ABSTRACT
This study set out to investigate the anti-inflammatory activity of Vangueria infausta, an edible wild fruit from Zimbabwe. The importance lies in the fact that this plant species could be developed as a low cost and effective therapeutic agent, with little or no side effects from natural sources. The fruit pulp of V. infausta was subjected to cold ethanol extraction to get crude extract. Flavonoids were isolated by thin layer chromatography (TLC) and unsaponifiable matter by liquid-liquid extraction using petroleum ether. The three were assayed by egg albumin denaturation and Nitric Oxide radical scavenging assays. Unsaponifiable fraction, crude ethanol extract and flavonoid fraction exhibited potent anti-inflammatory activity with a high of 93.12±0.03% (at 400 mg/L), 79.91±0.042% (at 800 mg/L) and 54.40±0.061% (at 800 mg/L), respectively in the egg albumin denaturation assay, whereas, in the nitric oxide (NO) radical scavenging assay it was respectively 68.99±0.058% (800 mg/L), 82.85±0.047% (at 800 mg/L) and 33.46±0.036% (at 800 mg/L). V. infausta crude extract and unsaponifiable fraction were superior to indomethacin and quercetin standards at lower concentrations in the egg albumin inhibition assay. This study shows that V. infausta possess potent anti-inflammatory phytochemicals that could be developed into anti-inflammatory drugs.
Key words: Vangueria infausta, unsaponifiable fraction, crude ethanol extract, flavonoids, anti-inflammatory activity, egg albumin assay, NO radical scavenging assay.
Inflammation is a pathophysiological response of living tissues to injury that leads to the local accumulation of plasmatic fluids and blood cells (Huang et al., 2011; Vazquez et al., 2011; Kandati et al., 2012). It involves a complex series of biochemical events closely related to pathogenesis of various ailments such as osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, migraine and acute gout (Huang et al., 2011; Vazquez et al., 2011; Kandati et al., 2012). It is usually characterized by redness, swollen joints and joint pain, stiffness and loss of joint function and can be acute or chronic inflammation (Kumar et al., 2013). The pathogenesis of many diseases and conditions, including several types of cancers, has been implicated in chronic inflammation (Moro et al., 2012). Currently, inflammation is being treated using non-steroidal anti-inflammatory drugs. These drugs, despite being used to treat this condition, are associated with undesirable side effects, including renal damage, hyperglycemia, hypertension, gastrointestinal ulceration and bleeding among others. In addition to the side effects, the greatest shortcomings of currently available potent synthetic drugs are their toxicity and resurfacing of symptoms after discontinuation (Bhagyasri et al., 2015). These modern pharmaceuticals are also out of reach of a large proportion of population in developing countries because they are expensive. It is against this background that the use of other sources of human knowledge be explored to provide common health benefits. Natural remedies to diseases are generally considered safe with little or no side effects. The screening of bioactive phytochemicals from plants has led to the discovery of new medicinal drugs with high efficacy in treatment and protection against diseases (Kumar et al., 2004; Sheeja and Kuttan, 2007). Vangueria infausta is an edible fruit that grows in the wild in Zimbabwe. It is commonly consumed among the rural folk who are normally marginalised from modern health delivery system. The tree flowers from September to November and fruits in the November to April period where the fruit is abundant. The aim of this project was to explore the potential of V. infausta, an edible wild fruit from Zimbabwe, as a possible source of anti-inflammatory agents and hence recommend its use for nutraceutical purposes.
Species collection
Ripe V. infausta fruits were collected from the Tsotsi Forest, in Insiza District of Bulawayo, Zimbabwe in January 2017. The fruits were identified by a worker at the National Herbarium of Zimbabwe, at the Harare Botanic gardens. The fruits are recorded under flora of Zimbabwe: individual record number 4211: V. infausta. The fruits were shade-dried for four weeks until constant weight was obtained. Pestle and mortar was used to grind the fruits to powder. Further grinding was done to reduce particle size using a grinding machine (Model: SM-45°C). The powder was stored in an air-tight plastic container until required for use.
Chemicals and standards
The standards used were indomethacin, an anti-inflammatory drug that was purchased from a local pharmacy and the flavonoid quercetin from Sigma Aldrich, South Africa. All other chemicals used were of analytical reagent grade and were also purchased from Sigma Aldrich, (South Africa) and Skylabs (South Africa).
Preparation of crude extract
15 g of powdered V. infausta sample were weighed using a Mettle Toledo digital analytical balance (model AB204-S, Ohio, USA) and mixed with 50 mL of analytical grade absolute ethanol in a 250 mL conical flask. This was done in triplicate and the samples were shaken for 30 min on a Labotec horizontal shaker (Midrand, South Africa). The samples were then filtered using Whatman No. 1 filter paper and placed in reagent bottles. The solvent maceration protocols were repeated three times and the collected filtrates were combined and concentrated under reduced pressure on a rotor vapour set at 40°C. This V. infausta sample is referred to as the crude ethanol extract in this study.
Extraction of the unsaponifiable fraction
The unsaponifiable fraction was extracted according to the method of Kovacs et al. (1979). 10 g of homogenized fruit samples were directly saponified in a round-bottom flask which had 25 mL of 50% KOH and 100 mL 95% ethanol. The mixture was refluxed for an hour with moderate stirring using a heating mantle and magnetic stirrer. The mixture was then cooled to room temperature and transferred to a separating funnel with the aid of 30 mL of 95% ethanol, 50 mL warm water and 50 mL cold water. The unsaponifiable fraction was extracted exhaustively 6 times with 150 mL portions of petroleum ether. The portions were then combined and washed with distilled water until soap-free and evaporated to dryness using a rotary evaporator at 40°C. The weight of concentrate was recorded as total unsaponifiable fraction (Jeong and Lachance, 2001). This sample of V. infausta is referred to as the unsaponifiable fraction in this study.
TLC isolation of flavonoids
Analytical thin-layer chromatography
This was done according to the method of Lihua et al. (2009) with some minor modifications. Thin layer chromatography (TLC) plates (10 x 1.5 cm) were activated by heating them at 100°C for about 10 min, and allowing them to cool to room temperature. Using a pencil and a ruler, pencil lines were drawn 1.5 cm from one edge of the plates. Extracts of samples were spotted on the pencil line using very thin capillary tubes. The plates were developed in a development chamber with a trial solvent. The solvent front was allowed to migrate up the TLC plate until it is about 1 cm from the top. The TLC plates were removed from the development chamber and the solvent front quickly marked with a pencil. They were air dried and then sprayed with 1% aluminium chloride solution, left to dry and then visualized under UV light at 365 nm. The positions of the flavonoids on the chromatograms were marked and captured on camera. The chloroform-methanol (10:1.25, v/v) gave the best separation of the spots.
Preparative thin-layer chromatography
Thick pre-coated silica gel plates measuring 20 cm x 20 cm were used. The solvent system used for the separation of phytochemicals was chloroform:methanol (10:1.25, v/v). Ethanol extracts of the fruit samples were deposited as a concentrated band 1.5 cm from the edge of the TLC plate and allowed to dry. The plates with dried samples were gently lowered into the development chamber, closed and left to develop. The plates were removed when the solvent had moved three quarters of the plates’ length and the position of the solvent front immediately marked with a pencil. The retention factor (Rf) values of the different bands were calculated using the equation:
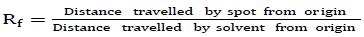
Using a previously reported method (Mittal, 2013) with some modifications the bands that tested positive for flavonoids in the analytical TLC were scratched off, combined together mixed with 5 ml of absolute ethanol, allowed to stand for 10 minutes, filtered with Whatman No.1 filter paper and filtrate collected in glass vials. This V. infausta sample is referred to as the flavonoid fraction in this study.
Preparation of standard solutions
Precisely 36.7 mg of quercetin were dissolved in 25 cm3 of methanol to form 1468 mg/L stock solution. This stock solution was serially diluted to give solutions of 800, 600, 400 and 200 mg/L. Similarly, 285.5 mg of indomethacin were also dissolved in 25 cm3 of methanol to make a stock solution of 11420 mg/L which was serially diluted to give solutions of 200, 150, 100 and 50 mg/L as well as the 800, 600, 400 and 200 mg/L solutions.
Preparation of sample solutions for anti-inflammatory assays
The recovered solutions of crude ethanol extracts, unsaponifiable fraction and flavonoids fraction were serially diluted to produce solutions of concentrations 800, 600, 400 and 200 mg/L of extract and assayed for anti-inflammatory activity.
Anti-inflammatory activity assays
Preparation of phosphate buffer saline
2.725 g of anhydrous sodium dihydrogen orthophosphate, 0.800 g disodium hydrogen orthophosphate and 22.500 g sodium chloride were weighed on a Mettler Toledo digital analytical balance (AB204-S, Ohio, USA) and dissolved in distilled water. The solution was diluted to the mark with distilled water in a 250 mL volumetric flask. The pH was adjusted to 7.4 using 0.1 N HCI or NaOH.
In vitro inhibition of egg albumin denaturation
The anti-inflammatory activity of V. infausta crude ethanol extract, unsaponifiable fraction and flavonoids fraction were determined in vitro for inhibition of denaturation of egg albumin (protein) according to the method of Mizushima and Kobayashi (1968) with some modifications. 0.2 mL of 1% egg albumin solution, 2 mL of sample extract or standard and 2.8 mL of phosphate buffered saline (pH 7.4) were mixed together to form a reaction mixture of total volume 5 mL. The control was made by mixing 2 mL of triple distilled water, 0.2 mL 1% egg albumin solution and 2.8 mL of phosphate buffered saline to make a total volume of 5 mL. The reaction mixtures were then incubated at 37±2°C for 30 min and heated in a water bath at 70±2°C for 15 min. After cooling, the absorbance was measured at 280 nm by UV/Vis spectrophotometer (Genesys10S, ThermoFisher Scientific Inc., USA) using triple distilled water as the blank. The percentage inhibition was calculated using the relationship:

Nitric oxide radical scavenging assay
This assay was done according to the method of Panda et al. (2009). The extracts were prepared and these were then serially diluted with distilled water to make concentrations from 200 to 800 mg/L. The freshly prepared solutions were refrigerated at 4°C for later use. Griess reagent was prepared by mixing equal amounts of 1% sulphanilamide in 2.5% phosphoric acid and 0.1% naphthylethylenediamine dihydrochloride in 2.5% phosphoric acid immediately before use. 0.5 mL of 10 mM sodium nitroprusside in phosphate buffered saline was mixed with 1 mL of the sample or standard in ethanol and incubated at 25°C for 180 min. The extract was mixed with an equal volume of freshly prepared Griess reagent. Control samples without the extracts or standard but with an equal volume of buffer were prepared in a similar manner as done in the test samples. The absorbance was measured at 546 nm using a Ultraviolet–visible (UV/Vis) spectrophotometer (Genesys10S, ThermoFisher Scientific Inc., USA) by using triple distilled water as blank. The percentage inhibition of the extract and standard was calculated and recorded. The percentage nitrite radical scavenging activity of the sample extracts or standard were calculated using the formula:

Statistical analysis
The results are expressed as mean ± standard deviation of three replicate measurements.
Table 1 shows the yield of crude ethanol extract, flavonoids fraction and unsaponifiable fraction of the V. infausta fruit.

Inhibition of egg albumin denaturation
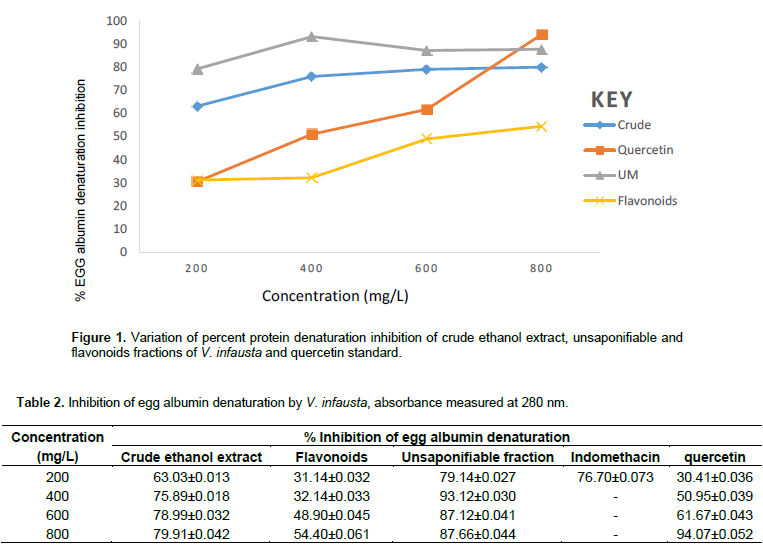
The results in Figure 1 and Table 2 show that percent protein (albumin) denaturation inhibition generally increases in a dose-dependent manner, rising gradually for the crude and flavonoid fractions of V. infausta as well as for the quercetin standard. However, for the unsaponifiable fraction percent inhibition rises steadily with concentration from 200 to 400 mg/mL, decrease steadily and becomes constant (Figure 1). The unsaponifiable fraction had the highest inhibition of heat induced protein (albumin) denaturation that varied from 79.14±0.027% at 200 mg/L to 93.12±0.03% at 400 mg/L, crude ethanol extract, had least percent inhibition of 63.03±0.013% at 200 mg/L and highest inhibition of 79.91±0.042% at 800 mg/L, flavonoids fraction had least percent inhibition of 31.14±0.032% at 200 mg/L and highest inhibition of 54.40±0.061% at 800 mg/L, standard quercetin had the least percent inhibition of 30.41±0.036% at 200 mg/L and highest inhibition of 94.04±0.052% at 800 mg/L and that of the standard indomethacin drug (not shown in Figure 1 since its concentration range was 50 to 200 mg/L) was 76.70±0.073% at 200 mg/L. Quercetin standard protein denaturation inhibitory activity surpasses that of the unsaponifiable fraction at high concentration, close to 800 mg/L (Figure 1). Crude ethanol extract had the second highest inhibition of egg albumin denaturation, which was, however, surpassed by that of standard quercetin around 700 mg/L. The flavonoids had the least percentage inhibition of egg albumin denaturation throughout the concentration range studied (200-800 mg/L).
NO radical scavenging activity
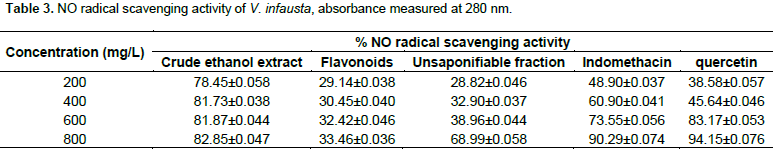
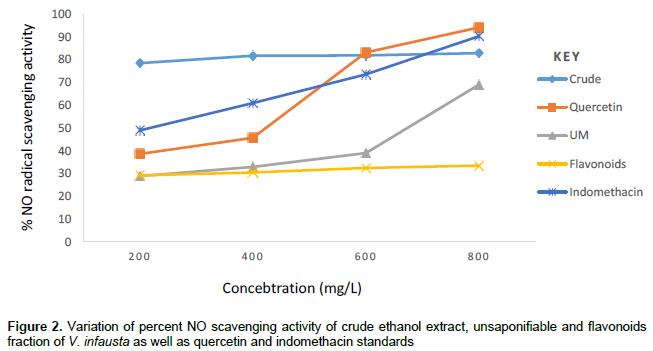
Table 3 illustrates that, crude ethanol extract of V. infausta had NO radical scavenging activity, varying from 78.45±0.058% at 200 mg/L to 82.85±0.047% at 800 mg/L, flavonoids fraction had mild NO radical scavenging activity varying from 29.14±0.038% at 200 mg/L to 33.46±0.036% at 800 mg/L, whereas, unsaponifiable fraction varied from 28.82±0.046% at 200 mg/L to 68.99±0.058% at 800 mg/L. Indomethacin (a drug used to treat inflammation) had NO radical scavenging activity that varied from 48.90±0.037% at 200 mg/L to 90.29±0.074% at 800 mg/L and quercetin standard (a typical flavonoid) ranged from 38.58±0.057% at 200 mg/L to 94.15±0.076% at 800 mg/L. Figure 2 shows that between 200 and 600mg/L, crude ethanol extract of V. infausta had the highest NO radical scavenging activity. Indomethacin has the second highest NO radical scavenging activity between 200 and about 500 mg/L, but surpasses that of crude ethanol extract of V. infausta at concentrations greater than 700 mg/L. However, above 500 mg/L quercetin standard NO radical scavenging activity surpasses that of indomethacin and at 600 mg/L it has almost the same radical scavenging activity as crude ethanol extract of V. infausta and surpasses it beyond this concentration. Interestingly, the unsaponifiable fraction of V. infausta, which had highest inhibition of protein (albumin) denaturation, has low nitric oxide (NO) radical scavenging activity which suddenly rises at high concentration beyond 600 mg/L. The flavonoids fraction of V. infausta has consistently low NO scavenging activity which seems to be constant throughout the concentration range studied (Figure 2).

Protein denaturation is the loss of biological functional properties of protein biomolecules. This protein denaturation is a well recorded cause of inflammatory and arthritic conditions such as osteoarthritis, spondylitis, cancer, rheumatoid arthritis, to name a few (Chandra et al., 2012; Stevens et al., 2005; Sangeetha and Vidhya, 2016). Auto-antigens produced in certain arthritic conditions are due to denaturation of proteins in vivo (Opie, 1962; Umapathy et al., 2010). Consequently, inhibiting protein denaturation may be helpful in preventing inflammatory conditions. Phytocompounds that prevent protein denaturation are therefore suitable targets for the development of anti-inflammatory drugs. The present study showed the in vitro anti-inflammatory activity (inhibition of protein denaturation) of V. infausta crude ethanol extract,as well as unsaponifiable and flavonoid fractions which were compared to quercetin and indomethacin standards. Figure 1 and Table 2 show that the crude and especially unsaponifiable fraction has very high inhibition of heat induced protein (albumin) denaturation over the range of concentrations studied (200 to 800 mg/L). The V. infausta flavonoids fraction also had significant anti-inflammatory activity (54.40±0.061%) but at high concentration (800 mg/L). The unsaponifiable fraction at 200 mg/L had inhibition (79.14±0.027%) that was comparable to indomethacin (76.70±0.073%). Inhibition of protein denaturation by quercetin standard was comparable to that of V. infausta unsaponifiable fraction and crude ethanol extract at concentrations above 700 mg/L (Figure 1). It is evident from this data that V. infausta contains phytocompounds that are suitable candidates for anti-inflammatory drug development. The unsaponifiable fraction is known to contain phytosterols, beta-carotenoids, tocopherols, hydrocarbons and terpenoids.
Terpenoids and steroids, the major constituents of the unsaponifiable fraction, are reported to possess anti-inflammatory activity (Perez, 2001). Terpenoids are reported to inhibit the development of chronic joint swelling (Bhagyasri et al., 2015). This could be the reason for the high protein denaturation inhibition of the unsaponifiable fraction of V. infausta. The likely constituents of phytocompounds in fruit of V. infausta are polyphenolic compounds, alkaloids, saponins, flavonoids, steroids and tannins. All these compounds are reported to have anti-inflammatory activity (Manach et al., 1996; Latha et al., 1998; Liu, 2003; Akindele and Adeyemi, 2007; Ilkay Orhan et al., 2007). Alkaloids containing pyridine ring system have striking anti-inflammatory activity, for example, berberine from berberis is used to treat rheumatisms (Bhagyasri et al., 2015). The in vitro anti-inflammatory activity of crude ethanol extract of V. infausta could be attributed to the synergistic effect of these compounds. Mbukwa et al. (2007) have isolated a number of flavonoids including quercetin from aerial parts of V. infausta. Flavonoids are believed to be the major anti-inflammatory agents in plant sources (Bhagyasri et al., 2015). Some are reported to act as phospholipase inhibitors and some act as TNF-α inhibitors in inflammatory conditions (Bhagyasri 2015). In addition, flavonoids are also known to inhibit cyclooxygenase and lipoxygenase pathways of arachidonic metabolism but this depends on their structure (Bhagyasri et al., 2015).
In this study, although standard quercetin showed a steady increase in anti-inflammatory activity with concentration, the cocktail of flavonoid fraction of V. infausta did not exhibit the same trend (Figure 1). This could be attributed to the fact that the flavonoids could be working synergistically against each other in anti-inflammatory activity or other factors which could be their chemical structures since mechanism of inhibition is dependent on structure as suggested by Bhagyasri et al. (2015). NO radical is a powerful pleiotropic link in physiological processes and a diffusible free radical in pathological conditions (Rintu et al., 2015). Nitric Oxide is produced in mammalian cells, and is responsible for various physiological processes, including fighting viruses and bacteria. However, excessive production of NO is associated with a number of ailments such as airway inflammation in asthma patients (Rao et al., 2016). The NO radical is known to react with superoxide radical anion (O2-) to form peroxynitrite (ONOO-), which is a cytotoxic molecule (Rintu et al., 2015). The protonated form of peroxynitrite, known as peroxynitrous acid (ONOOH) is a powerful oxidant (Malinski, 2007; Saumya et al., 2011). The damage caused by this powerful oxidant is through nitration or hydroxylation of aromatic compounds such as the amino acid tyrosine (Rintu et al., 2015). Peroxynitrite is reported to form an adduct with carbon dioxide dissolved in body fluids under physiologic conditions which causes oxidative damage to proteins in living systems (Sbazó et al., 2007).
In the Greiss assay, spontaneous decomposition of sodium nitroprusside in phosphate buffer generates NO radical which reacts with oxygen to form nitrite ions which are then estimated by UV-Vis after reaction with Greiss reagent. In the present study nitrite produced in the reaction mixture was reduced by V. infausta crude ethanol extract, the unsaponifiable fraction and the flavonoid fraction. This is due to anti-inflammatory phytocompounds which compete with oxygen to react with nitric oxide (Lalenti et al., 1993). The anti-inflammatory potency of V. infausta was evaluated for its NO radical scavenging activity. The NO radical scavenging activity of V. infausta crude ethanol extract was consistently high and constant varying from 78.45±0.058% at 200 mg/L to 82.85±0.047% at 800 mg/L (Figure 2). The crude ethanol extract is a cocktail of different phytocompounds, which are likely working synergistically to scavenge for the NO radical. The unsaponifiable fraction of V. infausta had low NO radical scavenging activity at concentrations varying from 28.82±0.046% at 200 mg/L to 38.96±0.059% at 600 mg/L but rising sharply to 68.99 ± 0.058% at 800 mg/L (Figure 2). This could be due to the fact that phytocompounds that act by NO radical scavenging mechanism increase with increase in extract concentration.
The NO radical scavenging assay of flavonoid fraction of V. infausta was consistently low and constant throughout the concentration range studied, varying from 29.14±0.038% at 200 mg/L to 33.46±0.036% at 800 mg/L. Both the quercetin standard and the indomethacin standard showed a gradual increase in NO radical scavenging activity with increase in standard concentration. The radical scavenging activity of V. infausta crude ethanol extract is superior to each of the standards over a wide concentration range studied (200 to 600 mg/L for quercetin, and 200 to 700mg/L for indomethacin). This signifies that V. infausta has anti-inflammatory phytocompounds that could be drug targets for further development to anti-inflammatory drugs. Although quercetin (a flavonoid standard) exhibits potent anti-inflammatory activity in both assays (Figures 1 and 2), it would be expected that the flavonoid extract of V. infausta exhibit the same or even better anti-inflammatory activity. It could be important to isolate and test anti-inflammatory activity of individual flavonoids. The result shown by flavonoids might imply that the anti-inflammatory activity exhibited by V. infausta is not due to one type of phytochemical only but a combination of them working in synergy. The result of the V. infausta unsaponifiable fraction in both assays reveals that the phytocompounds mostly exhibit inhibition of protein denaturation mechanism rather than NO radical scavenging activity mechanism. The crude ethanol extracts likely exhibit both mechanisms.
The study has shown that the crude ethanol extract and unsaponifiable fraction of V. infausta has significant anti-inflammatory activity as assessed by two assays, inhibition of heat induced protein denaturation and NO radical scavenging activity. Isolated flavonoids also show acceptable anti-inflammatory activity especially with the inhibition of protein denaturation assay at high concentrations. A comparison of anti-inflammatory activity of V. infausta and the standards quercetin (a flavonoid) and indomethacin (drug used to relieve inflammation), in the inhibition of protein denaturation, shows that unsaponifiable fraction and the crude ethanol extracts has potent anti-inflammatory phytochemicals that could be developed to anti-inflammatory drugs. The phytochemicals are effective especially at lower concentrations. The yields of unsaponifiable fraction, crude ethanol extracts and flavonoids (Table 1) show that it could be economically viable to extract anti-inflammatory phytocompounds from V. infausta.
The authors would like to express their sincere gratitude to the Bindura University of Science Education Research Board for providing funds for purchasing chemicals.
REFERENCES
|
Akindele AJ, Adeyemi OO (2007). Anti-inflammatory activity of aqueous leaf extract of Byrsocarpus coccineus. Fitoterapia 78:25-28.
Crossref
|
|
|
|
Bhagyasri Y, Lavakumar V, Divya Sree MS, Ashok Kumar CK (2015). An overview on anti-inflammatory activity of Indian herbal plants. Int. J. Res. Pharm. Nano Sci. 4(1):1-9.
|
|
|
|
|
Chandra S, Chatterjee P, Dey P, Bhattacharya S (2012). Evaluation of in vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pac. J. Trop. Biomed. 2(1):S178-S180.
Crossref
|
|
|
|
|
Huang N, Rizshsky L, Hauck C, Nikolau BJ, Murphy PA, Birt DF (2011). Identification of anti-inflammatory constituents in Hypericum perforatum and Hypericum gentianoides extracts using RAW 264.7 mouse macrophages. Phytochemistry 72:2015-2023.
Crossref
|
|
|
|
|
Jeong WS, Lachance PA (2001). Phytosterols and fatty acids in fig (Ficus carica, var. mission) fruit and tree components. J. Food Sci. 66(2):278-281.
Crossref
|
|
|
|
|
Kandati V, Govardhan P, Reddy CS, Ravinder Nath A, Reddy RR (2012). In vitro and in vivo anti-inflammatory activity of Syzygium alternifolium (wt) Walp. J. Med. Plants Res. 6:4995-5001.
Crossref
|
|
|
|
|
Kovacs MIP, Anderson WE, Ackman RG (1979). A simple method for the determination of cholesterol and some plant sterols in fishery-based food products. J. Food Sci. 44(5):1299-1305.
Crossref
|
|
|
|
|
Kumar BS, Saran GS, Mouna A, Kumar CN (2013). In vitro anti-inflammatory activity of Tankana churna. Food Feed Res. 40(1):17-20.
|
|
|
|
|
Kumar RA, Sridevi K, Kumar NV, Nanduri S, Rajagopal S (2004). Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 92(2 3):291-295.
|
|
|
|
|
Lalenti A, Moncada S, Di Rosa M (1993). Modulation of adjuvant arthritis by endogenous nitric oxide. Brit. J. Pharmacol. 110:701-706.
Crossref
|
|
|
|
|
Latha RM, Geetha T, Varalakshmi P (1998). Effect of Vernonia cinerea less flower extract in adjuvant-induced arthritis. Gen Pharmacol. 31:601-606.
Crossref
|
|
|
|
|
Lihua G, Tao W, Zhengtao W (2009). TLC bioautography-guided isolation of antioxidants from fruit of Perilla frutescens var. acuta. LWT- Food Sci. Technol. 42:131-136.
|
|
|
|
|
Liu RH (2003). Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 78:517S-520S.
Crossref
|
|
|
|
|
Malinski T (2007). Nitric oxide and nitroxidative stress in Alzheimer's disease. J. Alzheimer's Dis. 11:207-218.
Crossref
|
|
|
|
|
Manach C, Regerat F, Texeir O (1996). Bioavailability, metabolism and physiological impact of 4-oxo-flavonoids. Nutr. Res. 16:517-544.
Crossref
|
|
|
|
|
Mbukwa E, Chacha M, Majinda RRT (2007). Phytochemical constituents of Vangueria infausta: Their radical scavenging and anti-microbial activities. Arkivoc 9:104-112.
|
|
|
|
|
Mittal S (2013). Thin Layer chromatography and high pressure liquid chromatography profiling of plant extracts of Viola Ordorata Linn. Int. J. Pharm. Biol. Sci. 4(1)(B):542-549.
|
|
|
|
|
Mizushima Y, Kobayashi M (1968). Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J. Pharm. Pharmacol. 20:169 -173.
Crossref
|
|
|
|
|
Moro C, Palacios I, Lozano M, D'Arrigo M, Guillamón E, Villares A (2012). Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 130:350-355.
Crossref
|
|
|
|
|
Opie EL (1962). On the relationship between necrosis and inflammation to denaturation of protein. J. Exp. Med. 115:597-608.
Crossref
|
|
|
|
|
Orhan I, Kupeli E, Sener B, Yesilada E (2007). Appraisal of anti-inflammatory potential of clubmoss, Lycopodium clavatum L. J. Ethnopharmacol. 109:146-150.
Crossref
|
|
|
|
|
Panda BN, Raj AB, Shrivastava NR, Prathani AR (2009). The evaluation of nitric oxide scavenging activity of Acalyphaindica Linn Root. Asian J. Res. Chem. 2(2):148-150.
|
|
|
|
|
Perez RMG (2001). Anti-inflammatory activity of compounds isolated from plants. Review article. Sci. World J. 1:713-784.
Crossref
|
|
|
|
|
Rao USM, Ahmad BA, Mohd KS (2016). In vitro nitric oxide scavenging and anti-inflammatory activities of different solvent extracts of various parts of Musa paradisiaca. Malaysian J. Anal. Sci. 20(5):1191-1202.
Crossref
|
|
|
|
|
Rintu D, Shinjini M, Kaustab M, Pramathadhip P, Umesh PS, Banerjee ER (2015). Antioxidant and anti-inflammatory activities of different varieties of Piper leaf extracts (Piper betle L.). J Nutr. Food Sci. 5(5):1-15.
|
|
|
|
|
Sangeetha G, Vidhya R (2016). In vitro anti-inflammatory activity of different parts of Pedalium murex (L.). Int. J. Herb. Med. 4(3):31-36.
|
|
|
|
|
Saumya SM, Mahaboob BP, Basha P (2011). In vitro evaluation of free radical scavenging activities of Panax ginseng and Lagertroemia speciosa: a comparative analysis. Int. J. Pharm. Sci. 3:165-169.
|
|
|
|
|
Sbazó C, Ischiropoulos H, Radi R (2007). Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 6:662-680.
Crossref
|
|
|
|
|
Sheeja K, Kuttan G (2007). Activation of cytotoxic T lymphocyte responses and attenuation of tumor growth in vivo by Andrographis paniculata extract and Andrographolide. Immunopharmacol. Immunotoxicol. 29(1):81-93.
Crossref
|
|
|
|
|
Stevens RJ, Douglas KM, Saratzis AN, Kitas GD (2005). Inflammation and atherosclerosis in rheumatoid arthritis. Expert Rev. Mol. Med. 7(7):24.
Crossref
|
|
|
|
|
Umapathy P, Ndebia EJ, Meeme A, Adam B, Menziwa P, Nkeh-Chungag BN (2010). An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J. Med. Plants Res. 4:789-795.
|
|
|
|
|
Vazquez AI, Sanchez CM, Delgado NG, Alfonso AM, Ortega YS, Sanchez HC (2011). Anti-inflammatory and analgesic activities of red seaweed, Dichotomaria obtusata. Braz. J. Pharm. Sci. 47:111-8.
|
|