ABSTRACT
Myelo-protective and haematopoietic effects of seed extract fractions (SEF) of Phoenix dactylifera were investigated in Wistar rats. The acute toxicity of the SEF were determined in mice (n=12). Wistar rats (n=45), aged 2 to 3 months and weighing 150 to 220 g were grouped into 9, labeled (A to I). Groups A to D were intraperitoneally-induced for myelo-suppression with 3 mg/kg bodyweight (b.wt) of cyclophosphamide for 7 days. Groups A to Horally received graded-doses of SEF (A = (SEF1)100, B = (SEF1) 200, C = (SEF2) 100, D = (SEF2) 200, E = (SEF1) 100, F = (SEF1) 200, G = (SEF2) 100, and H = (SEF2) 200 mg/kg b.wt) for 21 days. Group I served as control. Blood samples (2.0 ml) were collected from each rat on days 8 and 15 into tri-potassium ethylene diaminetetraacetic acid anticoagulant containers and analyzed using haematological auto analyzer (Sysmex KX-21N) following manufacturers guideline. Bone marrow was collected from myelo-suppressed groups (B, D) and normal groups (F, H) on days 15 and 22 into fetal calf serum for cell count. The acute toxicity test revealed an oral LD50 of 2000 mg/kg b.wt. The SEF revealed flavonoids, saponins, tannin, proteins, reducing sugars and steroids. On day 8, the myelo-suppressed and normal groups revealed dose-dependentnon significant increase (p > 0.05) in haemoglobin, haematocrit, RBC and total WBC compared to control. On day 15, the myelo-suppressed and normal groups revealed dose- and time-dependent significant increase (p<0.05) in haemoglobin, haematocrit, RBC and total WBC and significant decrease (p < 0.05) in bone marrow cells of group B compared to control. Day 22 revealed significant increase (p < 0.05) in bone marrow cells of groups B, D, F and H compared to control. The observed effects indicate myelo-protective and haemopoietic potentials of the SEF in Wistar rats.
Key words: Phoenix dactylifera, anaemia, graded-doses, myelo-protection, haematopoietic.
Abbreviation:
SD, Standard deviation; SEF, seed extract fraction; b.wt, body weight; Hb, haemoglobin; Hct, haematocrit; RBC, red blood cell; MCHC, mean cell haemoglobin concentration; MCH, mean cell haemoglobin; MCV, mean cell volume; TWBC, total white blood cell
Treatment of oncology patients with cytotoxic drugs affect haematopoietic cells, especially the granulocyte-macrophage progenitors (CFU-GM) which results to neutropenia (Ozkan et al., 2005). Neutropenia is a decrease in circulating neutrophil in the peripheral blood. It is essential to introduce means to provide myelo-protective effects (Nichols et al., 1994). Biological response modifiers have been synthesized to circumvent this haematopoietic toxicity by the cytotoxic drugs. The colony-stimulating factors and interleukins regulate the proliferation of tumor-killing T lymphocytes and so called natural killer cells by inducing cell viability (Kiss et al., 2004).
Apoptosis of haematopoietic progenitors exposed to DNA-damaging drugs or γ-irradiation is mediated by p53 (Alyasiri et al., 2011). Furthermore, p53 appears to be a key regulator of the proliferation of hematopoietic progenitors, as p53 status influences both long- and short-term repopulation following bone marrow transplantation. Other approaches such as employing herbal medicine with a view to myelo-protection have equally been pursued (Ufelle et al., 2011).
Phoenix dactylifera possess numerous medicinal properties and is used in the treatment of stroke, building up body weight, help in slowing ageing and in treatment of toothache (Biglari et al., 2008). The extract of the date have exhibited anti-diabetic, anti- inflammatory and anti-oxidant activity (Michael et al., 2013; Rahmani et al., 2014).
Most anti-neoplastic agents are known to cause myelo-suppression. It has also been demonstrated that various crude and fractions of Vitex doniana leaves extracts have myelo-protective activity in cyclophosphamide-induced myelo-toxicity (Ufelle et al., 2011). There is paucity of data on the myelo-protective and haematopoietic effects of SEF of Phoenix dactylifera. The aim of this study was to investigate the myelo-protective effects of SEF of P. dactylifera in myelo-suppressed and normal Wistar rats. The specific objectives were to determine the acute toxicity (LD50) of the seed extract in mice, fractionate the extract using column chromatography and Gas Chromatography Mass Spectrometry (GC-MS), haematological parameters and bone marrow count of the myelo-suppressed and normal Wistar rats after oral administration of SEF of P. dactylifera.
Collection of the plant materials
The fruits of P. dactylifera were bought from Daji market, Sokoto, Sokoto State, Nigeria. It was authenticated by a taxonomist in the Department of Plant Science and Biotechnology, University of Nigeria Nsukka Campus, Nigeria. A voucher specimen (UNH-M3) was kept in the herbarium unit for future reference.
Animal housing
Wistar rats (n=45) were purchased and housed in the Animal House of College of Medicine, University of Nigeria Enugu Campus. They were allowed to acclimatize for two weeks and fed with commercially available rat feed and have access to water and feed and have access to water and feed ad libitum.Wistar rats were handled in this study according to International guidelines for handling experimental animals by American Physiological Society (APS).
Preparation of extract
The seeds (150 g) were harvested from its fruits, shade-dried and grinded into fine power and then soaked in 2.5L of methanol for 48 h. Filtration was carried out using what man Number 1 filter paper. The filtrate was evaporated to dryness. The dried extract(18.75 g) was scrapped out of the stainless bowl giving a percentage yield of 12.5 %. Ten (10)grams of the extract was dissolved in 100 ml of distilled water to get a concentration of 100 mg/ml, ready for use.
Acute toxicity test: (median lethal dose, LD50)
This was performed on mice according to the procedure described by Lorke (1983). The LD50 was performed in two stages. In the first stage, 3 groups of 3 mice each were treated with 10, 100 and 1000 mg/kg b.wt of the extract and observed for number of deaths in 24 h. Based on the percentage survival rates, 4 mice were treated with 1500, 2000, 2500 and 3000 mg/kg b.wt of extract in the second stage and the number of deaths in 24 h recorded. The LD50 was calculated as the geometric mean of the highest non-lethal and the lowest lethal doses.
Column chromatography was performed according to the method described by Still et al. (1978).
Phytochemical analysis
Phytochemical analysis of seed extract fractions of P. dactylifera were done in the Department of Pharmacognosy, University of Nigeria, Nsukka, Nigeria with the method described by Ioan (1984). In general tests for the presence or absence of phytochemical compounds using the above methods involve the addition of an appropriate standard chemical agent to the extract in a test-tube and shaken vigorously or gently as the case may be. Gentle heat may sometimes be required.
Gas chromatography-mass spectrometry (GC-MS sample preparation)
.
Extract (0.02 g) was dissolved into 10.0 ml of methylene chloride in GC-MS sample vial. A screw cap and septa (red side facing out) was placed onto the sample vial.The sample vial was placed into the sample tray provided for GC-MS samples.The information requested for the sample was printed onto the log in sheet for the sample tray.GC-MS-QP 2010 PLUS SHIMADZU JAPAN was used to separate the methanol seeds extract of P. dactylifera after column chromatography and named the different compounds (Alon and Amirav, 2006).
Experimental design
The acute toxicity of the SEF were determined in mice (n=12). Wistar rats (n=45), aged 2 to 3 months and weighing 150 to 220 g were grouped into 9, labeled (A to I). Groups A to D were intraperitoneally-induced for myelo-suppression with 3 mg/kg bodyweight (b.wt) of cyclophosphamide for 7 days. Groups A to H orally received graded-doses of SEF (A = (SEF1)100, B = (SEF1) 200, C = (SEF2) 100, D = (SEF2) 200, E = (SEF1) 100, F = (SEF1) 200, G = (SEF2) 100, and H = (SEF2) 200 mg/kg b.wt) for 21 days. Group I served as control.
Sample collection
Blood samples (2.0 ml) were collected from each rat on days 8 and 15 into tri-potassium ethylene diaminetetraacetic acid anticoagulant containers for haematological analysis. Bone marrow was collected from myelo-suppressed groups (B, D) and normal groups (F, H) on days 15 and 22 into fetal calf serum for bone marrow cell count.
Haematological analysis
This was analyzed using haematological auto analyzer (Sysmex KX-21N) following manufacturers guideline.
Bone marrow cell count
Principle: The fetal calf serum makes the bone marrow cells non-adhesive and easy for visual count microscopically (Pawar et al., 2006).
Procedure
Bone marrow was harvested from the femur and suspended in
RPMI containing 2 ml of10% fetal calf serum (FCS).The bone marrow cell was counted in a haemocytometer using white blood cell dilution buffer (1% glacial acetic acid) in phosphate buffered saline (PBS) and expressed as total live cells per femur.
Statistical analysis
The data were subjected to descriptive statistics in statistical package for social science computer software version 20 using analysis of variance and student’s t-test at 95% confidence interval. Probability value of less than 0.05 was considered significant.
P. dactylifera possess numerous medicinal properties and is used in the treatment of many ailments (Onuh et al., 2012). The extract of date has been reported to possess anti-ulcer, hepato-protective, anti-diarrheal effects. Whereas the methanol and aqueous extract of the date have exhibited anti-inflammatory and anti-oxidant activity by significantly increasing the plasma levels of vitamin C, E and A (Zhang et al., 2013). Dates are being consumed in modern cultures for the pleasant flavor, odour, and their biting texture in addition to their
use for flavoring foods, beverages and medicine (Al-shahib and Marshall, 2003). The fruit is a natural source of folic acid, an important micronutrient and independent risk factor for cardiovascular disease because of its tannin content; it is used medicinally as a detersive and astringent in intestinal trouble. Due to paucity of data on the myelo-protective and haematopoietic properties of SEF of P. dactylifera, this study was designed to investigate the myelo-protective and haematopoietic effects of SEF of P. dactylifera in myelo-suppressed and normal Wistar rats.
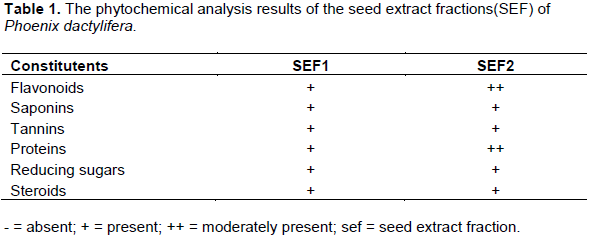
The acute toxicity test revealed an oral LD50 of 2000 mg/kg b.wt in mice. The observed high LD50 of the SEF indicate its safety for consumption. The phytochemical analysis of SEF revealed flavonoids, saponins, tannin, proteins, reducing sugars and steroids Table 1. This indicates the pharmacological potentials of SEF of P. dactylifera.
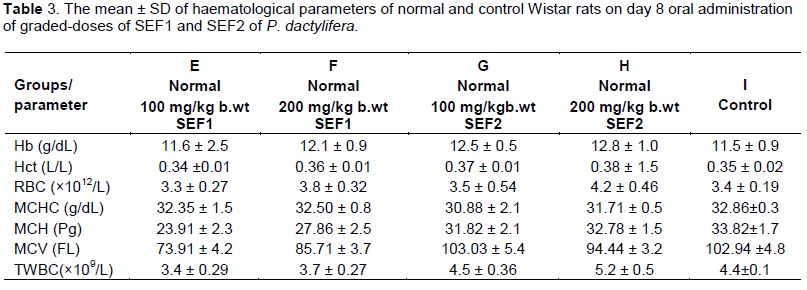
On day 8, the myelo-suppressed groups (A and B) that received graded-doses of SEF 1 revealed significant decrease in haemoglobin, haematocrit, RBC and total WBC when compared with the control. However, the myelo-suppressed groups (C and D) that received graded-doses of SEF 2 revealed dose-dependent increases compared to A and B but were lower than the control values Table 2. The progressive increases in the haematological parameters indicate myelo-protective effect which was more pronounced in SEF 2. The more pronounced effects of SEF 2 may be due to the differences in the concentrations and molecular weight to their chemical constituents. The SEF 2 revealed smaller molecular weight compounds than SEF 1. The observed lower values than the control might be due to the effect of cyclophosphamide that was used to induce myelo-suppression. The observed effects may also be attributed to the duration of SEF administration at the stage and time of sample collection which may not be enough to cause significant effects. The normal groups (E to H) revealed progressive increases in haemoglobin, haematocrit, RBC and total WBC which were not significant when compared with the control Table 3. This may also be attributed to the duration of SEF administration at the stage and time of sample collection which may not be enough to cause significant effects.

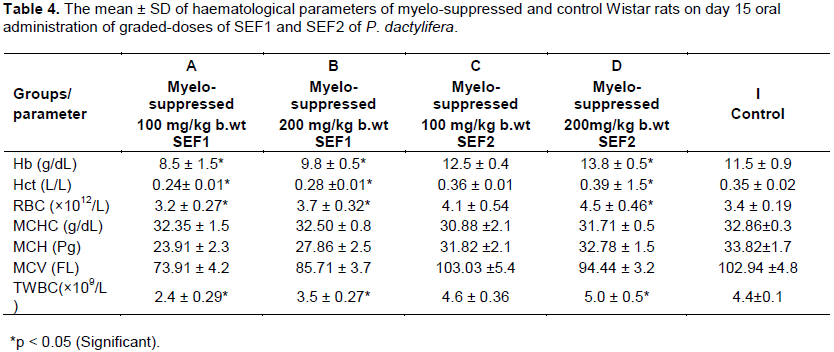
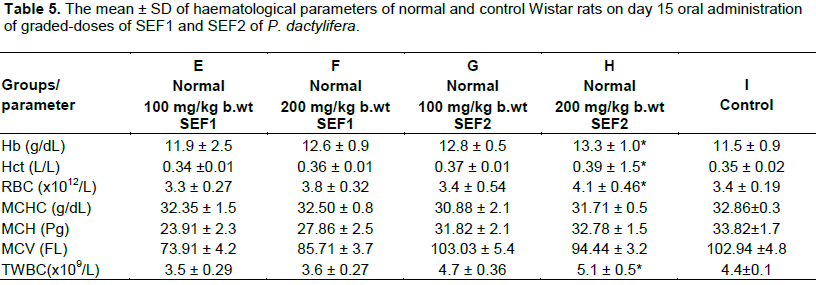
On day 15, the myelo-suppressed groups (A and B) revealed significant decrease in haemoglobin, haematocrit, RBC and total WBC when compared with the control. This might be that the constituents SEF could not correct the myelo-suppressive actions of the cyclophosphamide at this stage. However, the parameters recorded progressive increases at increasing dosage of SEF and when day 15 was compared with day 8. The observed effects were dose and time-dependent. This indicates myelo-protective action by the extract (Ragab et al, 2013). The haemoglobin, haematocrit, RBC and total WBC of the myelo-suppressed group C that received lower dose of SEF 2 was not significant but increased significantly in myelo-suppressed group D that received higher dose of SEF 2 Table 4.The SEF might have stimulated erythropoietin production for haematopoiesis as well as the immune system for the leucocytosis. Themyelo-protective effect manifests at increased concentration of SEF. The haemoglobin, haematocrit, RBC and total WBC of normal groups (E and F) that received graded-doses of SEF 1 and group G that received lower dose of SEF 2 were not significant but group H that received higher dose of SEF 2 increased significantly when compared with the control Table 5. The observed effects indicate haematopoietic potentials which was more noticeable in SEF 2. This may be due to the higher concentration and smaller molecular weight compounds contents of SEF 2.
On day 15, bone marrow cell count decreased significantly in group B rats that received SEF 1 when compared with the control. This might be due to duration of extract administration. On day 22, groups B, D, F and H revealed significant increase in bone marrow cell count when compared with the control. The bone marrow cell count of rats that received SEF 2 was higher than those that received SEF 1 Table 6. The observed effects indicate myelo-protective and haemopoietic potentials of the SEF. The SEF might be stimulating the liver to synthesize more erythropoietin to cause haematopoiesis. The observed effects were more consistent in SEF2 probably due to the smaller molecular weight compounds in SEF2 which may have easier penetration into the tissue to cause the observed effects. The GC-MS of SEF 1 and SEF 2 of Phoenix datiliferawith their Formulae, molecular weights and compound names are shown in Figures 1 to 12.
In conclusion, this study has demonstrated myelo-protective and haematopoietic properties by SEF as shown by the observed progressive increases in the parameters of both the myelo-suppressed and normal Wister rats.
The authors have not declared any conflict of interests.
REFERENCES
|
Alon T, Amirav A (2006)."Isotope Abundance Analysis Method and Software for Improved Sample Identification with the Supersonic GC-MS". Rapid Commun. Mass Spectrom. 20:2579-2588.
Crossref
|
|
|
|
Al-Shahib W, Marshall RJ (2003). The fruit of date palm: it's possible use as best food for the future. Int. J. Food Sci. Nutr. 54(4):237-259.
Crossref
|
|
|
|
|
Alyasiri NS, Mehdi SJ, Alam M.S, Ali A, Mandal AK, Gupta S, Singh I, Rizvi MMA (2011). PTEN mediated AKT activation contributes to the reduced apoptosis among Indian oral squamous cell carcinoma patients. J. Cancer Res. Clin. Oncol. 138:103-109.
Crossref
|
|
|
|
|
Biglari F, Abbas FM, Alkarkhi, Azhar ME (2008). Antioxidant activity and phenolic content of various date palms (Phoenix dactylifera) fruits from Iran. Food Chem. 107:1636-1641.
Crossref
|
|
|
|
|
Ioan C (1984). Methodology for Analysis of Vegetable Drugs. Faculty of Pharma,Eucharest Romania. pp. 20-30.
|
|
|
|
|
Kiss C, Benko I, Kovacs P (2004). Leukemic cells and the cytokine patchwork. Pediatr. Blood Cancer 42(2):113-21.
Crossref
|
|
|
|
|
Lorke DA (1983). A new approach to practical acute toxicity testing. Arch. Toxicol. 53:275-289.
Crossref
|
|
|
|
|
Michael HN, Salib JY, Eskander EF (2013). Bioactivity of diosmetin glycosides isolated from the epicarp of date fruits, Phoenix dactylifera, on the biochemical profile of alloxan diabetic male rats. Phytother. Res. 27:699-704.
Crossref
|
|
|
|
|
Nichols CR, Fox, EP, Roth BJ, Williams SD, Loehrer PJ, Einhorn LH (1994). Incidence of neutropenic fever in patients treated with standard – dose combination chemotherapy for small cell lung cancer and the cost impart of treatment with granulocyte colony stimulating factor. J. Clin. Oncol. 12(6):1245-1250.
|
|
|
|
|
Onuh SN, Ukaejiofor EO, Achukwu PU, Ufelle SA, Okwuosa CN, Chukwuka CJ (2012). Hematopoietic activity &effect of crude fruit extract of phoenix dactylifera on peripheral blood parameters. Int. J. Biol. Med. Res. 3(2):1720-1723.
|
|
|
|
|
Ozkan K, Turkkan E, Ender K, Mutlu D, Murat A, Nalan B, Abdulmecit Y, Osman M (2005). 5 Fluorouracil, epirubicin and cisplatin in the treatment of metastatic gastric carcinoma: a retrospective analysis of 68 Patients. Indian J. Cancer 42(2):85-88.
Crossref
|
|
|
|
|
Pawar RS, Jain AP, Kashaw SK, Singhai AK (2006). Haematopoietic activity of Asteracantha longifoliaon cyclophosphamide-induced bone marrow suppression. Indian J. Pharmacol. Sci. 68:337-340.
Crossref
|
|
|
|
|
Ragab AR, Elkablawy MA, Sheik BY, Baraka HN (2013). Antioxidant and tissue-protective studies on Ajwa extract: dates from Al Madinah Al-Monwarah, Saudia Arabia. J. Environ. Anal. Toxicol. 3:2161-0525.
|
|
|
|
|
Rahmani AH, Aly SM, Ali H, Babiker AY, Srikar S, Khan AA (2014). Therapeutic effects of date fruits (Phoenix dactylifera) in the prevention of diseases via modulation of anti-inflammatory, anti-oxidant and anti-tumour activity. Int. J. Clin. Exp. Med. 7(3):483-91.
|
|
|
|
|
Still WC, Kahn M, Mitra A (1978). Chromatography. J. Organ. Chem. 43(14):2923 -2925.
Crossref
|
|
|
|
|
Ufelle SA, Ukaejiofo EO, Ghasi S, Achukwu PU, Udeani TKC, Neboh EE (2011). Myelo-protective activity of aqueous and methanolic leaf extracts of vitexdoniana in cyclophosphamide-induced myelo-suppression in wistar rats. Int. J. Biol. Med. Res. 2(1):409-414.
|
|
|
|
|
Zhang CR, Aldosari SA, Vidyasagar PS, Nair KM, Nair MG (2013). Antioxidant and anti-inflammatory assays confirm bioactive compounds in Ajwa Date fruit. J Agric. Food Chem. 61:5834-5840.
Crossref
|
|