ABSTRACT
An important chlorophyll-derived diterpenoid essential oil, phytol (PYT) bio-metabolite, phytanic acid (PA) has a number of pathophysiological contributions. The PA metabolism and its plasma levels associated phenomena are continuously being researched on. This study aims to look at the complete current scenario of PA. The findings suggest that PA has anti-diabetic, cytotoxic, anticancer and anti-teratogenic activities. Although PA-mediated Refsum’s Diseases (RD), Sjogren-Larsson Syndrome (SLS) and prostate cancer are still controversial; Zellweger's Disease Hyperpipecolic Academia (ZDHA), Rhizomelic Chondrodysplasia Punctata (RCDP), Leber Disease (LD) and oxidative stress leading to mitochondrial and cardiac complications are also evident. In conclusion, PA may be a good biomarker of some pathophysiological phenomena and can be used for medico-pharmaceutical functions.
Key words: Phytanic acid, phytol bio-metabolite, biomarkers, pathophysiological contributions.
Abbreviation:
ACSVL1/VLCS, Membrane-bound enzyme at the ER; AMACR, α-methylacyl-CoA racemase; ARD, adult Refsum’s disease; ERD, early Refsum’s disease; FAO, fatty acid oxidase; HDL, high density lipoproteins; IDDM, insulin dependent diabetes mellitus; IRD, infantile Refsum’s disease; LD, Leber disease; NALD, neonatal adrenoleucodystrophy; PA, phytanic acid; PPAR, proliferator-activated receptor; PYT, phytol; PAHX, phytanoyl-CoA 2-hydroxylase; RBC, red blood corpuscles; RCDP, rhizomelic chondrodysplasia punctata; ROS, reactive oxygen species; RXR, retinoid X receptor; SLS, Sjogren Larsson syndrome; ZDHA, Zellweger's disease hyperpipecolic academia; 3-MAA, 3-methyl-adipic acid; ADH, aldehyde dehydrogenase; AMP, adenosine mono-phosphate; ATP, adenosine tri-phosphate; AV, arterio-ventricular; CoA-SH, co-enzyme-A-thiol; CYP450, - cytochrome P-450; CVS, cardio vascular system; DNA, deoxy ribonucleic acid; ER, endoplasmic reticulum; FAD, flavin adenine di-nucleotide; FADH, reduced flavin adenine di-nucleotide; FALDH, fatty aldehyde dehydrogenase; FL, follicular lymphoma; GRP40, G-protein-coupled receptor-40; HACL1, 2-hydroxyphytanoyl-CoA lyase; LBL, large B-cell lymphoma; NAD, nicotinamide adenine dinucleotide; NAD+, oxidized nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; NADP, reduced nicotinamide adenine di-nucleotide phosphate; NADP+, oxidized nicotinamide adenine dinucleotide phosphate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; NHL, non-Hodgkin lymphoma; PDH, pristanal dehydrogenase; PHYH, phytanoyl-CoA α-hydroxylase; PHYH/PAHX, phytanoyl-CoA 2-hydrolase; PMP34, peroxisomal membrane protein-34; PXMP2, peroxisomal membrane protein-2; RAR, retinoic acid receptor; Res, retinyl esters; SCPx, sterol carrier protein; UDP, uridine di-phosphate.
Chemically, 3,7,11,15-tetramethylhexadecanoic acid (phytanic acid) is a phytol (PYT) degraded and saturated branched-chain fatty acid, which is a C20 oxygenated hydrocarbon. It is made up of a C16 backbone and 4 methyl (-CH3) groups. Phytanic acid (PA) is a good substrate for chemical synthesis and is found in both marine and terrestrial food chains (Islam et al., 2015).
From the earlier evidence, elevated serum PA levels are characterized by a number of childhood complications as well as the most well-known incident, Refsum’s Disease (RD). PA may be associated with the production of formic acid, cardiac complications, Sjogren-Larsson Syndrome (SLS), cytotoxicity, lymphomas (Islam et al., 2015) as well as neurological damages (Nagai, 2015) due to its elevated serum levels. The high plasma PA levels are also evident in Rhizomelic Chondrodysplasia Punctata (RCDP), chronic polyneuropathy, bilateral shortening of the proximal phalanges, ichthyosis, progressive ataxia and disarthria (Islam et al., 2015).
However, PA, due to its specificaction on receptors, retinoid X receptor (RXR) and proliferator-activated receptor–gamma (PPARγ), is now considered to have a number of beneficial physiological effects such as - cellular growth and differentiation, reproduction, embryonic development (Islam et al., 2015), management of type 2 (insulin dependent) diabetes (Che et al., 2013; Elmazar et al., 2013) and cholesterol lowering (Peter et al., 2014), respectively. In addition, there are also talks for PA mediated anti-teratogenic and mitochondrial membrane permeability (Islam et al., 2015) capabilities. Although PA interceded prostate cancer is controversial (Kataria et al., 2015), there is a notion for anti-mammary cancer potentials of some PA derivatives (Liska et al., 2012).
This study aims to make a comprehensive review on the chlorophyll-derived PYT bio-metabolite, PA.
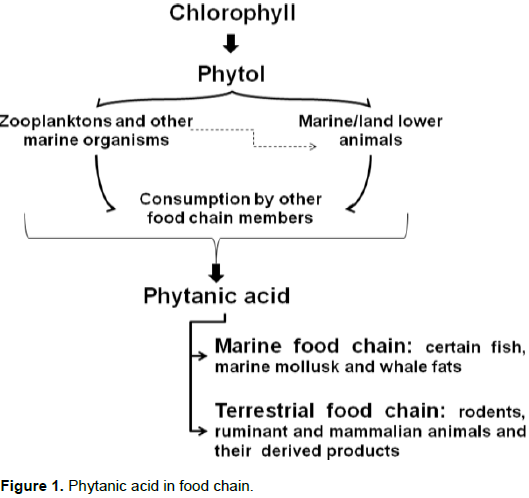
Sources and synthesis In rats, about 95% of intact chlorophyll passes through the digestive system, leaving only 5% of the PYT available for conversion to PA. PA is especially abundant in the contents of ruminant animals (cows and sheep) such as fat, liver, plasma, milk, butter, cheese, meat and rumen; although there is no evidence in poultry meat. However, PA is also present in pigs (non-ruminant). PA amount ranges from 0.01 to 0.3% of the total fatty acid pool, but can exceed the 10% mark in milk from cows fed with silage, the fermented grass that is used as winter-feed for cattle. High amounts were also detected in Antarctic krill (1.4%), plankton, mollusks, fish oil, whale oil and milk. In addition, high levels of PA are present in earthworms up to 3.5%. However, almost no PA is present in vegetables (Islam et al., 2015). PA in the food chain is shown in Figure 1.
PA is degraded by a process called α-oxidation, because a methyl group on its C3 position makes β-oxidation impossible and the hydroxylation of PA at C2 is the first step of α-oxidation. Pristanic acid (PRA) (2,6,10,14-tetramethylpentadecanoic acid) is the C19 α-oxidation product of PA. At first, by the help of phytanoyl-CoA hydroxylase, PYT converts to α-oxidation of phytanoyl-CoA. This results in 2-hydroxyphytanoyl-CoA, followed by shortening of one carbon to aldehyde, pristanal (2,6,10,14-tetramethylpentadecanal) and finally to PRA in liver and fibroblasts in humans (Wanders et al., 2011; Islam et al. 2015).
It is noted that PYT is converted into PA in the microsomes of ER, which is rich in fatty acid oxidation (FAO) enzymes. This is done by the subsequent action of alcohol and aldehyde dehydrogenase (ADH) enzymes, where acyl-CoA synthetase helps to get phytanoyl-CoA (a bona fide intermediate of PYT), then a reductase enzyme reduces it to phytanoyl-CoA. Phytanoyl-CoA is further broken down via the peroxisomal α-oxidation to PA. However, PA can be obtained after reduction of PYT to dihyro-PYT leading to phytanal. PA occurrence and metabolism pathways are depicted in Figure 2 (Wanders et al., 2011).
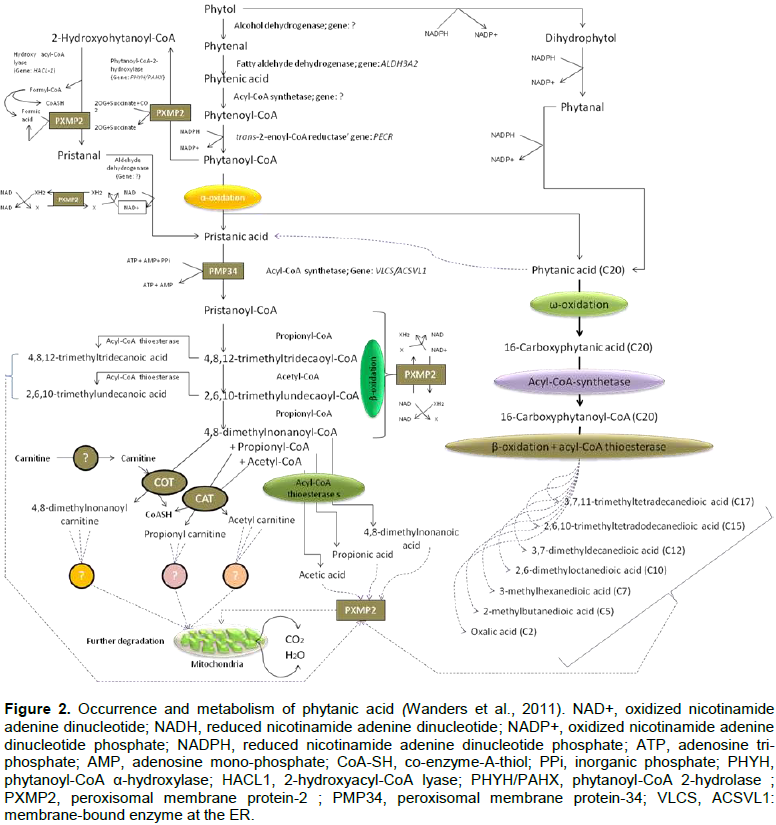
Once the α-oxidation product, PRA occurs, it is now converted to pristanoyl-CoA in peroxisomes. However, peroxisomal membrane is semi-permeable with compounds having molecular wt. 300 to 400 Da; thus allowing both of them through the porin PXMP2; whereas larger Mw compounds like phytanoyl-CoA require specific carrier proteins including PMP34 for ATP. Thus, both of them may act as starting materials. 2-hydroxyphytanoyl-CoA is the primate in the α-oxidation pathway followed by pristanal, PRA and pristanoyl-CoA. The latter is ready for subsequent β-oxidation in peroxisomes and mitochondria. It undergoes three cycles of β-oxidation in peroxisomes. In a study, fibroblasts from patients with a deficiency at the level of mitochondrial carnitine/acylcarnitine shows an accumulation of 4,8-dimethylnonanoylcarnitine. Finally, the end-products of pristanoyl-CoA β-oxidation are subsequently shuttled to mitochondria for further degradation following two distinct pathways: 1) carnitine-dependent and 2) carnitine-independent routes. Although the ultimate products in the former case are un-clear, CoA esters (acyl-CoA thioesterases) present in peroxisomes are thought to be responsible for various short and medium chain fatty acids (Wanders et al., 2011) (Figure 2).
Digestion and absorption
PA is nearly completely absorbed in the small intestine of ruminants and non-ruminants. Pancreatic lipase and phospholipases A2 and B in the digestive tract release PA from dietary triglycerides or other lipids, which are incorporated into mixed micelles. Micelles then transfer their contents into enterocytes, thus leading to PA incorporation into triglycerides and other lipids. They are exported with chylomicrons, just like other long-chain fatty acids (Wierzbicki et al., 1999).
Transport and cellular uptake
PA is transported with all major lipoproteins and enterscells through receptor-mediated uptake. There is a possibility of reverse transport from tissue deposits via high-density lipoproteins (HDL) (Wierzbicki et al., 1999).
Storage, excretion, and regulation
The triglycerides in adipose tissue are thought to be the major storage sites of PA and its oxidation product, PRA (Chambraud et al., 1999).
Excretion is still controversial, but it passes through the same means as the fatty acid intermediates occur (Chambraud et al., 1999).
Regulation may involve phytanoyl-CoA dioxygenase through intracellular signaling, since it is the specific target of the immunosuppressant binding protein immunophilin, FKBP52 (Chambraud et al., 1999).
Metabolism profile in human and/or other animals
According to Little et al. (2002), human UDP-glucuronosyl transferases (UGTs) are responsible for PA glucuronidation in the liver, stomach and intestinal microsomes. However, Gloerich et al. (2007) demonstrated that peroxisome proliferator-activated receptor-alpha (PPARα) is responsible for PYT metabolism to PA in mice (Watkins et al., 2010).
Ruminants and great captive apes/cohort of humans (e.g. chimpanzees, bonobos, gorillas, and orangutans) after the fermentation of ingested plant materials by gut microbes can liberate PA and accumulate in fats. Otherwise, the members of marine food chain can accumulate PA by ingesting zooplankton and/or krill, which are rich in PYT and chlorophyll-related precursors (Wanders et al., 2011).
PA can be obtained as RBC bound stuff (Watkins et al., 2010), which could serve as a biomarker for evaluating digestive health (Moser et al., 2013) and influence the functions of nervous, cardiovascular, and skeletal systems in human and great apes (Watkins et al., 2010).
PATHO-PHYSIOLOGIC CONTRIBUTIONS
Childhood complications
Infantile PA storage disease (IRD), ZDHA or NALD have been depicted with characteristic facial dysmorphism, sensorineural hearing loss, severe visual impairment, retinitis pigmentosa, hypotonia, hepatomegaly, and shunted growth at an elevated serum PA and PA-oxidase levels (Islam et al., 2015).
PA in Refsum’s disease (RD)
RD is termed PA storage disease categorized as Early Refsum’s Disease (ERD) and Adult Refsum’s Disease (ARD). It is more common phenomenon in elevated PA levels (Mukherji et al., 2002) where PA is accumulated in lipids of liver, kidney, muscle and urine of patients (Klenk and Kahlke 1963). RD (heredopathia atactica polyneuritiformis) was first described by Sigvald Refsum (neurologist), in 1946. The symptoms are characterized by retinitis pigmentosa, peripheral neuropathy, cerebellar ataxia and elevated protein concentrations in the cerebrospinal fluid in the absence of an increased number of cells. In addition, anosmia was found in nearly all RD patients; while deafness, ichthyosis shortened metacarpals or metatarsals, and cardiac arrhythmias were also regularly present (Wierzbicki, 2007). ARD is mainly characterized by defective α-oxidation (Wierzbicki, 2007) but normal Ω-oxidation at β-end of PA to 3-methyl-adipic acid (3-MAA), thus an increase in the clearance of PA (Wanders and Komen, 2007). This ω-oxidation pathway is NADPH (reduced nicotinamide adenine di-nucleotide phosphate); it is dependent and can be inhibited by imidazole derivatives (Komen et al., 2005) such as ketoconazole, clotrimazole, bifonazole, miconazole and CO (carbon monoxide) but these are the powerful inhibitors of cytochrome P-450 (CYP450); thus CYP450 may be involved in PA metabolism (Matsunaga et al., 1998). CYP4 (Xu et al., 2006) CYP4F3A, CYP4F3B, CYP4F2, and CYP4A11 are the important clearance mediator enzymes (Komen and Wanders, 2006). There is a methyl group at the β-position of PA, which hinders its α-oxidation. However, the urine of RD patients revealed the presence of 3-methyladipic acid and 3,6-dimethyl octanedioic acid, which are presumed intermediates due to PA ω-oxidation. This has increased the interest in the research of its ω-oxidation route. In a study done on mouse model, there was a possible pathway for ω-oxidation of PA where the gene encoding phytanoyl-CoA 2-hydroxylase was disrupted. Briefly, ω-oxidation of PA yields phytane-1,16-dioic acid upon activation which may be degraded by β-oxidation from the ω-end to produce the different acyl-CoA esters and corresponding free fatty acids after thioesterase-mediated cleavage of the CoA esters (Wanders et al., 2011) (Figure 2). Ultimate result is the corresponding short and medium chain fatty acids, which after further mitochondrial oxidation may produce water and carbon-di-oxide.
PA in cytotoxic and oxidative stress
A cytotoxic effect in astroglia cells was addressed to a combined action on Ca2+ regulation, mitochondrial depolarization, and increased reactive oxygen species (ROS) generation in the brain (Kahlert et al., 2005), where there is a decline of Ca2+ loading and severe release of CYPc (Schönfeld et al., 2006). Thus by apoptosis in mitochondrial route, it initiates cell death (Reiser et al., 2006). PA stimulates ROS generation by inactivating aconitase and oxidation of the mitochondrial glutathione pool, thus resulting in oxidative damage (Schönfeld and Reiser, 2006), the eventual characterization of the impairment of mitochondrial respiration and homeostasis (Busanello et al., 2013). According to Busanello et al. (2010), PA disrupts Na+K+-ATPase activity and the electron flow through the respiratory chain in the brain cortex of the PA disordered patients, resulting in neurological damage and raised body PA levels. Moreover, both the PA and PRA mediated Ca2+ deregulation can stimulate the free fatty acid receptor GPR40 (G-protein-coupled receptor), which is the signaling cascade of the toxicity of these two acids (Kruska and Reiser, 2011). Conversely, Nagai (2015) claimed that PA induces mitochondrial abnormality and cell death by the activation of Hdac2, 3 in Neuro2a cells, thus the neuronal damage in RD with associated peroxisomal disorders and the accumulation of PA in tissues and body fluids. However, the PA (>1 mM) in RD can perturb normal lipid homeostasis through PPARα gene leading to lipid degradation, which might explain the loss of adipose tissue in this category of patients (Gloerich et al., 2005).
PA in other phenomena
Along with RCDP, other well-known peroxisomal disorder, Leber Disease (LD) also has been reported with elevated PA levels in tissues and body fluids (Schönfeld, 2004). Later an elevated serum PA concentration was reported in an older patient (age 47 yrs) with poor nearsighted vision characterized by chronic polyneuropathy, bilateral shortening of the proximal phalanges, and ichthyosis (Yamamoto et al., 1995). A lofty plasma PA and PRA levels was also demonstrated in the progressive ataxia and disarthria (Clayton et al., 1996). Patients suffering from RD have the deficiency of PA α-hydroxylase namely phytanoyl-CoA hydroxylase (Zomer et al., 2000a) and more specifically phytanoyl-CoA2 hydroxylase (Wanders et al., 2011) in peroxisomes; on the other hand, the RCDP patients experience α-hydroxylation of PA as well as decarboxylation of α-hydroxy-PA deficiency (Pahan et al., 1996). Now, it is evident that transport and biochemical pathways of PA are mediated by phytanoyl-CoA 2-hydroxylase (PAHX) and 2-hydroxyphytanoyl-CoA lyase in peroxisomes (Wierzbicki et al., 2002). Thus, not only α-hydroxylation of PA but also decarboxylation of α-hydroxy-PA complications may raise plasma PA levels. According to Verhoeven et al. (1998), the toxic substance, formic acid is a decarboxylation product by the α-oxidation of PA.
PA in retinoid cells and type 2 diabetes (IDDM)
Now, it is evident that PA is a true physiological ligand for PPARα, PPARδ and PPARγ subtypes as well as the retinoid nuclear receptor, RXR (retinoid X receptor) (De Keyser, 2006). The oxidative metabolites of retinoic acid (RA) and all trans-forms of it are essential for cellular growth and differentiation, reproduction, and embryonic development (Arnhold et al., 2002). PA being the RXR-agonist stimulates the intestinal CYP26 gene (P450RAI) expression and metabolism of all-trans-RA in intestinal cells (Zomer et al., 2002b). In addition, PA by its stimulatory and influencing actions on nuclear receptors and gene expression can mediate cell differentiation especially adipocyte (both brown and white) in which high expression of phytanoyl-CoA hydroxylase occurs (Schluter et al., 2002). On the other hand, PA like thiazolidinedione drugs apparently stimulates the transcriptional activity of PPARs/RXR heterodimers, which is responsible for 2-deoxy-D-glucose uptake in hepatocytes in liver and metabolism. This suggests its potential role in the management of IDDM (Manuel, 2012). It may occur at no or inadequate insulin concentrations (Che et al., 2013); thus there is an indication for insulin resistance pathways by activation of nuclear receptors and heterodimerization of RXR with PPARγ (Elmazar et al., 2013).
PA as anti-teratogenic agent
Retinoids and their receptors are involved both in normal and abnormal embryonic development (Chambon, 1993). It is evident that all trans-RA and its natural precursor, retinol are teratogenic in a wider range of species (Lammer and Scott 1994). A research done on albino mice (matted female) demonstrated that PA greatly reduced the oxidative metabolism and teratogenic effects of retinol. As the ligand selective retinoid receptors, RAR (retinoic acid receptor) and RXR are required for efficient DNA binding and transactivation of target genes responsible for teratogenic effects, PA is evident for its action in this pathway (Arnhold et al., 2002). Tang et al. (2007). demonstrated that PA could increase the levels of retinyl esters (REs) in retinal cells and thus the inhibition of cell proliferation. However, a teratogenic potential was demonstrated by co-administration of a natural RXR ligand with a synthetic RAR agonist (Am580), thus this activity of PA is still contentious (Elmazar and Nau, 2004).
Hypo-/hyper-PA and their ultimate role in physiology
A low plasma PA (hypo-PA) level was claimed due to the deficiency of the enzyme, α-methylacyl-CoA racemase (Wierzbicki et al., 2002). Hypo-PA levels cause hypersensitization of mitochondria thus results in rapid permeability transition, which eventually increases membrane H+ conductance and disturbs the protein-linked functions in energy coupling; and finally a reduction of ATP supply is the consequence of de-energized mitochondria. In this short term PA toxicity, a depolarization occurs by the stimulation of non-phosphorylating oxygen uptake and inhibition of the reduction of tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (Schönfeld et al., 2004). Moreover, the nonesterified PA in the high (hyper-PA) tissue levels increases the mitochondrial inner membrane permeability by opening the permeability transition pore, and initiating the release of endogenous Mg++ along with increasing H+ conductance (protonophoric action); however it slightly stimulates the conductance of the inner membrane to K+ and Cl- (Komen et al., 2007).
PA in prostate and mammary cancers
Prostate cancer is substantiated in linking risk with polymorphisms in the α-methylacyl-CoA racemase (AMACR) gene and branched-chain fatty acids. PA may be one of them (Wright et al., 2012) but there is no causal link between circulating PA levels and the prostate cancer (Kataria et al. 2015). On the other hand, PA precursor (PYT) co-treatment with vitamin D analogue has been pointed out for anti-mammary cancer potential (Liska et al., 2012).
PA in cardiac complications
PA can be readily incorporated in phospolipid fraction of retinal cells in human (Young et al., 2011) and myocardial membranes in mice. The latter one is responsible for bradycardia and impaired AV nodal as well as intraventricular impulse conduction, which may lead to a sudden cardiac death (Monnig et al., 2004).
PA in other diseases
Patients suffering from SLS are characterized by ichthyosis, mental retardation and spastic diplegia or tetraplegia thought to be accumulation of long-chain aliphatic alcohols; it is evident that there is a deficiency in microsomal enzyme, FALDH that is the ultimate result of elevated PA levels in SLS patients. However, it is still controversial whether PA contributes to produce this disease or not (Willemsen et al., 2004). A vast intake of dairy fat can modify the plasma PA levels (Werner et al., 2011) and according to Ollberding et al. (2013), much consumption of beef milk rich in phytol is associated with increased risk of some lymphoma such as - large B-cell lymphoma (LBL), follicular lymphoma (FL) and non-Hodgkin lymphoma (NHL).
Miscellaneous
PA is evident for a synthetic aid of penicillin and cephalosporin derivatives mainly glutaryl-7-amino-cephalo PA (Xu, 2004) and some fatty acids (Rudolf, 2012). In addition, it has topical applicability (Vollhardt, 2010) and is used as a recovering agent for the byproduct methyl chloride of glyphosate acid in chemical industry (Ji, 2003), demonstrating PA as a versatile organic bio-metabolite and use thereof.
DEMONSTRATIVE CONJUNCTURES
Although there is no evidence that the PA content of normal diets affects human health, its elevated levels in body tissue fluids are coined for its contribution to RD, NHL, cardiac death, eurological damages, and few childhood complications. Still, PA contribution to the SLS patients is controversial. Moreover, hypo-PA level in body is evident for de-energization and eventually loss of mitochondrial homeostasis. The anti-teratogenic effects of PA are still controversial but there are evidences for its management of type 2 diabetes (Wanders et al., 2011; Islam et al., 2015).
As the methyl group at C3 position makes PA unable to β-oxidation difficulty, the α- and ω-oxidations are well known (Figure 3). Patients’ patho-physiology such as deficiency of metabolic enzymes or mutation in the genes of the enzymes are responsible for high plasma-PA levels of patients suffering from RD, SLS, RCDP and ZHDA(Wanders et al., 2011; Islam et al., 2015).
Thus, toxicity due to PA in body elevation may be a controversial and an advance research topic. It has been depicted that PA levels in body fluids may be considered as a biomarker for the patients’ benchmarking as PA complications including the digestive anomalies. Thus, phytanic acid may be an interesting bio-metabolite with medical and pharmaceutical considerations.
Phytanic acid may be considered as an important biomarker for some diseases such as RD, SLS, RCDP and ZDHA. However, having anti-diabetic, cytotoxic and anti-cancer, anti-teratogenic and cosmetic like activities makes it an interesting topic from medico-pharmaceutical view-point.
The authors have not declared any conflict of interests.
REFERENCES
|
Arnhold T, Elmazar MM, Nau H (2002). Prevention of vitamin A teratogenesis by phytol or phytanic acid results from reduced metabolism of retinol to the teratogenic metabolite, all-trans-retinoic acid. Toxicol. Sci. 66:274-282.
Crossref
|
|
|
|
Busanello EN, Amaral AU, Tonin AM, Zanatta A, Viegas CM, Vargas CR, Wajner M (2013). Disruption of mitochondrial homeostasis by phytanic acid in cerebellum of young rats. Cerebellum 12:362-369.
Crossref
|
|
|
|
|
Busanello EN, Viegas CM, Moura AP, Tonin AM, Grings M, Vargas CR, Wajner M (2010). In vitro evidence that phytanic acid compromises Na(+),K(+)-ATPase activity and the electron flow through the respiratory chain in brain cortex from young rats. Brain Res. 1352:231-238.
Crossref
|
|
|
|
|
Chambon P (1993). The molecular and genetic dissection of retinoid signaling pathway. Gene 135:223-228.
Crossref
|
|
|
|
|
Chambraud B, Radanyi C, Camonis JR, Rajkowski K, Schumacher M, Baulieu EE (1999). Immunophilins, Refsum disease, and lupus nephritis: The peroxisomal enzyme phytanoyl-CoA alpha-hydroxylase is a new FKBP-associated protein. Proceed. Nat. Acad. Sci. 96:2104-2109.
Crossref
|
|
|
|
|
Che BN, Oksbjerg N, Hellgren LI, Nielsen JH, Young JF (2013). Phytanic acid stimulates glucose uptake in a model of skeletal muscles, the primary porcine myotubes. Lipids Health Dis. 12:14.
Crossref
|
|
|
|
|
Clayton PT, Johnson AW, Mills KA, Lynes GW, Wilson J, Casteels M, Mannaerts G (1996). Ataxia associated with increased plasma concentrations of pristanic acid, phytanic acid and C27 bile acids but normal fibroblast branched-chain fatty acid oxidation. J. Inherit. Metabol. Dis. 19:761-768.
Crossref
|
|
|
|
|
De Keyser L (2006). Livestock products with an increased PPAR/RXR heterodimer activator level. US0167096.
|
|
|
|
|
Elmazar MM, El-Abhar HS, Schaalan MF, Farag NA (2013). Phytol/Phytanic acid and insulin resistance: potential role of phytanic acid proven by docking simulation and modulation of biochemical alterations. PLoS One 8:e45638.
Crossref
|
|
|
|
|
Elmazar MM, Nau H (2004). Potentiation of the teratogenic effects induced by coadministration of retinoic acid or phytanic acid/phytol with synthetic retinoid receptor ligands. Arch. Toxicol. 78:660-668.
Crossref
|
|
|
|
|
Gloerich J, van den Brink DM, Ruiter JP, van Vlies N, Vaz FM, Wanders RJ, Ferdinandusse S (2007). Metabolism of phytol to phytanic acid in the mouse, and the role of PPARalpha in its regulation. J. Lipid Res. 48:77-85.
Crossref
|
|
|
|
|
Gloerich J, van Vlies N, Jansen GA, Denis S, Ruiter JP, van Werkhoven MA, Duran M, Vaz FM, Wanders RJ, Ferdinandusse S (2005). A phytol-enriched diet induces changes in fatty acid metabolism in mice both via PPARalpha- dependent and -independent pathways. J. Lipid Res. 46:716-726.
Crossref
|
|
|
|
|
Islam MT, de Alencar MV, da Conceição Machado K, da Conceição Machado K, de Carvalho Melo-Cavalcante AA, de Sousa DP, de Freitas RM (2015). Phytol in a pharma-medico-stance. Chem. Biol. Interact. 240:60-73.
Crossref
|
|
|
|
|
Ji C (2003). Technique for cleanly recovering byproduct methyl chloride of glyphosate acid. CN1446782.
|
|
|
|
|
Kahlert S, Schönfeld P, Reiser G (2005). The Refsum disease marker phytanic acid, a branched chain fatty acid, affects Ca2+ homeostasis and mitochondria, and reduces cell viability in rat hippocampal astrocytes. Neurobiol. Dis. 18:110-118.
Crossref
|
|
|
|
|
Kataria Y, Wright M, Deaton RJ, Rueter EE, Rybicki BA, Moser AB, Ananthanrayanan V, Gann PH (2015). Dietary influences on tissue concentrations of phytanic acid and AMACR expression in the benign human prostate. Prostate 75:200-210.
Crossref
|
|
|
|
|
Klenk E, Kahlke W (1963). Über das Vorkommen der 3.7.11.15-Tetramethyl-hexadecansäure (Phytansäure) in den Cholesterinestern und anderen Lipoidfraktionen der Organe bei einem Krankheitsfall unbekannter Genese (Verdacht auf Heredopathia atactica polyneuritiformis [Refsum-Syndrom]). In: Hoppe Seylers Z Physiol Chem. 333: S133-139.
Crossref
|
|
|
|
|
Komen JC, Distelmaier F, Koopman WJ, Wanders RJ, Smeitink J, Willems PH (2007). Phytanic acid impairs mitochondrial respiration through protonophoric action. Cell. Mol. Life Sci. 64:3271-3281.
Crossref
|
|
|
|
|
Komen JC, Duran M, Wanders RJ (2005). Characterization of phytanic acid omega hydroxylation in human liver microsomes. Mol. Genet. Metabol. 85:190-195.
Crossref
|
|
|
|
|
Komen JC, Wanders RJ (2006). Identification of the cytochrome P450 enzymes responsible for the omega-hydroxylation of phytanic acid. FEBS Lett. 580:3794-3798.
Crossref
|
|
|
|
|
Kruska N, Reiser G (2011). Phytanic acid and pristanic acid, branched-chain fatty acids associated with Refsum disease and other inherited peroxisomal disorders, mediate intracellular Ca2+ signaling through activation of free fatty acid receptor GPR40. Neurobiol. Dis. 43:465-472.
Crossref
|
|
|
|
|
Liska J, Macejova D, Ondkova S, Brtko J (2012). Morphology of 1-methyl-1-nitrosourea induced rat mammary tumours after treatment with precursor of phytanic acid or its combination with vitamin D analogue. Endocrinol. Regul. 46:21-26.
Crossref
|
|
|
|
|
Little JM, Williams L, Xu J, Radominska-Pandya A (2002). Glucuronidation of the dietary fatty acids, phytanic acid and docosahexaenoic acid, by human UDP-glucuronosyltransferases. Drug Metabol. Disposition 30:531-533.
Crossref
|
|
|
|
|
Manuel CLJ (2012). Compositions rich in omega-3 fatty acids with a low content in phytanic acid. EP2429317.
|
|
|
|
|
Matsunaga I, Sumimoto T, Kusunose E, Ichihara K (1998). Phytanic acid alpha-hydroxylation by bacterial cytochrome P450. Lipids 33:1213-1216.
Crossref
|
|
|
|
|
Monnig G, Wiekowski J, Kirchhof P, Stypmann J, Plenz G, Fabritz L, Bruns HJ, Eckardt L, Assmann G, Haverkamp W, Breithardt G, Seedorf U (2004). Phytanic acid accumulation is associated with conduction delay and sudden cardiac death in sterol carrier protein-2/sterol carrier protein-x deficient mice. J. Cardiovasc. Electrophysiol. 15:1310-1316.
Crossref
|
|
|
|
|
Moser AB, Hey J, Dranchak PK, Karaman MW, Zhao J, Cox LA, Ryder OA, Hacia JG (2013). Diverse captive non-human primates with phytanic acid-deficient diets rich in plant products have substantial phytanic acid levels in their red blood cells. Lipids Health Dis. 12:10.
Crossref
|
|
|
|
|
Mukherji M, Kershaw NJ, Schofield CJ, Wierzbicki AS, Lloyd MD (2002). Utilization of sterol carrier protein-2 by phytanoyl-CoA 2-hydroxylase in the peroxisomal alpha oxidation of phytanic acid. Chem. Biol. 9:597-605.
Crossref
|
|
|
|
|
Nagai K (2015). Phytanic acid induces Neuro2a cell death via histone deacetylase activation and mitochondrial dysfunction. Neurotoxicol. Teratol. 48:33-39.
Crossref
|
|
|
|
|
Lammer EJ, Scott WJ (1994). Teratogenicity of vitamin A and retinoids. In vitamin A Health and Disease (R. Blomhoff, Ed.), Marcel Dekker, New York; pp. 615-664.
|
|
|
|
|
Ollberding NJ, Aschebrook-Kilfoy B, Caces DB, Wright ME, Weisenburger DD, Smith SM, Chiu BC (2013). Phytanic acid and the risk of non-Hodgkin lymphoma. Carcinogen. 34:170-175.
Crossref
|
|
|
|
|
Pahan K, Khan M, Singh I (1996). Phytanic acid oxidation: normal activation and transport yet defective alpha-hydroxylation of phytanic acid in peroxisomes from Refsum disease and rhizomelic chondrodysplasia punctata. J. Lipid Res. 37:1137-1143.
|
|
|
|
|
Peter O, Malin H, Rikard H (2014). Phytol as a cholesterol lowering agent. US073703.
|
|
|
|
|
Reiser G, Schönfeld P, Kahlert S (2006). Mechanism of toxicity of the branched-chain fatty acid phytanic acid, a marker of Refsum disease, in astrocytes involves mitochondrial impairment. Int. J. Dev. Neurosci. 24:113-122.
Crossref
|
|
|
|
|
Rudolf K (2012). Fatty acid fractionation process, fatty acid products and use thereof. EP2464240.
|
|
|
|
|
Schluter A, Giralt M, Iglesias R, Villarroya F (2002). Phytanic acid, but not pristanic acid, mediates the positive effects of phytol derivatives on brown adipocyte differentiation. FEBS Lett. 517:83-86.
Crossref
|
|
|
|
|
Schönfeld P, Kahlert S, Reiser G (2004). In brain mitochondria the branched-chain fatty acid phytanic acid impairs energy transduction and sensitizes for permeability transition. Biochem. J. 383:121-128.
Crossref
|
|
|
|
|
Schönfeld P, Kahlert S, Reiser G (2006). A study of the cytotoxicity of branched-chain phytanic acid with mitochondria and rat brain astrocytes. Exp. Gerontol. 41:688-696.
Crossref
|
|
|
|
|
Schönfeld P, Reiser G (2006). Rotenone-like action of the branched-chain phytanic acid induces oxidative stress in mitochondria. J. Biol. Chem. 281:7136-7142.
Crossref
|
|
|
|
|
Tang XH, Suh MJ, Li R, Gudas LJ (2007). Cell proliferation inhibition and alterations in retinol esterification induced by phytanic acid and docosahexaenoic acid. J. Lipid Res. 48:165-176.
Crossref
|
|
|
|
|
Verhoeven NM, Wanders RJ, Poll-The BT, Saudubray JM, Jakobs C (1998). The metabolism of phytanic acid and pristanic acid in man: a review. J. Inherit. Metabol. Dis. 21:697-728.
Crossref
|
|
|
|
|
Vollhardt JH (2010). Topical agents containing phytanic acid or a derivative thereof. CN101829028.
|
|
|
|
|
Wanders RJ, Komen JC (2007). Peroxisomes, Refsum's disease and the alpha- and omega-oxidation of phytanic acid. Biochem. Soc. Trans. 35:865-869.
Crossref
|
|
|
|
|
Wanders RJA, Komen J, Ferdinandusse S (2011). Phytanic acid metabolism in health and disease. Biochim. Biophys. Acta 1811:498-507.
Crossref
|
|
|
|
|
Watkins PA, Moser AB, Toomer CB, Steinberg SJ, Moser HW, Karaman MW, Ramaswamy K, Siegmund KD, Lee DR, Ely JJ, Ryder OA, Hacia JG (2010). Identification of differences in human and great ape phytanic acid metabolism that could influence gene expression profiles and physiological functions. BMC Physiol. 10:19.
Crossref
|
|
|
|
|
Werner LB, Hellgren LI, Raff M, Jensen SK, Petersen RA, Drachmann T, Tholstrup T (2011). Effect of dairy fat on plasma phytanic acid in healthy volunteers - a randomized controlled study. Lipids Health Dis. 10:95.
Crossref
|
|
|
|
|
Wierzbicki AS, Lloyd MD, Schofield CJ, Feher MD, Gibberd FB (2002). Refsum's disease: a peroxisomal disorder affecting phytanic acid alpha-oxidation. J. Neurochem. 80:727-735.
Crossref
|
|
|
|
|
Wierzbicki AS, Sankaralingam A, Lumb PJ, Hardman TC, Sidey MC, Gibberd FB (1999). Transport of phytanic acid on lipoproteins in Refsum disease. J. Inherit. Metabol. Dis. 22:29-36.
Crossref
|
|
|
|
|
Wierzbicki AS (2007). Peroxisomal disorders affecting phytanic acid alpha-oxidation: a review. Biochem. Soc. Trans. 35:881-886.
Crossref
|
|
|
|
|
Willemsen MA, Van Der Graaf M, Van Der Knaap MS, Heerschap A, Van Domburg PH, Gabreëls FJ, Rotteveel JJ (2004). MR imaging and proton MR spectroscopic studies in Sjögren-Larsson syndrome: characterization of the leukoencephalopathy. AJNR Am. J. Neuroradiol. 25:649-657.
|
|
|
|
|
Wright ME, Bowen P, Virtamo J, Albanes D, Gann PH (2012). Estimated phytanic acid intake and prostate cancer risk: a prospective cohort study. Int. J. Cancer 131:1396-1406.
Crossref
|
|
|
|
|
Xu F, Ng VY, Kroetz DL, de Montellano PR (2006). CYP4 isoform specificity in the omega-hydroxylation of phytanic acid, a potential route to elimination of the causative agent of Refsum's disease. J. Pharmacol. Exp. Ther. 318:835-839.
Crossref
|
|
|
|
|
Xu G (2004). Crystallizing method for 7-amino cephalo phytanic acid. CN1511956.
|
|
|
|
|
Yamamoto S, Onozu H, Yamada N, Hayasaka S, Watanabe A (1995). Mild retinal changes in a 47-year-old patient with phytanic acid storage disease. Ophthalmologica 209:251-255.
Crossref
|
|
|
|
|
Young SP, Johnson AW, Muller DP (2011). Effects of phytanic acid on the vitamin E status, lipid composition and physical properties of retinal cell membranes: implications for adult Refsum disease. Clin. Sci. 101:697-705.
Crossref
|
|
|
|
|
Zomer AW, Jansen GA, Van Der Burg B, Verhoeven NM, Jakobs C, Van Der Saag PT, Wanders RJ, Poll-The BT (2000a). Poll-The BT. Phytanoyl-CoA hydroxylase activity is induced by phytanic acid. Eur. J. Biochem. 267:4063-4067.
Crossref
|
|
|
|
|
Zomer AW, van Der Burg B, Jansen GA, Wanders RJ, Poll-The BT, van Der Saag PT (2000b). Pristanic acid and phytanic acid: naturally occurring ligands for the nuclear receptor peroxisome proliferator-activated receptor alpha. J. Lipid Res. 41:1801-1807.
|
|