ABSTRACT
Nanoparticles are of current interest because of their emerging understanding effects on human health. Many developed nanoparticles were created based upon the current application. The application of nanoparticles in drug delivery of pharmaceuticals offers many advantages on the treatment strategies and therapy outcomes. Poly-beta-hydroxyl-butyrate (PHB) nanoparticles are very versatile and its design could be tailored to the needs of individual drug. Biodegradable polymeric PHB are able to provide controlled release of the encapsulated drug and it could be prolonged or enhanced. The emergence of PHB group of polymers as a potential cheap biomaterial may also become an interesting alternative in the production and controlled release manner. The Global Tuberculosis Report published by WHO revealed an increasing incidence of drug resistance and patients need to be treated for the period of 18 to 24 months using second line, anti- tuberculosis (TB) drug. Rifampicin, an anti tuberculosis drug, was chosen to incorporate in both types of nanoparticle production and it is delivered to the patients for wound healing (Tuberculosis patients) by microencapsulation. The nanoparticles were developed using single emulsion evaporation formed into the microspheres with regular or irregular morphology and the varying size efficiencies and particle size distributions. The microencapsulation of drug with PHB was coated onto the cotton gauze for wound healing. Physical and chemical analysis for he developed cotton gauze for wound healing.
Key words: Poly-beta-hydroxyl-butyrate (PHB) nanoparticles, cotton gauze, drug, tuberculosis.
During last decade, pharmaceutical companies and other scientists have carried out extensive research on drug delivery. Patients with pulmonary tuberculosis and extra pulmonary tuberculosis with local skin rashes such as pruritus, with or without erythema which does not cure completely with a single dose, but it needs prolonged treatment with antituberculosis drug Rifampicin. Rifampicin inhibits the gene transcription of mycobacteria by blocking the DNA-dependent RNA polymerase, which prevents the bacillus from synthesizing messenger RNA and protein, causing cell death. My aim of this study is to concentrate and design the cotton gauze with drug recommended to tuberculosis patients wound healing. The design of cotton gauze has two advantages, one with synthesis of natural polymer, poly-beta-hydroxyl-butyrate (PHB), as nanoparticles and next with encapsulation of drug with polymer and developed onto the cotton gauze. PHB extracted from Bacillus species, stored as a lipid storage under adverse conditions. The natural biopolymers-PHB that are synthesized and catabolized by microorganisms particularly bacteria (Witholt and Kessler, 2002; Akar et al., 2006; Berlanga et al., 2006). Many studies were carried out on development of PHB nanoparticles for drug delivery. In the research, the area of interest is in the simple biodegradable polymeric nanoparticles such as PHB which is selected and encapsulated with drug (rifampicin) developed onto cotton gauze, and is able to provide controlled release of encapsulated drug, consequently resulting in the prolonged or enhanced efficacy of the drug (Praveen et al., 2015). The emergence of PHB group of polymer as potential cheap biomaterial may also become an interesting alternative in the production of controlled release matter.
The research investigated the potential of prolonged nanoparticles from PHB copolymer, which is a type of nanoparticle; it also investigated the formulation for the development of nanoparticle and the production of coated nanoparticle by single emulsion evaporation. The study also revealed coating of developed nanoparticle onto the cotton gauze for wound healing. Tuberculosis (TB), is a ubiquitous high contagious chronic granulomas bacterial infection caused by Mycobacterium tuberculosis that infects over 8 million people worldwide and 2 million death annually (Pandey et al., 2003). According to WHO Post-2016, survey report on TB-patients revealed about 30 countries, with 10,000 infected patients per year. Out of 30 countries, 14 countries were top TB burden country listed as Angola, China, DPR Korea, DR Congo, Ethiopia, India, Indonesia, Kenya, Mozambique, Myanmar, Papua New Guinea, South Africa, Thailand, and Zimbabwe. Rifampicin (RIF), an anti tuberculosis drug was chosen to incorporate in nanoparticle encapsulation. RIF has low solubility and high permeability with high dose and is classified as class II drug in Biopharmaceutical Classification System (BCS). Rifampicin is the drug of choice for pre oral administration using nanoprecipitation technique. The nanoprecipitation method is also called solvent displacement or interfacial deposition where the drug solution in a water miscible organic solvent is mixed with an aqueous solution containing a surfactant. Upon mixing, the supersaturated solution leads to nucleation and size of drug particles, which may be stabilized by surfactant. The study also reveals that the characterisation of the nanoparticles and developed nanoparticles with drug on to the cotton gauze for wound healing. The developed cotton gauze can be applied to the local chronic wounds.
Isolation of PHB producing organisms from soil samples
Different soil samples were collected and from each soil samples, 1 g of soil sample was suspended in 9 ml sterile distilled water and shaken vigorously for 2 min. The diluted soil samples were heated at 60°C for 30 min in water bath. The liquid with the sample was serially diluted and plated on nutrient agar medium. The plates were incubated at 37°C for 24 to 48 h (Yilmaz et al., 2005). The isolated colonies were selected and subcultured on minimal agar medium for further studies (Cappuccino, 1992).
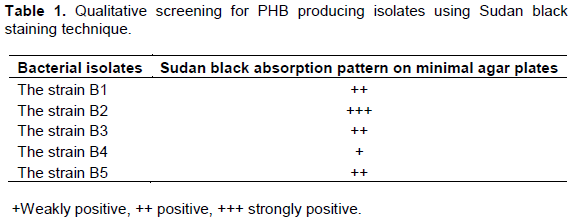
Qualitative screening for the production of PHB using Sudan black staining technique
As a qualitative screening, the production of PHB was determined by plate screening method, the Sudan black staining technique. The cultures were grown on the minimal media supplied with glucose (1%) as a sole carbon source for two days at 40°C. After incubation, the plates were flooded with 0.3% of Sudan black solution and kept undisturbed for 20 min. Solution was drained off. Viable colony staining technique was selected in order to reveal the different pattern of Sudan black absorption seen on the agar plates such as maximum and moderate absorption. PHB was extracted using chloroform extraction method (Mekala et al., 2011).
Development of PHB nanoparticles by nanoencapsulation method
PHB nanoparticles are created by nanoencapsulation method by using polycaprolactone (PCL) as biodegradable polyester with a low melting point of around 60°C.
Development of PHB nanoparticles without drug
About 1 g of PHB powder was dissolved in 5 ml chloroform and mix thoroughly to suspension of about 0.1% PCL was added and the mixture was heated with magnetic stirrer. About 100 ml of 0.1% sodium alginate was added to the mixture and stirred with magnetic stirrer for about 15 to 30 min. The preparation solution was loaded in a syringe and poured onto the beaker/plate containing about 1 mol calcium chloride solution. PHB nanoparticles are formed without drug.
Development of PHB nanoparticles with rifampicin
About 1 g of PHB was dissolved in chloroform (5 ml), suspended to 0.1% of PCL and heated with magnetic stirrer. 100 ml of 0.1% sodium alginate was added to the mixture and stirrer for 15 to 30 min. The prepared mixture was loaded in the syringe and sprayed on the plate containing 1 ml of calcium chloride. The same procedure was repeated for 0.2, 0.4, 0.6, and 0.8 mg concentrations and the PHB nanoparticles are encapsulated with rifampicin.
Development of PHB nanoparticles
About 1 g of PHB powder was mixed with 150 mg of propylene glycol and was dissolved in 5 ml chloroform and mixed separately. The dispersion was added to 10 ml of aqueous ethanol solution (70%). After 5 min, the mixture of organic solvents were removed by evaporation at 35°C under normal pressure and centrifuged at 10000 for 20 min. The supernatant were removed and pellet was washed with water and dried at room temperature, collect the dried powder for SEM image to observe nanoparticles.
Development of gauze with PHB nanoparticle without drug
A modified method of was used for polymer coating on cotton gauze surface. In brief, PHB (2 g) was dissolved in 0.5% v/v aqueous acetic acid solution by stirring for 1 h at 60°C. Sodium alginate (2.0 g) was added to the PHB solution and stirred for 10 min. The cotton gauze was dipped into the PHB-sodium alginate solution and kept for drying at 80°C for 5 min (Shanmugasundaram, 2012).
Development of cotton gauze with PHB nanoparticle with rifampicin
PHB solution was prepared by stirring a dispersion of PHB (2 g) in 0.5% (v/v) aqueous acetic acid solution for 1 h at 60°C and about 1 g of rifampicin drug and about 2 g of sodium alginate polymer was added to the PHB solution and stirred for 10 min. The gauze was dipped in the solution and dried in room temperature for 2 h.
Characterisation of developed PHB nanoparticles with rifampicin
Physical characterisation of developed PHB nanoparticles with rifampicin
The morphological appearance of developed PHB nanoparticles with rifampicin was observed by Scanning Electron Microscope. The samples were sonicated at 20 KHZ for 3 cycles of 5 min each. After sample preparation, the photographs of the sample were taken by Scanning Electron Microscope (Model-JEOL-6390 under the magnification 500 and 1300X, accelerating at the voltage 0.5 to 30 kV).
Chemical characterisation of developed PHB nanoparticles with rifampicin
Fourier transform infrared (FTIR) spectroscopy was a form of vibrational spectroscopy, the sample was irradiated with infrared radiation from an infrared source, and absorption of the radiation stimulates vibrational motions by depositing quanta of energy into vibrational modes. The changes in the vibration motion gave rise to bands in the vibrational spectrum; each spectral band was characterized by its frequency and amplitude (Sacksteder et al., 2001). The PHB, Rifampicin, and developed PHB nanoparticle with Rifampicin were subjected to FTIR spectroscopic analysis.
Characterisation of developed PHB nanoparticles with rifampicin on cotton gauze
Physical characterisation of developed PHB nanoparticles with rifampicin on cotton gauze
The morphological appearance of developed PHB nanoparticles with rifampicin coated cotton gauze was observed by Scanning Electron Microscope. The samples were sonicated at 20 KHZ for 3 cycles of 5 min each. After sample preparation, the photographs of the sample were taken by Scanning Electron Microscope (Model-JEOL-6390 under the magnification 500 and 1300X, accelerating at the voltage 0.5 to 30 kV).
Wash fastness
The samples were taken for about 6”×2” and treated by LAUNDER-O-METER. Staple multi-fiber test fabric along one edge of technical face of sample. Sample was set aside. About 150 ml of water and 0.225 g of detergent (0.15% w/w of liquor) were added to each canister. About 50 steel balls were added into canister. Blank gasket was placed into canister lid. Sample was pressed into lid and lid was closed. Canister was clamped. Then rotor was started and run for 2 min at 410°C to pre-heat the canister and the solution. Now the cover of one canister was unclamped. The samples were added to each canister in the row. After finishing, the row was re-clamped again. Rotor was manually turned to the next row. The process was repeated until all samples were loaded. Then canisters were removed and each sample contents were added to separate beaker. Each sample was rinsed 3 times each for a minute with de-ionized water, and excess water was removed. Sample was dried in oven (106°F or 71°C) for 1 h before evaluation (AATCC, 110106).
Thickness
The other physical properties of cotton gauze such as thickness were measured as per IS 7702-75.
Chemical characterisation of developed PHB nanoparticles with rifampicin on cotton gauze
The developed PHB nanoparticle coated on rifampicin cotton gauze was subjected to FTIR spectroscopic analysis. The IR spectrum of the sample represented the total chemical composition, because every chemical compound in the sample made its own distinct contribution to the absorbance spectrum. The distinction of an individual spectrum, which was determined by the chemical structure of each component and the degree to which each component contributes to the spectrum was directly related to the concentrations of the components of the sample. Spectra were recorded in 4000 to 400 cm-1 range.
The results and discussion focuses on the qualitative and quantitative screening of PHB from soil samples. PHB created as nanoparticle with the drug rifampicin was developed as an encapsulated drug with PHB onto the cotton gauze for wound healing. The physical and chemical characterisation before and after the developed cotton gauze was done by FTIR and SEM, which proves the bonding of encapsulated nanoparticles.
Isolation of PHB producing microorganisms from soil sample
All the five isolates from soil samples were observed under the direct dilution and plating on minimal agar supplemented with 2% glucose. The stored PHB granules are synthesized by the microorganisms, when the cell surroundings contain an unbalanced growth condition such as limited concentration of O, N, P, S, or trace elements, such as Mg, Ca, Fe and high carbon concentration (Lee, 1996; Sudesh et al., 2000).
Qualitative and quantitative screening for the production of PHB
Strains B1 to B5 were observed for the presence of lipophilic PHB granules. On comparison with all the 5 isolates, the strain B2 was observed to be more lipophilic as a dark gray colour on minimal media due to the absorption of Sudan black stain. Selection of strains was done based on the Sudan black absorption pattern (+++) by the PHB granules as tabulated in Table 1.Neema and Kumari (2013) reported the use of Sudan black B for screening of novel lipid producing isolates from secondary sludge and soil. They confirmed the greater value of dye and modified the procedure for demonstrating intracellular fatty material present in bacteria by preparing microscopic slides of bacteria stained with alcoholic Sudan black B solution.
Quantitative analysis
Extraction of PHB-chloroform by extraction method: The extraction of PHB by chloroform extraction method results in three phases. Three phases were obtained from the upper phase which consisted of hypochlorite solution was removed and the middle phase (chloroform containing undisturbed cells) was separated by filtration from the bottom phase (chloroform with PHB). The filtrate was subjected to chloroform evaporation at 100°C in the water bath and remain as PHB crystal (Mekala et al., 2011).
Development of PHB nanoparticles and nanoencapsulation with rifampicin
PHB nanoparticle was developed by emulsification-solvent evaporation and polymerization of nanoparticle as nanospheres. The dried PHB nanoparticles with rifampicin appeared in powdery form. Formation of nanospheres depends upon the initial PHB, alcohol and rifampicin concentration. The average particle size and increases in the particle size is most probably due to the presence of PHB encapsulated into nanospheres. More amount of PHB firmly absorbed and encapsulated with rifampicin increases the size of the nanoparticles.
Characterisation of developed PHB nanoparticles with rifampicin
The physical characterisation of developed PHB nanoparticles with rifampicin and control sample were done by Scanning Electron Microscopy (SEM) and chemical characteristics of developed PHB nanoparticles with rifampicin were investigated by FTIR analysis.
Physical characterisation of developed PHB nanoparticles with rifampicin on cotton gauze
The developed PHB nanoparticles and rifampicin onto the cotton gauze were subjected to SEM analysis. SEM was used to investigate the morphology of developed polymer and drug coated onto the cotton gauze under -5 to 300,000 magnification, accelerating 0.5 to 30 kv revealed the property as smooth, moderateuniformity. The size and increased number of the spherical structures influence the impact strength of the developed PHB nanoparticles with drug rifampicin. Cui et al. (2006) reported Scanning Electron Microphotograph of polymethacrylic acid nanoparticles containing lamivudine; the nanoparticles appear as a discrete spherical structure without aggregation in different magnification. The PLGA nanoparticles containing paclitaxel appears to be homogeneous, smooth, moderate uniformity, spherical in shape and did not cause aggregation of particles after lyophilization and these particles were readily redispersible which is confirmed by surface morphology studies reported by Ranjith et al. (2012). The magnification of the prepared cotton gauze with polymer revealed their presence and the efficiency of coating method. Under optimal condition, the magnification of the specimen will be appropriate and a high resolution image can be produced. The SEM image created with the used specimen at higher magnification can be a good evidence for the coated substance presence. The similar was reported by Gomes et al. (2010).The developed cotton gauze with rifampicin and PHB under SEM analysis is as shown in Plates 1, 2 and 3.
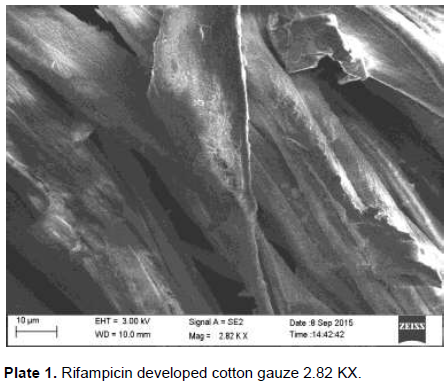
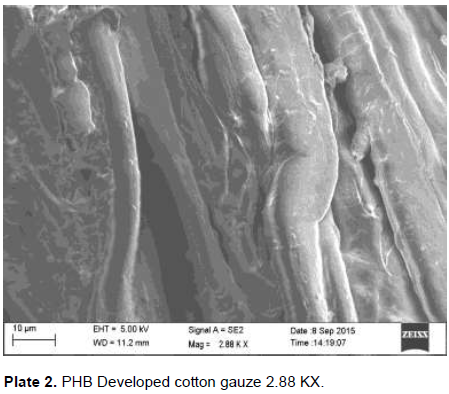
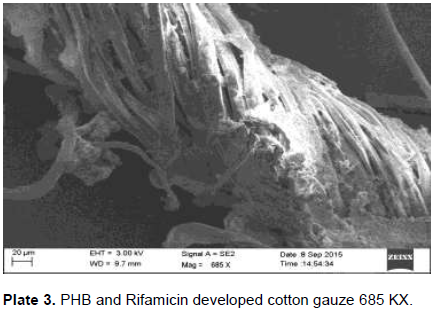
Thickness
Thickness observed for cotton gauze coated with PHB gauze, rifampicin gauze, PHB and rifampicin gauze revealed that dissolved polymer and the thickness of the polymer solution is slightly denser than the water consistency (Table 2). The area density was measured using GSM cutter method as per ASTM D3775. Thickness and stiffness of the fabric were measured as per ASTM D1777-96 and ASTM D6828 standard methods, respectively. The thickness of the cotton gauze after bonding with PHB and rifampicin is stronger (2.15 mm), when compared with the control gauze, it shows increased thickness and the thickness depends upon the bonding of the polymer to the cotton gauze.

Chemical characterisation of developed PHB nanoparticles with rifampicin
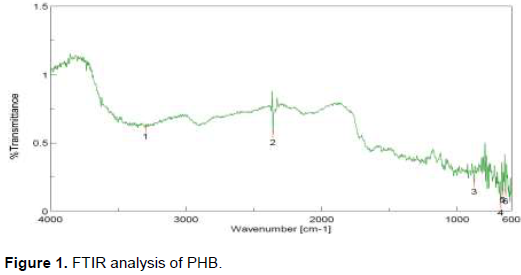
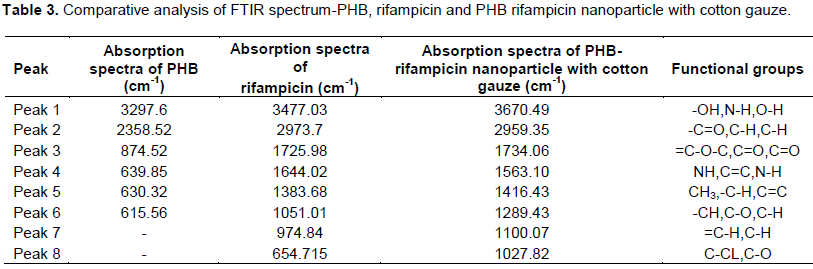
The crude P(3HB-CO-4HB) copolymer showed an intense absorption spectra at peak 1 (3297.6 cm-1) and revealed the presence of functional group –OH. The peak 2 (2358.52 cm-1) revealed the presence of functional group –C=O. The FTIR-spectra at 874.52 cm-1 (peak3) revealed the =C-O-C group. The absorption spectra at 639.85 cm-1 (peak 4) revealed the presence of functional group NH. The peak 5 at 630.32 cm-1 revealed the presence of functional group –CH3 and the peak 6 (15.56 cm-1) revealed the presence of –CH group. The FTIR analysis confirms the presence of functional group present in PHB (Figure 1).
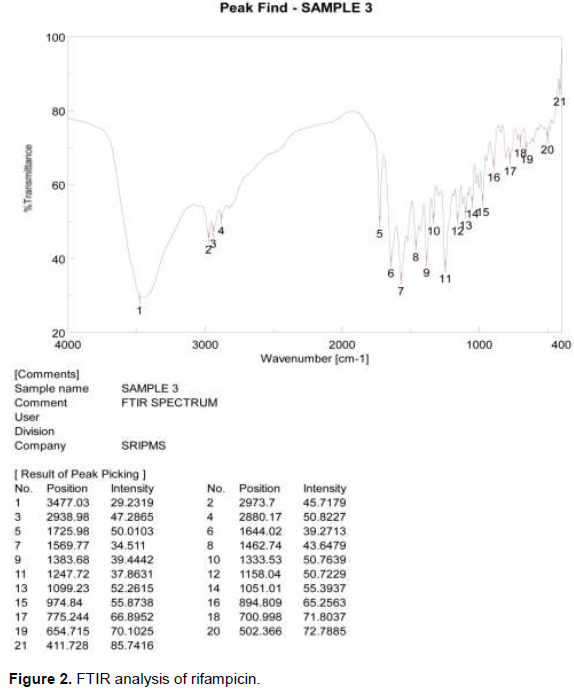
FTIR analysis of rifampicin
FTIR spectra predicted the presence of functional groups of rifampicin and are as shown in Figure 2. The FTIR spectrum of rifampicin reveals the absorption spectra at 3477.03 cm2 corresponding to N-H group. The absorption spectra absorbed at 2973.7 cm2 were attributed to C-H group. The absorption spectra at 1725.98 cm2 reveal the presence of functional group C=O. The absorption spectra at 1644.02 cm2 reveal the presence of functional group C=C. Similar absorption spectra were reported by Granja et al. (2004). Absorption spectra at 1383.68 cm2 reveals the functional group –C-H, absorption spectra at 1051.01 cm2 attribute the functional group C-O, functional group =C-H absorbed from the absorption spectra at 974.84 cm2, and functional group C-Cl absorbed from the absorption spectra at 654.715 cm2. Similar absorption spectrum was reported by Amirah et al. (2014). The FTIR analysis confirms the presence of functional group present in rifampicin.
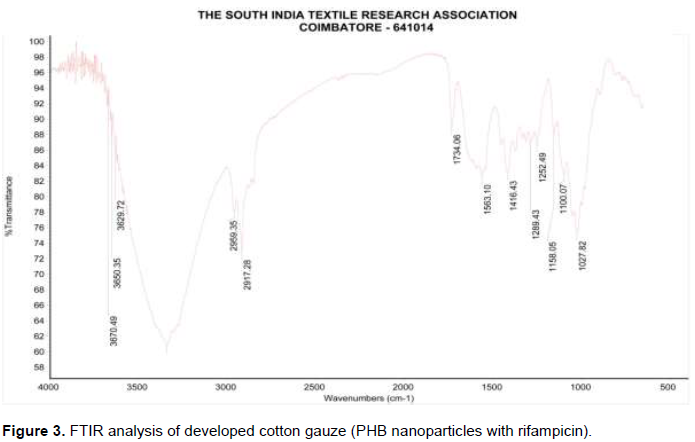
The FTIR spectrum of PHB and rifampicin on the developed PHB cotton gauze attributes several characteristic peaks at 3670.49 cm2 which reveals the presence of O-H group. Absorption stretching at 2959.35 cm2 attributes the presence of C-H group. Absorption spectrum absorbed at 1734.06 cm2 reveals the presence of C=O functional group. Absorption spectrum at 1563.10 cm2 attributes the presence of N-H functional group. Absorption spectrum at 1416.43 cm2 reveals the presence of C=C. Absorption spectrum at 1289.43 attributes the presence of C-H functional group. Stretching of C-H functional group reveals absorption spectrum at 1100.07 cm2. Absorption spectrum at 1027.82 cm2 reveals the presence of C-O functional group (Figure 3). The main peaks associated with P (3HB-co-4HB) copolymer could also be seen in the rifampicin-loaded nanoparticles spectrum, such as the absorption peaks at 1724 cm-1 and 1172 cm. Similar absorption spectrum was reported by Amirah et al. (2014). FTIR analysis confirms the presence of functional group of PHB and rifampicin present on the developed cotton gauze (Table 3).
In comparison, absorption spectra of PHB nanoparticle with rifampicin revealed that similar functional group was observed in the developed cotton gauze. FTIR spectra predicted the presence of functional groups of PHB, that is, aliphatic C-H, =O stretching, =C-H deformation, =C-H, =CH, ans =C-O. PHB and copolymers are known to contain these functional groups (Raveendran et al., 2011). FTIR spectra predicted the presence of functional groups of PHB (Mustafa et al., 2000), that is, aliphatic C-H, =O stretching, =C-H deformation, =C-H, =CH, and =C-O.
Wash fastness test
Colour fastness to washing was done for PHB and rifampicin coated cotton gauze to evaluate the reduction of colour.
The test result ensures the persistence of bio efficacy by bound polymer (PHB) with drug rifampicin to cotton
gauze after certain number of washes. To evaluate the durability of antibacterial effect and the persistence of coated polymers after washing, the developed cotton gauze were washed with AATCC standard reference detergent without bleaching agent (WOB). The samples were rinsed with warm water; air dried and tested further (Table 4).After completion of chemical and physical analysis, the developed cotton gauze was subjected to CAM test-Chorioallantoic membrane inoculation.
In conclusion, the novel PHB as a biopolymer from Bacillus species, has a huge potential to be developed as nanoparticles with rifampicin. Though, rifampicin has more advantages on oral administration, but this research focuses on developing the nanoparticle with drug and is applied on to the cotton gauze for skin wound dressing. The FTIR analysis reveals the presence of both the functional group of PHB and rifampicin onto the developed cotton gauze. The SEM image reveals the bonding of the development nanoparticles coated onto the cotton gauze. The physical test results such as thickness and wash fastness attribute the stability of the cotton gauze for wound dressing.
The authors have not declared any conflict of interests.
REFERENCES
|
Akar A, Akkaya EU, Yesiladali SK, Celikyilmaz G, Cokqor EU, Tamerler C (2006). Accumulation of polyhydroxyalkanoates by Microlunatus phosphovorus under various growth conditions. J. Ind. Microbiol. Biotechnol. 33:215-220.
Crossref
|
|
|
|
Amirah MG, Amirul AA, Habibah (2014). Formulation and Characterization of Rifampicin-loaded P (3HB-CO-4HB) Nanoparticle. Int. J. Pharma. Phar. Sci. 6(4):141-146.
|
|
|
|
|
Berlanga M, Montero MT, Fernandez-Borrell J, Guerrero R (2006). Rapid spectrofluorometric screening of poly-hydroxyalkanoate-producing bacteria from microbial mats. Int. Microbiol. 25(4):471-476.
|
|
|
|
|
Cappuccino JG, Sherman N (1992). Microbiology a laboratory manual. Third edition, the Benjamin/Cummings publishing company,Inc, Californis 3:127-178.
|
|
|
|
|
Cui F, Oian F, Yin C (2006). Preparation and characterization of mucoadhesive polymer- Coated nanoparticles. Int. J. Pharm. 31:154-161.
Crossref
|
|
|
|
|
Gomes AP, Mano A, Queirozand Gouveia IC (2010). Assessment of bacteria – Textile interaction using Scanning Electron Microcopy. A Study on LbL Chitosan/Alginate Coated cotton. In Microscopy: Science, Technology, Application and Education, Mendez – Vilas, A. and .Diaz (Eds.). Formatex Research Center, Badajoz, Spain. pp. 286-292.
|
|
|
|
|
Granja PL, Silva AIN, Borges JP, Barrias CC, Amaral IF (2004). Preparation and Characterization of Injectable Chitosan Hydroxyapatite Microspheres. J. Key Eng. Mater. pp. 254-256:573-6.
|
|
|
|
|
Lee Y (1996). Bacterial polyhydroxyalkanoates. J. Biotechnol. Biol. Eng. 49:1-14.
Crossref
|
|
|
|
|
Neema PM, Kumari A (2013). Isolation of lipid producing yeast and fungi from secondary sewage sludge and soil. Aus. J. Basic Appl. Sci. 7(9):283-288.
|
|
|
|
|
Mekala M, Rajendran R, Anjali PG, Kirthika S (2011). Isolation, identification, Extraction and optimization of Poly-Beta-Hydroxybutyrate producing Bacillus from soil using the cheap substrate jack fruit seed powder. Dev. Micro. Mole. Biol . Res. In pub. 2(2):85-92.
|
|
|
|
|
Praveen K, Parthasarathi K, Kulkarni A, Atul S (2015). Pharmaceutical application of nanoparticles in drug delivery system. J. Chem. Pharm. Res. 7(8): 703-712.
|
|
|
|
|
Ramsay JA, Berger E, Voyer R, Chavarile C, Ramsay BA (1994). Extraction of poly-3-hydroxy butyrate using chlorinated solvents. J. Biotechnol. Tech. 8:583-594.
|
|
|
|
|
Ranjith K, Gopal V, Aravind KG, Karthik A, Nasee M, Sreenivasa R (2012). PLGA 50:50 nanoparticles of paclitaxel: Development, in vitro anti-tumor activity in BT-549 cells and in vivo evaluation. J. Mater. Sci. 35(2):319-326.
|
|
|
|
|
Raveendran S, Balakrishnan A, Parameswaran B, Sreelatha KD, Ramachandran KB, Carlos R, Ashok P (2011). Production and Characterization of Poly-3-hydroxybutyrate from Crude Glycerol by Bacillus sphaericus NII 0838 and Improving Its Thermal Properties by Blending with Other Polymers. J. Braz. Arc. Biol. Technol. 54(4):783-794.
Crossref
|
|
|
|
|
Sacksteder C, Barry BA (2001). Fourier Transform Infrared Spectroscopy: A Molecular Approach to an Organism. J. Phycs. 37:197-199.
Crossref
|
|
|
|
|
Shanmugasundaram OL (2012). Development and characterization of cotton and organic cotton gauze fabric coated with biopolymer. Ind. J. Fibre. Tex. Res. 37:146-150.
|
|
|
|
|
Sudesh K, Abe H, Doi Y (2000). Synthesis, structure and properties of Polyhydroxyalkanoates: biological polyester. J. Poly. Sci. 25:1503-1555.
Crossref
|
|
|
|
|
Witholt B, Kessler B (2002). Perspectives of medium-chain length poly (hydroxyalkanotes), a versatile set of bacterial bioplastics. Curr. Opin. Biotechnol. 10:279-285.
Crossref
|
|
|
|
|
Yilmaz M, Soran H, Beyatli Y (2005). Determination of Poly-β-hydroxybutyrate (PHB) production by some Bacillus spp. Wld. J. Micro. Biotechnol. 21:565-566.
Crossref
|
|