Management of rheumatic disorders are limited by the inability to provide long-term drug delivery due to the irritation to gastric mucosa and poor availability of drugs, which may be overcome by prolonging the drug release. The nanostructured carriers (NP)based gel was developed as potential topical system for aceclofenac topical delivery. In the present study, chitosan-sodium alginate nanoparticles were investigated using a novel gel base for the prolonged topical delivery NSAID aceclofenac. A modified ionotropic gelation method was used to produce aceclofenac loaded nano particulate systems. Drug content, particle properties such as size, size distribution and zeta potential were determined. The nano particulate system was encapsulated using a novel hydrophilic polymer pemulen in different proportions. The nanoparticles remained within the colloidal range and it was uniformly dispersed in pemulen gel preparation. The gels were evaluated for spredability, extrudability and in vitro, in vivo studies. The optimized formulations were characterized by FT-IR, DSC. In conclusion, nanoparticle based gel could be a promising vehicle for topical delivery of aceclofenac and the novel gel base pemulen can be used for the incorporation topical drugs.
Aceclofenac is a cyclooxygenase inhibitor used in the treatment of osteoarthritis, rheumatoid arthritis and in management of acute pain in adults. It shows high anti-inflammatory and analgesic activities with the incidence of gastric side effects and high therapeutic index (Fei et al., 2012). The mean plasma elimination half-life is around 2 h and is administered at a dose of 100 mg twice daily. The shorter biological half-life and dosing frequency more than once a day for prolonged period results in gastric irritation (Batheja et al., 2013).
Nanoparticles are novel drug delivery systems having the capability to release the drug at an optimum rate at the desired site of action. Nano particular formulations provide the liberty to use a wide range of polymers like synthetic, natural, biodegradable and non-biodegradable polymers. (Harshad et al., 2013). Chitosan and alginate are two biopolymers that have received much attention and have been extensively studied for such use
Chitosan being a cationic polymer has been used for the production nanoparticles by ionotropic gelation with negatively charged polymers and there are many chitosan-polyanion complexes that have been investigated as drug delivery systems for drugs, proteins with encouraging results (Shahin et al., 2011)
Nano particles are promising delivery carriers that have been utilized for formulation and delivery of various drugs. For topical administration, they are usually incorporated into gel or cream to increase their residence time, their stability and characteristics. The topical use of nanoparticles improves the drug bioavailability by prolonging the residence time of drugs applied topically or by enhancing the passing of drugs through the epithelial cells by opening the tight junctions between epithelial cells and also to reduce the side effect of the drug (Luigi et al., 1995; Alessandro et al., 2015).
Pemulen is a polymeric emulsifier composed of a block copolymer consisting of a poly acrylic acid similar to the Carbopol used to make aqueous and solvent gels. Pemulen is part of a class of copolymers, referred to as acrylate/C10-30 alkyl acrylate cross polymers which is cross linked with a long-chained methacrylate having a lipophilic regions as the methacrylate as well as hydrophilic regions composed of the acrylic acid. Cross-linked polymers can swell in water up to 1,000 times their original volume to form a gel (soomneka et al., 1995).
Pemulen polymers deposit an occlusive layer on the skin, delivering the topical medication in the form of low-irritancy formulation with elegant skin feel. Pemulen polymer also be used for high-clarity topical gels (Isabelle
, 2014; Elgadir et al., 2015). The current study aimed at developing nano particulate topical formulation of aceclofenac using a novel polymer pemulen as a gel base for topical delivery.
Aceclofenac was obtained as a gift sample from MSN Lab Pvt Ltd. Hyderabad and pemulen was gift from Strides Lab, Bangalore. Methylparaben, Propylparaben and polyethylene glycol was procured from Merck Chemicals, Mumbai. Sodium Alginate was Procured from SD Fine Chemicals Mumbai and chitosan was collected as a gift sample from central institute of Fisheries Technology, Kochin. All the solvents used were analytical grade and double distilled water was used for experimental work.
Experimental
The pemulen based transdermal delivery system was developed by encapsulating sodium alginate based aceclofenac nanoparticles in pemulen gel base. Sodium alginate nanoparticles were prepared employing ionotropic pregelation method and further they were encapsulated in a gel using pemulen as a base and the efficiency of gel base in delivery of aceclofenac was evaluated.
Preparation and evaluation of sodium alginate nanoparticles
Sodium alginate nanoparticle formulated employing two-step process based on ionotropic pre-gelation method with modifications from ideal preparation. The nanoparticles of aceclofenac were prepared based preâ€gelation of poly anion with calcium chloride followed by poly cationic crosslinking technique.
The nanoparticles were developed using 0.0063% w/v sodium alginate solution. Drug was incorporated at the rate 1 mg/mL to sodium alginate solution in step one itself, before adding to calcium chloride for gelation. About Calcium chloride solution (7.5 mL) was added drop wise to sodium alginate solution (117.5mL, 0.0063% w/v) to induce gelation. It was stirred continuously for 60 min to get the alginate pre gel. Then different concentrations of 25 mL of chitosan solution was added drop wise to the pre gel along with constant stirring for 90 min. Nanoparticles were concentrated by centrifugation at 11,500 rpm for 40 min. Nanoparticles thus formed were analyzed for particle size (Soomneka et al., 1995).
The volumes of the acetic acid and sodium hydroxide were selected in a way to adjust the final pH at around 5.9 to 6, to ensure the solubility of both aceclofenac and chitosan and the stability of the prepared nanoparticles (Monika Schäfer-Korting et al., 2007; Lippacher et al., 2001).
Formulation of aceclofenac pemulen gel
Pemulen at different concentration (0.5, 1 and 2% w/w) were used to formulate different topical formulation of aceclofenac Different formulations of pemulen based aceclofenac gel is given in Table 1. Pemulen was dispersed in water with preservatives methyl paraben and propyl paraben which were dissolved in freshly boiled and cooled water.
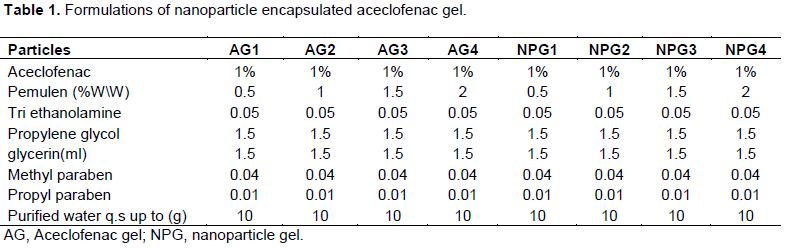
Aceclofenac was dissolved in propylene glycol and glycerin mixture and it was added to the preservative mixture and stirred well. Then the aqueous solution was added to the pemulen dispersion with stirring to get the uniform gel consistency. The pH of gel was adjusted within the range of 6.8 to 7.2 by adding triethanolamine. The uniform dispersion was packed in collapsible tubes for further studies (Simovic et al., 2007).
Preparation of nano particle encapsulated pemulen gel
The optimized batch of the nano particle dispersion dispersed into different concentration of the pemulen gel. Pemulen was soaked in preservative solution for overnight as specified previously. The nanoparticles equivalent to 100 mg of aceclofenac was dispersed in glycerin propylene glycol mixture and mixed with pemulen gel to get the uniform consistency of pemulen gel. Later the pH was adjusted with tri ethanolamine. Three batches of pemulen gels were prepared and subject to physical and chemical analysis.
Evaluation studies
Zeta size
The average particle size and poly dispersity index of the nanoparticles were determined by photon correlation spectroscopy using a Zeta sizer DTS version 5.03 (Malvern Instrument, Worcestershire, England). The samples of nanoparticles dispersions were diluted to four times their volume with 0.05 M NaCl. The particle size is represented by the average (diameter) of the Gaussian distribution function in the logarithmic axis mode.
Zeta potential
Zeta potential is an abbreviation for electro kinetic potential in colloidal systems. Zeta potential is electric potential in the interfacial double layer at the location of the slipping plane versus a point in the bulk fluid away from the interface. The surface charge (Zeta potential) was determined by measuring the electrophoretic mobility of the nanoparticles using a Malvern zeta sizer (Malvern instrument, UK). Samples were prepared by diluting with distilled water.
Physical analysis of pemulen gel
The following physical analysis was carried out for pemulen based aceclofenac and its nanoparticle encapsulated topical formulations .The organoleptic features of the pemulen gels were examined at the same temperature, lighting and packaging condition to assess variation in appearance, phase separation and color.
pH measurements
One gram of each pemulen gel formulation was weighed and dispersed with 25 ml of distilled water. After homogenization, the pH of the sample was measured with meter. The test was conducted as triplicate.
Spreadability of gel
For gel dosage form good spredability value is one of the important properties. Spredability gives indication of gel ability to spread on skin part. Spreading value decide the therapeutic efficiency of gel. Spredability apparatus contains wooden block having two glass plates. Initially gel sample was placed between the two glass plates. Weight near about 300 g was putted on top plate which expelled the air and form uniform gel layer. Afterwards 100 g weight was put to drag top plate by 10 cm using stringe attached to hook. Time required to move upper plate by 10 cm distance was noted, lesser the time required for dragging the upper plate better is the spredability value. Speradability value was determined with the help of formula:
S = M × L / T
Where, S is the spreadability value, L is the length of the glass slide, M is the weight tied to the upper plate, and T is the time taken to separate the glass slides. The test was conducted as triplicate for all formulations.
Extrudability
A good gel formulation should extrude easily from the container. In this test, a closed collapsible tube containing 10 g gel was passed firmly at crimped end. When the cap was removed, gel extrudes until pressure was dissipates. The weight in grams required to extrude 0.5 cm ribbon of gel in 10 s was determined. The results for each formulation were recorded as extrusion pressure in grams.
In-vitro drug diffusion study
The diffusion study of the nanoparticle gel and conventional gel using pemulen were carried out in Franz diffusion cell. The cellophane membrane was soaked in phosphate buffer for 24 h and it was mounted on the diffusion cell for further studies. Gel sample containing 100 mg of aceclofenac was taken on the semi-permeable dialysis membrane presoaked overnight in the freshly prepared diffusion medium. The donor compartment was kept in contact with a receptor compartment and the temperature was maintained at 37±0.1°C. The buffer solution was kept on the receptor side. At time intervals of 0.5, 1, 2, 3, 4, 5 and 6 h, 2 ml of sample was withdrawn and replaced by fresh medium and the drug concentration on the receptor fluid was determined spectro photometrically at 248 nm against appropriate blank (Sangvi et al., 2007).
Ex-vivo diffusion study
Ex vivo diffusion study for nanoparticle gel and conventional gel were performed on the goat skin using Franz Diffusion cells. Fresh goat hairless skin was obtained from a local slaughter - house. The skin was separated by removing the underlying fat and loose tissues. The membrane was washed with distilled water and then with 0.1 N NaoH and then wrapped in aluminium foil. The film was stored in freeze until further use. For ex vivo permeation studies, skins were allowed to hydrate for 1 h with phosphate buffer before being mounted on the Franz diffusion cell with the stratum corneum (Sharma et al., 2011).
Drug release study was performed on the excised skin using Franz diffusion cell. The skin was cleaned with water and then swabbed with cotton immersed in phosphate buffer. The gel formulations loaded with 10 mg of aceclofenac and its equivalent nanoparticles gel were placed on the excised skin. Stratum corneum was placed towards donor compartment and dermal towards receptor compartment. Phosphate buffer solution (pH 6.8) used as diffusion medium (37 ± 0.5°C). At time intervals of 0.5, 1, 2, 3, 4, 5, 6 and 12 h and replaced by fresh medium 2 ml sample were withdrawn and drug content was determined by UV spectrophotometer. For determination of drug deposition diffusion cell was dismantled, skin was removed. Excess drug present on the skin was removed. Afterwards skin was cut into small pieces which were placed in methanol undergo sonication for 1 h. Drug deposition in the skin was checked by using UV spectrophotometer.
Characterization studies
Fourier transform infra-red spectroscopy
About 5 mg of sample was mixed thoroughly with 100 mg of KBr IR powder and compacted under vacuum at a pressure of about 6000 kg/cm² for 3 min. The resultant disc was mounted in a suitable holder in a Schimadzu model 8033 IR spectrophotometer and the IR spectrum was recorded from 4000 to 625 cm-1 in a scan time of 12 min. The resultant spectra were compared for any spectral changes. The resultant spectra were compared for any spectral changes.
Differential scanning calorimetry
Differential scanning calorimetric thermo grams of sample were recorded in Mettler Toledo cu and k irradiation, analyzer equipped with a monitor and printer. The instrument was calibrated with indium as a standard. Accurately weighed 2.5 mg of samples were placed in open flat bottom, pierced aluminium sample pan. The melting point, peak maxima, appearance of any new peak and peak shape was noted.
Anti-inflammatory activity
The anti-inflammatory activity of the nanoparticle encapsulated pemulen gel was assessed employing carrageenan induced paw oedema method. Adult male Wistar rats, weighing between 128 to 160 g, maintained in standard laboratory conditions, at temperature 25 ± 1°C and relative humidity 55 ± 5%. These rats are randomly divided into 3 groups with 6 in each group.
Experimental Procedure: Wister rats were weighed and marks were made on right hind paw behind tibiatarsal junction. Each rat was placed in an observation chamber for 20 min to minimize stress related behaviors. Inflammation was induced by injecting 0.1 ml of 1% w/v carrageenan solution dispersed normal saline subcutaneously into the sub-plantar surface of the right paw of the rat. After one hour about 0.5 g of the gel formulations of plain aceclofenac and nanoparticle encapsulated gel was gently rubbed onto the plantar surface of the right hind paw 50 times with the index finger. All rats were subsequently returned to the observation chamber. The formulation was given as per the following groups.
Group - I: Control (Inflamed, treated with gel base only).
Group - II: Standard (Inflamed, treated with the plain aceclofenac gel)
Group - III: Sample (inflamed, treated with the nanoparticle encapsulated pemulen gel).
The inflammatory response was assessed by measuring the volume of the paw at different periodical intervals after carrageenan administration, using plethysmometer.
The percentage inhibition of paw volume for each rat in treated groups was calculated and expressed as mean ± SD percent inhibition of paw volume. The single ANOVA test was applied to test the significance.
The preparation of sodium alginate aceclofenac nanoparticles was based on ionotropic gelation process using aqueous phases of the poly cationic chitosan and poly anionic alginate. The optimized nanoparticles of aceclofenac were found to be fine, spherical and free flowing powders. The optimized nanoparticles showed a drug content in the range of 90.76±0.55 to 99.45 ±0.55%. The particle size varied from 287.89 to 356.87 nm. Highest encapsulation efficiency of 87.32% and a smaller particle size particle size of 187.2 nm were taken as criteria for selection of optimized formulation.
Zeta size
Zeta size distribution of the optimized chitosan-alginate nanoparticles given in Figure 1. The particle size distribution of the prepared nanoparticles was determined by using a zeta sizer. The results revealed that the particle size distribution was found to be uniform and the size was found to increase with addition of chitosan.
The particle diameter (z-average) for the optimized chitosan-alginate nanoparticles was approximately 187.4 nm (Figure 1). It is noteworthy that the hydrodynamic diameter of the particles measured by light scattering is smaller than the size estimated from microscopy particularly because of high swelling capacity of chitosan-alginate nanoparticles.
Zeta potential
The Zeta potential of optimized aceclofenac loaded alginate chitosan nanoparticles is given in Figure 2. For the optimized nano formulation, value of zeta potential was found to be -35.6 mV. This relatively high positive value was due to cationic nature of Chitosan and Alg/Chi ratio. The net positive zeta potential indicates the presence of free surface amino groups on the nanoparticulate delivery system It indicates that the synthesized drug loaded nanoparticles could be expected to be stable for long time.
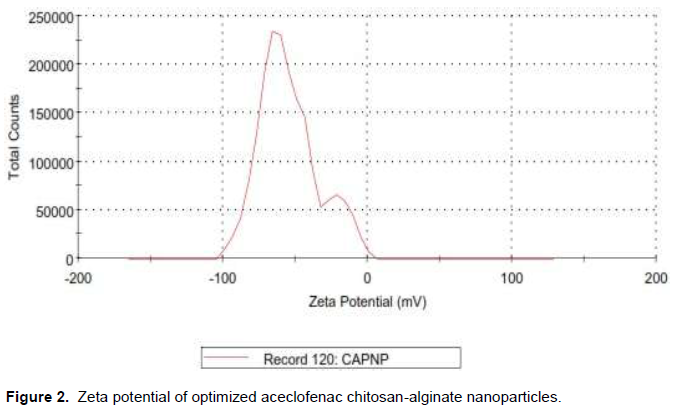
The stability of colloidal systems is directly related to the magnitude of their zeta potential. In general, if the value of the particle zeta potential is large, the colloidal system will be stable. Conversely, if the particle zeta potential is relatively small, the colloidal system will agglomerate. The surface charge of the particles is of substantial importance in all the production steps of these particles because the efficiency of the delivery system is directly related to the establishment of electrostatic interactions.
Evaluation of pemulen gels
All topical pemulen based formulation aceclofenac gels were clear, homogenous without any phase separation. The gels were transparent, non-greasy without any gritty particles. Spreadability data indicate that the gel is easily spreadable by a small amount of shear. Consistency reflects the capacity of the gel to get ejected in uniform and desired quantity when the tube is squeezed. The gel formulations were homogeneous in texture and fell within a pH range of 6.8 to 7.4 which is within the normal skin pH.
The aceclofenac and its nanoparticle encapsulated gels were smooth, elegant and transparent. The gel showed homogenous gel without any grittiness. The nanoparticle encapsulated gel showed uniform distribution of nanoparticles.
pH Determination
The pH values of all developed formulae was in range 6 to 7 which is considered acceptable to avoid the risk of irritation upon application to the skin. The results propose that the nano particle-based semi-solid formulation is acceptable for topical application.
Drug content
Drug Content determination results are Tabulated in Table 2 after various formulation of aceclofenac gel the drug content of the formulated gel was estimated and the results were in the official limits with range of 9.5 to 9.99 mg/g gel. The drug content determination also showed that the drug was uniformly distributed throughout the gel.
Extrudability and spreadability
The extrusion of gel is importantparameter during application andfor the patientcompliance.Extrudibility of gel formulations with high concentration of gelling agent was found satisfactory while with low concentration of gelling agents good extrudability was observed. Spredability plays an important role in patient compliance and help in uniform application of gel to the skin. Pharmaceuticallyacceptable geltakes less time to spreads and will have high spreadibiliy. The spreadability of formulation was decreased as the concentration of Pemulen was increased. The spreadibility of formulation was decreased as the concentration of Pemulen was increased it was assumed that the increased viscosity at higher concentration of pemulen was responsible for decreased spreadability.
In vitro drug release studies
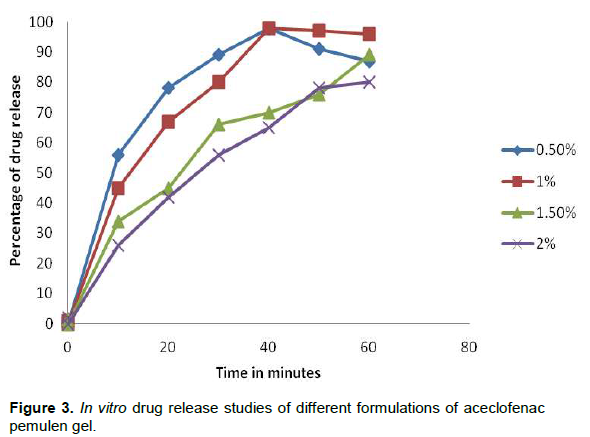
The in vitro drug release studies of the aceclofenac gel containing different proportions of pemulen is shown in Figure 3. As the concentration of pemulen in the dispersion increased, the dissolution rate also decreased. Among all the formulation containing 1% of pemulen showed complete release of drug in 30 min whereas increased proportions of pemulen showed slower and incomplete drug release. This could be attributed to the fact that very high quantity in the dispersion may be responsible for impeding the drug diffusion through the dispersion matrix thus bringing about a reduction in drug release profile from the formulated system.
The comparative profile of optimized pemulen with its nanoparticulate dispersion shows that the nanoparticles showed delayed release for a period of 6 hs shown in Figure 4. It was presumed to be due to controlled, slower release of the drug from nano particulate particle. The plain aceclofenac nano particle showed more than 75% drug diffused while the nano particulate based gel showed decreased drug release. While the gel formulation containing 2% of pemulen showed decreased drug release than other formulation which was assumed to be due to the high viscosity of gel.
Kinetics of drug release
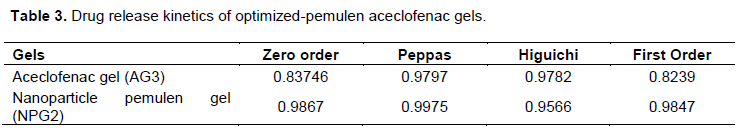
The comparative release constants of aceclofenac and nano particle encapsulated gel are given in Table 3. The release kinetics data indicated that the release of drug from nano particle best fits to zero order release model because the correlation coefficient values were higher in case of zero order equation and the release from gel NPG2 fits to Higuchi model. The release rate is independent of the concentration of the drug. The release exponent value of Korsmeyer-Peppas signifies that the mechanism of drug release from both gel and nanoparticle gel follows anomalous diffusion.
Ex vivo drug permeation studies
Ex vivo permeation of aceclofenac from nano particle encapsulated gel through excised rat skin was assessed and correlated with plain aceclofenac gel.
The amount of drug permeated through rat skin from aceclofenac nanoparticle incorporated gels is given in Figure 5. Nano particle dispersion gel exhibited the greatest cumulative amount of drug permeation in 12 h. The cumulative amount of drug permeated at the end of 12 h was found to be greater for aceclofenac nanoparticle
enriched pemulen gels than the aceclofenac gel nanoparticle encapsulated pemulen gels released drug slowly when compared with plain ACN gels accounted for by the time drug takes to diffuse through the gel. The slower release of drug from nanoparticle gels maintained the drug concentration for longer period. The formulations possessed a sustained drug release, but the sustained effect was more pronounced with nanoparticle enriched gel formulations.
The amount of ACN penetrated into dermis from NP gel at 12 h was nearly 2 fold that of plain aceclofenac gel. The small size and close interaction between NPs and the stratum corneum are the possible reasons for increase in the drug amount penetrating into the viable skin. The increase amount of drug in the dermis is also related to the occlusion properties. It was assumed that hydration of stratum corneum can increase, which can facilitate drug penetration of transdermal drug delivery.
Characterization studies
FTIR spectra of aceclofenac encapsulated pemulen gel
The FTIR spectra of aceclofenac (Figure 6) showed distinct sharp peaks at 3317 and 3272 indicate the presence of a primary amine and broad peaks near 2919 including 1921 may be due to CH stretching of CH2 groups, carbonyl group vibration at 1770 and 1716. Peaks at 1589, 1577 and 1508 indicates the presence of C=C ring stretching. The characteristic peak of 1723 was observed due to the pemulen. All these principal IR peaks of aceclofenac and pemulen were also observed in FTIR spectrum of aceclofenac gel. No extra peaks were found in spectrum of aceclofenac gels indicating absence of interaction or instability in gel formation.
DSC studies nanoparticle encapsulated gel
The DSC of acecclofenac showed a sharp endothermic peak at 149.40°C corresponding to the melting point of acecofenac. The DSC of aceclofenac and its optimized nanoparticle gel is given in Figure 7. The DSC scan of physical mixture also showed a sharp melting at 152.87 due to the melting of pemulen and a melting point at 272 is due to melting of sodium alginate and at 122.7°C due to chitosan melting. The DSC of optimized formulation with no additional peak indicating that the acecclofenac did not undergo any crystal modification or degradation. The gel formulation showed a melting point at 137°C indicates slight alteration of melting point it was assumed that the interaction of sodium alginate with chitosan was responsible for alteration of melting point. The intensity was decreased due to the dilution in pemulen gel.
As the DSC scan does not showed significant changes it can be concluded that the aceclofenac does not showed any crystalline changes.
Anti-inflammatory activity aceclofenac nanoparticle gel
Anti-inflammatory activity of aceclofenac and its sodium alginate nanoparticles gel in pemulen is reported in Figure 8. The anti-inflammatory activity of nanoparticles in comparison with pure drug was evaluated on the basis of ability to inhibit the edema produced in hind paw after challenging with carrageenan.
The sodium alginate nanoparticles of aceclofenac gel showed delayed action as compared to pure drug. A peak of inhibition of 31.6 and 54.3% was observed after 6 h for plain drug and nanoparticles respectively. The difference was significant showing significance with a p value less than 0.005% indicating that the nanoparticles gel of aceclofenac showed an improvement in anti-inflammatory activity for prolonged period on topical application.