. cepa inhibited hatching of 100% of eggs of A. caninum between 10,000 and 2,500 ug/ml and 100% of eggs of T. canis between 10,000 and 1,250 ug/ml while that of A. sativum inhibited hatching of 100% of A. caninum eggs between 10,000 and 5,000 ug/ml. However the ethanol extract of A. sativum did not have the same effect on the development of T. canis eggs at these concentrations. The ethanol extracts of both A. cepa and A. sativum affected the survival of 100% of A. caninum larvae at a concentration of 156 ug/ml and above. The water extracts of the three plants had moderate effects on the eggs and the larvae of both parasites. The results indicate that the ethanol extracts of A. cepa and A. sativum have anthelmintic properties which should be investigated further to support the ethnoveterinary use of the plants as anthelmintics for control and treatment of worm infestation in dogs.
Key words: Allium cepa, Allium sativum, Anthelmintic activity, Ancylostoma caninum, Jatropha curcas Toxocara canis.
The common varieties of Allium cepa (onion) locally known as kitunguu and Allium sativum locally known as kitunguu sumu are widely grown for their culinary uses. The leaves of A. sativum are long, narrow and flat like grass. The bulb (the only part with culinary uses) is of a compound nature, consisting of numerous bulblets, known technically as 'cloves,' grouped together between the membranous scales and enclosed within a whitish skin, which holds them as in a sack. The flowers of A. sativum are placed at the end of a stalk rising direct from the bulb and are whitish, grouped together in a globular head, or umbel, with an enclosing kind of leaf or spathae, and among them are small bulbils (Grieve, 2006). A cepa is a perennial herb which is strong smelling when crushed. Its roots are adventitious and fibrous. It has an underground stem modified into a tunicated bulb consisting of reduced stem and axillary buds surrounded by inner fleshy scale leaves and outer membranous dry scales. Leaves are radical, alternate, sessile, simple cylindrical, hollow green and parallel veined foliage with a fleshy sheathing base arising from the underground stem (Ranjitkar, 2003). The terminal inflorescence develops from the ring-like apical meristem. It has scapes, one to several, generally elongate well above the leaves and range in height from 30 cm to more than 100 cm (Ross, 2001)
Both A. sativum and A. cepa were sourced from Uthiru Market located approximately 25 km from Nairobi City Center and their bulbs were used for the experiment. Jatropha curcas is a monoecious plant with unisexual or occasionally hermaphrodite flowers. It has a gynoecium with three slender styles which are connate to about two-thirds of their length, dilating to massive bifurcate stigma. It grows to the size of a small tree or a large shrub, which can reach a height of 3 to 5 m. The branches contain latex. It forms five roots from seedlings, one central and four peripheral. A tap root is not usually formed by vegetatively propagated plants. Leaves have 5 to 7 lobes, hypostomatic and stomata are of paracytic (Rubiaceous) type. It has inflorescence each yielding a bunch of approximately 10 or more ovoid fruits. The seeds are black and the seed weight per 1000 is about 727 g (Kumar and Sharma, 2008).
J. curcas was sourced from the outskirts of Ngong Hills in Kajiado County, approximately 30 km South of Nairobi. The leaves were used for the experiment. Ascarids, Toxocara canis (dog ascarid worm) and Ancylostoma caninum (dog hookworm) are among the most frequently observed helminth parasites in dogs in Kenya (Kagira and Kanyari, 2000). Both T. canis and A. caninum are also known to cause helminthiasis in human beings (Dickson, 2003; Juergen and Paul, 2003). The adult stage of the ascarid worm lives in the small intestines of the dog from where their eggs are passed into the environment through feces. Over a period of 2 to 6 weeks the eggs develop to the infective third stage larvae (Azam et al., 2012). Humans and dogs are infected by ingesting eggs containing the third stage larvae (Overgaauw, 1997; Kasai, 1995) or less frequently tissues dormant larvae in tissues of a paratenic hosts. Once ingested the larvae hatch and migrate through blood vessels into soft tissues (Hill et al., 1985). Puppies from infected bitches are born infected through prenatal transmission. Toxocara canis infections in humans are usually mild and asymptomatic in two major forms; visceral toxocariasis and ocular toxocariais. The adult stage of the hookworm is also common in the dog intestines attaching to the linings with hook like teeth and from where they shed eggs to the environment through feces. Animals get infected by ingesting third stage (L3) infective larvae or by the infective larvae attaching and burrowing into the skin from where they migrate to the small intestines, mature, mate and lay eggs. Puppies can also be infected through their nursing mother’s milk. Conventional prophylaxis and treatment is based upon routine administration of febantel and pyrantel pamoate to pregnant bitches and puppies. Extracts of A. Sativum have shown in vitro anthelmintic efficacy against Fasciola gigantica (Kumar, 2014), Schistosoma mansoni (Mantawy et al., 2012) and Hemonchus contortus (Zafar, 2014). Crude ethnolic and aqueous extracts of A. cepa bulbs showed strong anthelmintic activity on Pheretima posthuma (earthworm), the earthworms were paralysed within 20 min and died within 30 min of exposure (Abhijeet et al., 2012). For J. curcas, hexane, ethyl acetate and ethanol extracts obtained from the seeds were found to have in vitro efficacy against Haemonchus contortus (Monterio et al., 2011) while the aqueous latex of leaves of the plant was found to have similar efficacy against Pheretima posthuma (Hitesh et al., 2014). However, none of these extracts is available in the market for use in treatment of helminthosis because more studies are required to establish the active ingredients, modes of action and safety. This study was designed to evaluate the in vitro efficacy of ethanol and aqueous extracts from bulbs of A. sativum and A. cepa and from leaves of J. curcas against T. canis and A. caninum parasites.
Experimental animals
Two mongrel male puppies aged between 6 and 10 weeks, with an average weight of 2.2 kg and showing signs of natural strongyle and ascarid infection were purchased from households in Ndumboini village in Kabete. During the experiment the puppies were housed and fed together in dog kennels at the Faculty of Veterinary Medicine of the University of Nairobi. Screening of the puppies for helminth infection was done by microscopic examination of fecal smears from each puppy. Both strongyle and ascarid eggs were observed.
Plant collection and identification
Both Alliums are commercially grown for culinary uses and were bought from Uthiru Market in Kabete, Nairobi. The J. curcas was collected from Ngong Forest in Kajiado County where it grows naturally as a shrub. All the plant materials were identified at the Kenya National Museums Herbarium in Nairobi.
Preparation of extracts
The bulbs of the Alliums and leaves of the J. curcas were oven dried separately at 60°C for 48 to 72 h. Each of the dry plant materials was ground to a fine powder, sealed in paper bags and kept in cool dry place. An aqueous extract of the leaves of J. curcas was obtained following the method described by Juliana et al. (2014). A total of 900 ml of distilled water was added to 100 g of dry powder and the mixture was heated at 100°C for 15 min. After cooling, the mixture was centrifuged before filtration through a No.1 filter paper (M&N 615 12.5CM) in order to separate the residues. The filtered solution was then freeze dried to obtain a light green colored powder with a recovery rate of 0.94% relative to dry powder.
The ethanol extract of leaves of J. curcas was prepared according to Ekundayo and Ekekwe (2013). I L of 99.5% ethanol was mixed with 100 g of dried leaf powder and agitated several times for a period of 72 h. The mixture was filtered through Whatman No. 1 filter paper into a clean beaker and then evaporated over a sand bathe at 80°C. Drying was completed in an oven at 40°C to prevent the dry extract from sticking to the walls of the container. This yielded a dark green (near black) colored paste with a recovery rate of 2.61%.
Aqueous extracts of both A. sativum and A. cepa were obtained as described by Mikhail (2009). A total of 100 g of dry powder from ground dry bulbs of each of the Allium was mixed with 900 ml of distilled water and heated in water bath at 100°C for 30 min. After cooling, the mixture was filtered through Whatman No. 1 filter paper and the filtrate was centrifuged before decanting the supernatant into a clean beaker. The extracts were freeze dried. A. sativum yielded a yellowish colored crystalline powder with a recovery rate of 18.25% while A cepa yielded a sticky dark tan (dark brown) colored solid with a recovery rate of 16.75%.
Ethanol extracts of Alliums were obtained according to Akintobi et al. (2013). A total of 100 g of each powder was mixed with 400 ml of 99.5% ethanol and agitated several times for a period of 72 h. The mixture was filtered through a Whatman No. 1 filter paper into a clean beaker and then evaporated over a sand bathe at 80°C. Drying was completed in an oven at 40°C to prevent the dry extract from sticking to the walls of the container. The A sativum ethanol extract yielded a mid-brown colored paste with a recovery rate of 0.39% while the A cepa ethanol extract yielded a maroon colored paste with a recovery rate of 2.03%.
Determination of effects on hatching
This was done using the Egg Hatch Assay as described by Le Jambre (1976) and used by other researchers (Coles et al. 2006) with minor modifications.
Determination of effects on survival of larvae (Larval Motility Assay)
This observation was only made on larvae of A. caninum. This is because they hatch into free larvae unlike those of T. canis which hatch within the egg shells making it difficult to observe their movements. Survival of larvae was determined after hatching by calculating the percentage of larvae that were motile (alive) and those that were not (dead) out of the total larvae that hatched (Thoithi et al., 2002).
Identification of the eggs
The eggs of Ancylostoma caninum are typical strongyle with an ovoid shape and a thin wall. They measure about 40x65 µm, and contain already 4 to 16 cells when shed with the feces. The eggs of Toxocara canis are almost spherical, brown in color and measuring about 50x85 µm. They have a thick wall with a rough surface, and contain a single cell when in feces. (www.parasitipedia.net)
Preparation of the eggs solution
According to Coles et al. (2006) eggs intended for the hatching test should be used within 3 h of collection because sensitivity decreases with age of the eggs. To obtain fresh fecal samples, the puppies were given dog food pellets at 8 am on the day of harvesting, after starving them for 12 to 18 h; this ensures that they defecate within an hour of feeding
releasing fresh samples.
About 200 g of a pooled fecal sample was mixed into a suspension with saturated saline solution (350 g of NaCl in 1,000 ml of tap water) and allowed to settle for 30 minutes. A plastic petri dish was placed on the surface of the suspension in order to allow the eggs adhere to the bottom of the dish. The eggs were recovered by washing off with distilled water then centrifuged twice in order to remove the excess salt. 15 ml of distilled water was added to the final residue and mixed well. 50 µl of this solution were dropped into three microscope slides and a cover slip placed onto each and then observed under a light microscope at X10 magnification in order to count the number of strongyle and ascarid eggs in each slide. The average estimated number of eggs per 50 µl of the parasite egg solution was 173 strongyle and 27 ascarid eggs.
Exposing the eggs to the extracts
The extracts were reconstituted by adding 40 mg of each dry extract into 2 ml of distilled water in order to make a stock solution. The aqueous extracts of each of the plant was dissolved readily in water while the ethanol extracts of A. sativum and A. cepa dissolved after raising the temperature of the mixture in a water bath at 40°C for 15 min and shaking vigorously. The ethanol extract of J. curcas could not dissolve in water even after shaking or raising the temperature. Therefore it was not used for any tests.
The stock of 20,000 ug/ml of each plant extract was diluted serially to give 10,000, 5,000, 2,500, 1,250, 625, 312.5 and 156.25 ug/ml in a microtitre plate in order to obtain 150 µl of each dilution per well. A total of 50 µl of the parasite solution was added into each well to make a final mixture of 200ul of parasite eggs and extract in each well. Further dilutions of 78.13, 39.06, 19.53, 9.77, 4.88, 2.44, 1.22 and 0.61 ug/ml were done for ethanol extracts of both A. cepa and A. sativum and for the aqueous extract of A. cepa. For the positive control a similar dilution was made as for the drug preparation (Vermic TotalTM) containing a combination of febantel and pyrantel permeate which are both sensitive to A. caninum and T. canis. For the negative controls, one well had 200 µl of the parasite egg solution and another one had 150 µl of 99.5% ethanol mixed with 50 µl of the parasite egg solution. The microtitre plate cover was replaced, wrapped up with aluminum foil and incubated for 2 weeks. Observations were made under an inverted microscope after 48 h and again after 96 h for A. caninum eggs then on alternate days up to 2 weeks for T. canis eggs. This is because A. caninum eggs usually hatch after 48 to 72 h into free larvae while T. canis eggs develop to a fully larvated egg over a period of 2 to 6 weeks and does not break into free larvae (Dena et al., 2012). The readings were done by counting the number of free A. caninum larvae and the number of developing T. Canis eggs. The percentage inhibition was calculated using the following formula;
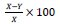
Where: X is the estimated total number of eggs per 50 µl; Y is the number of hatched or developing eggs.
Table 1 shows the percentage inhibition of egg hatching and effects on motility of larvae of each of the six extracts on A. caninum. Table 2 shows the percentage Egg Hatch Inhibition on T. canis and Table 3 shows the dose response on further dilution of aqueous and ethanol extracts of A. cepa and ethanol extract of A. sativum. The aqueous and ethanol extracts of A. cepa inhibited hatching of eggs of both A. caninum and T. canis and has moderate effect on survival of larvae as shown in Tables 1 and 2. However, the ethanol extract gave higher percentage inhibition rates at lower concentrations than the aqueous extract. This suggests that there was a better dose response for the ethanol extract of A. cepa. Ethanol extract of A. sativum also inhibited hatching of eggs of both parasites and had moderate effect on survival of larvae. Both aqueous extracts of A. sativum and J. curcas did not have effect on the eggs and on survival of larvae as shown in Tables 1 and 2. Figures 1 and 2 compare the percentage inhibition of ethanol extracts of A. cepa and A. sativum on A. caninum and T. canis respectively. Both show that the ethanol extract of A. cepa has a higher dose response. None of the extracts was as active as the combination drug VERMIC TOTALTM at equal concentrations.
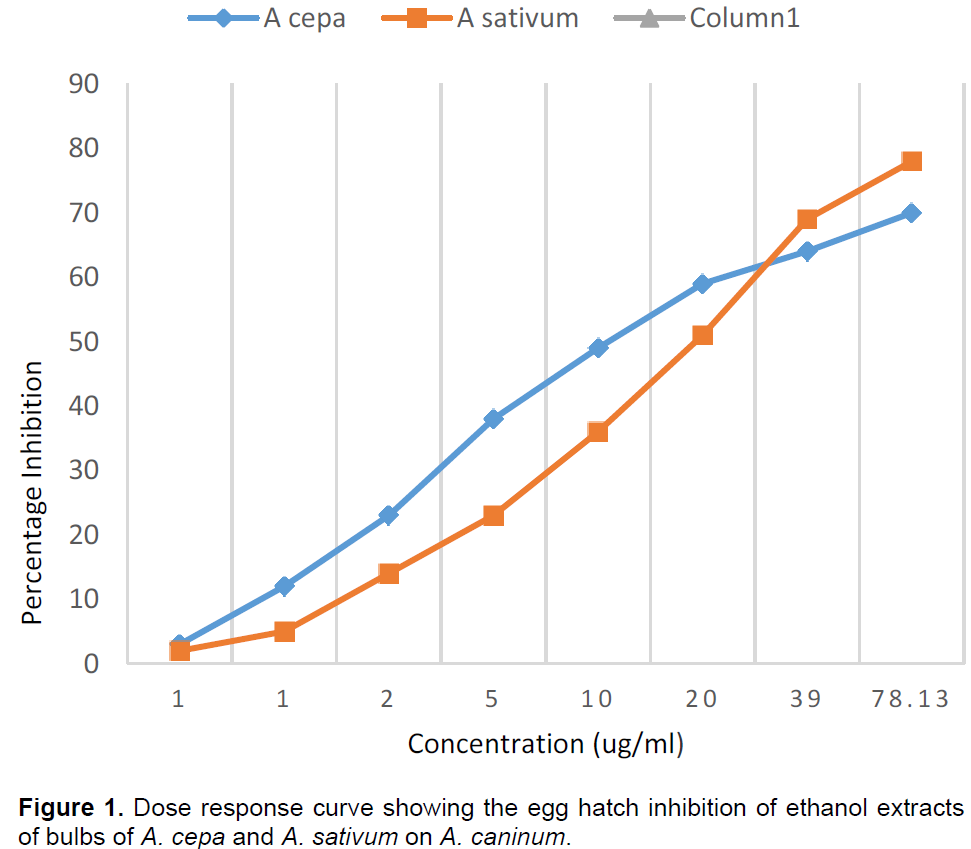
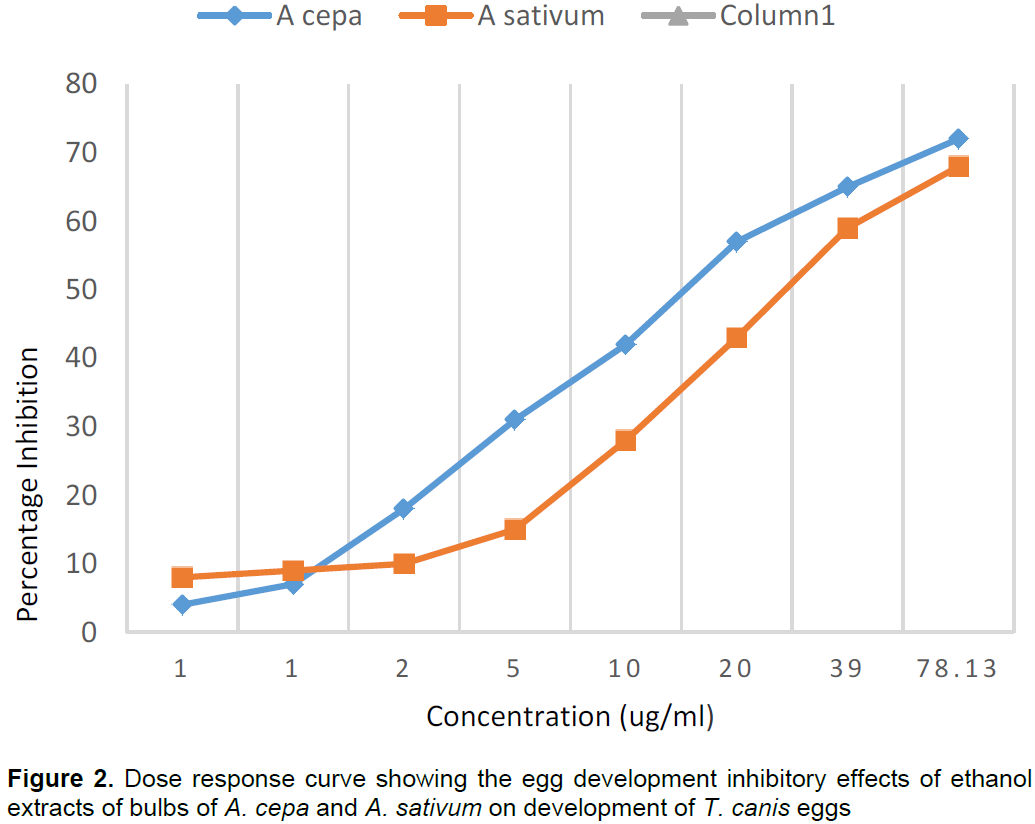
This work reports for the first time the in vitro anthelmintic activity of the water and ethanol extract of bulbs of A. cepa and A. sativum as well as the leaves of J curcas on A. caninum and T. canis. The results indicate that the ethanol extracts of A. cepa and A. sativum have anthelmintic properties against Ancylostoma caninum and Toxocara canis. There was a complete inhibition of Egg Hatching and Egg Development up to a dilution of 1,250 ug/ml for the A. cepa ethanol extract after which a dose response was observed. This percentage inhibition was higher compared to that of the ethanol extract of A. sativum suggesting that the A. cepa extract has a higher anthelmintic efficacy.
Both extracts showed good anthelmintic activity since they affected the survival of larvae. However, none of these extracts was as active as Vermic TotalTM (Febantel and Pyrantel Permoate). The aqueous extract of J. curcas had no effect in terms of inhibition of egg hatching or development but had high activity against survival of the larvae. The sequential activity on both eggs and larvae as shown by the ethanol extracts of A. cepa and A. sativum as well as the aqueous extract of A. cepa, is comparable to that of commonly used anthelmintic drugs like benzimidazoles which are active against eggs, larvae and adult stages of nematode worms (Behm and Bryant, 1983). The results support the work of other researchers who reported that crude extracts of A. cepa and A. sativum have anthelmintic properties (Abhijeet et al., 2012; Mantawy et al., 2012; Kumar, 2014; Zafar, 2014). Dimethyl Sulfoxide (DMSO) or Tween80 can be used to concentrate organic solvent dry extracts which cannot be reconstituted in water. However according to Luciana et al. (2011) worms can tolerate up to 2% DMSO or Tween 80 although even these solvents at 2% cannot dissolve extracts made with pure ethanol. Therefore this study did not use DMSO or Tween 80 to reconstitute the ethanol extract of J. curcas but opted to remove it from the experiment.
The use of 20,000 ug/ml as the concentration of stock solutions for serial dilution of each extract was based on methods described by Thoithi et al. (2002). Higher concentrations of aqueous extracts of A. sativum and J. curcas may show better effect on egg hatching and survival of larvae. More work can be done to test the in vitro effects of these extracts at higher concentration ranges. Further pharmacological work should be carried out on the efficacy of the ethanol extracts of A. cepa and A. sativum in puppies to determine the toxic effects in mice in order to get further information that can support the use of the medicines for control and treatment of worm infestations in dogs.
The authors have not declared any conflict of interest.
REFERENCES
|
Abhijeet B, Mudreka G, Chirag P, Manoj A, Deepa S, Pallavi A (2012). Anthelmintic Activities of the Crude Extracts of Allium cepa Bulbs and Elletatria cardomomum Seeds. Res. J. Pharm. Biol. Chem. Sci. 3(1):50.
|
|
|
|
Akintobi OA, Nwanze JC, Ogele JO, Idowu AA, Onianwa O, Okonko IO (2013). Antimicrobial Activity of Allium sativum (Garlic) Extract against Some Selected Pathogenic Bacteria. J. Nat. Sci. 11(1).
|
|
|
|
|
Azam DU, Said A, Abd-Allah GA, Morgan ER (2012). Temperature and the development and survival of infective Toxocara canis larvae. J. Parasitol. Res. 110:649-656.
Crossref
|
|
|
|
|
Behm CA, Bryant C (1983). The Mode of Action of some Modern Anthelmintics. In. Resistance in Nematodes to Anthelmintic Drugs.
|
|
|
|
|
Coles GC, Jackson F, Pomroy WE, Prichard RK, Von Samson-Himmelstjerna G, Silvestre A, Taylor MA, Vercruysse J (2006). The detection of anthelmintic resistance in nematodes of veterinary importance. J. Vet. Parasitol. 136:167-185.
Crossref
|
|
|
|
|
Dickson D (2003). Toxocariasis: Clinical Aspects, Epidemiology, Medical Ecology, and Molecular Aspects. J. Clin. Microbiol. Rev. 16(2):265-272
Crossref
|
|
|
|
|
Eds. N Anderson and P. Walter. CSIRO Division of Animal Health, Australian Wool Corporation Technical Report.
|
|
|
|
|
Ekundayo EO, Ekekwe JN (2013). Antibacterial activity of leaves extract of Jatropha curcas and Euphorbia heterophylla. J. Microb. Res. 7(44):5097-5100
|
|
|
|
|
Grieve M (2006). Garlic. Botanical.Com [Retrieved April 4, 2016]
|
|
|
|
|
Hitesh KP, Manisha J, Bhagat SR (2014). Anthelmintic Activity of latex of Jatropha curcas (ratanjot). Int. J. Pharm. Sci. Res. 5(3)
|
|
|
|
|
Juergen KL, Paul P (2003). Experimental human infection with the dog hookworm, Ancylostoma caninum. Med. J. Austr. 178(2):69-71.
|
|
|
|
|
Juliana FS, Thiago S, Yamara AS, Bárbara C, Rafael BG, Arnóbio A, Hugo AO, Ivanise MM, Silvana MZ, Matheus FF (2014). Aqueous Leaf Extract of Jatropha gossypiifolia L. (Euphorbiaceae) Inhibits Enzymatic and Biological Actions of Bothrops jararaca Snake Venom. PLOS ONE.
|
|
|
|
|
Kumar A, Sharma S (2008). An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.). Rev. Ind. Crops Prod. 28(1):1-10.
Crossref
|
|
|
|
|
Luciana MK, Jorge FS, Ferreira, Anne MZ, Carol M, David S, Lindsay, Ana CS, Chagas Alessandro FTA (2011). Caenorhabditis elegans as a model to screen plant extracts and compounds as natural anthelmintics for veterinary use. Vet. Parasitol. 182(2-4):264-268.
Crossref
|
|
|
|
|
Mantawy MM, Aly HF, Zayed N, Fahmy ZH (2012). Antioxidant and Schistosomicidal effect of Allium sativum and Allium cepa against Schistosoma mansoni different stages. Eur. Rev. Med. Pharmacol. Sci. 7:16.
|
|
|
|
|
Ranjitkar HD (1995). A Handbook of Practical Botany. Arun Kumar Ranjitkar, Kathmandu P 165.
|
|
|
|
|
Ross IA (2001). Medicinal Plants of the world: Chemical Constituents. Traditional and Modern Medicinal Uses. Humana Press, Totowa. 2:1-9
Crossref
|
|
|
|
|
Thoithi GN, Maingi N, Karume D, Gathumbi PK, Mwangi JW, Kibwage IO (2002). Anthelmitnic and other Pharmacological activities of the Root Bark Extract of Albizia anthelmintica Brongn. East and Central Afr. J. Pharm. Sci. 5:60-66.
|
|