Antioxidant capacity, phenolic content, antimicrobial and cytotoxic properties of extracts and fractions from flowers and fruits of G. uruguensis were evaluated. Total phenolic content ranged between 406.8 ± 20.47 and 57.98 ± 1.734 gallic acid equivalent in mg g-1 (mg GAE g-1) samples. Ethyl acetate and butanol fractions from flowers had the greatest rates in phenolic content and antioxidant activity in 1,1-diphenyl-2-picrylhydrazyl (DPPH) and thiobarbituric acid reactive substances (TBARS) assays. Pearson´s coefficient showed a strong association between total phenolic compounds in DPPH (r = - 0.83, p < 0.01) and TBARS (r = 0.85, p < 0.01). Eleven out of the 12 samples tested were toxic in the brine shrimp assay (LC50 < 100 µg mL-1). Antimicrobial susceptibility profile revealed flower remaining fraction and fruit extract with moderate activity against Enterococcus faecalis. Results indicate that G. uruguensis is a good source of compounds with antioxidant and cytotoxic activities. Further studies to identify compounds causing these activities are recommended.
The Rubiaceae family has approximately 650 genera and 13,000 species, predominantly distributed throughout the tropical regions (Delprete and Jardim, 2012). Rubiaceae plants demonstrate several secondary metabolites and important biological activities, such as caffeine in Coffea arabica L., quinine in Cinchona pubescens Vahl. and emetin in Psychotria ipecuanha (Brot.) Stokes. The three compounds mentioned above are among the 30 substances isolated from the most important plants for medicinal use (Gerlach et al., 2010). In Brazil, the plants of the Rubiaceae family are popularly used in the manufacture of phytotherapics, such as herbal medicine prepared from Uncaria tomentosa (Wild.) DC., popularly known as cat's claw (WHO, 1997; BRASIL, 2013).
Members of the Guettarda genus are distributed between East Africa and the islands of the Indian and Pacific Oceans to Neotropical regions (Achille et al., 2006). In Brazil, twenty species are distributed in the Amazon, Caatinga, Savannah, Atlantic Rainforest and Pampa regions (Barbosa, 2015). In fact, several species from this genus have been traditionally used as medicine, particularly against inflammatory diseases (Albuquerque et al., 2007; Capasso et al., 1998; Agra et al., 2007; Agra et al., 2008; Matos, 1997; Brandão, 1985).
Phytochemical investigations on the genus revealed several compounds, including alkaloids (Kan-Fan and Husson, 1979; Capasso et al., 1998), iridoids (Inouye et al., 1988; Ferrari et al., 1986; Naressi et al., 2015), triterpenes (Aquino et al., 1988; Aquino et al., 1989; Bhattcharyya and Almeida, 1985) and other classes of substances, such as phenolic esters (Oliveira et al., 2008; Testa et al., 2012; Naressi et al., 2015).
Several kinds of Guettarda extracts have been reported for their biological activities, including anti-inflammatory, antioxidant, antiviral, antimicrobial and anti-convulsive properties (Pina et al., 2012; Duarte et al., 2014; Barros et al., 2012; Saravana et al., 2009).
Guettarda uruguensis Cham. & Schltdl. (Rubiaceae), a species commonly known as velvetseed, has eatable fruits (Corrêa and Penna, 1984; Kunkel, 1984) and sweet-scented flowers (Kinupp, 2007). Since species of the genus have been employed from time immemorial for therapeutic purposes, with acknowledged pharmacological activities, and since studies on the biological activities of its fruits and flowers are scarce, current study determines the antioxidant capacity, phenolic content, antimicrobial and cytotoxic proprieties of extracts and organic fractions of the flowers and fruits of G. uruguensis.
Plant material
Flowers and fruits of G. uruguensis were collected in Curitiba, Brazil, between November 2012 and February 2013. Plant material was identified by botanist José Tadeu Weidlich Motta of the Municipal Botanic Museum of Curitiba. A voucher specimen was deposited and registered under MBM 386376.
Chemicals
Ascorbic acid, butylated hydroxytoluene (BHT), 1,1-diphenyl-2-picrylhydrazyl (DPPH), Folin-Ciocalteu reagent, gallic acid, caffeic acid, rutin, tris, thiobarbituric acid (TBA), sodium dodecyl sulfate (SDS), ammonium molybdate, sodium phosphate and carbonate sodium were purchased from Sigma Co. (St. Louis,MO, USA). All other chemicals used were of analytical grade.
Extraction and liquid/liquid partition
Dried flowers (306 g) were extracted by ethanol (5 L) in a Soxhlet extractor. Ethanol (FLE, 58.2 g) was extracted under reduced pressure in a rotatory evaporator by removing solvents. Partition was performed in a modified Soxhlet extractor (Carvalho et al., 2009), employing solvents within an increasing polarity scale. Hexane (FLH, 2.2 g), chloroform (FLC, 0.5 g),ethyl acetate (FLA, 3.7 g), butanol (FLB, 14.5 g) and remaining fractions (FLR, 27.3 g) were extracted. Fractions of dried fruits (200 g) were also extracted in modified Soxhlet (Carvalho et al., 2009) with solvents in an increasingly polarity scale. Hexane (FRH, 2.7 g), chloroform (FRC, 1.5 g), ethyl acetate (FRA, 0.8 g) and remaining fraction (FRR, 24.3 g) were extracted. Dried flowers (145 g) were extracted with ethanol (1.5 L) at room temperature (three times a week). The extract was concentrated in a vacuum at 40°C, and the crude alkaloid fraction (FLALC, 0.3 g) was obtained by classical methods (Batista et al., 1996). Dried flowers (200 g) were infused in hot water (1 L) for 60 min. The procedure was repeated three times. The combined extract (FLAQ, 32.6 g) was collected by filtration, concentrated under reduced pressure, and lyophilized.
Total phenolic content
Total phenolic contents of flowers and fruits were determined by modified Folin-Ciocalteu method (Singleton and Rossi, 1965). Aliquots of ethanol extracts and its fractions (200 μL), dissolved in methanol, were added to Folin-Ciocalteu reagent (200 μL), sodium carbonate saturated solution (400 μL) and distilled water (3.2 mL). Absorbance was measured at 760 nm after 30 min. A standard curve was prepared with gallic acid at a concentration range between 2.5 and 20 µg mL-1 (y = 0.0392x – 0.0583, r = 0.9964). Total phenolic content was expressed as gallic acid equivalent (GAE) in mg g-1 samples.
HPLC fingerprint of flower and fruit ethyl acetate fractions
A fingerprint HPLC analysis of ethyl acetate fractions was performed with high performance liquid chromatography (Merck-Hitachi LaChrom Elite®HPLC System) equipped with a pump (L-2130), UV–VIS detector (DAD L-2450), rheodyne manual injector (loop 20 μL). The fractions were dissolved in methanol and filtered with Milli pore membrane (0.45 mM pore diameter). The samples diluted in methanol (10 mg.mL-1) were eluted using column Waters Xterra® reverse phase column C18 5 µm (4.6 x 250 mm). Total run time was 43 min with mobile phase A: methanol, B: acid phase, gradient elution: 0 to 40 min: 20 to 100% A, 40 to 43 min 100% A. Methanol used was HPLC grade (TEDIA) and acid phase was composed of 1% acetic acid. Flow rate at 1 mL min-1 and an injection volume of 20 µL were employed and peak was detected at 329 nm.
Evaluation of antioxidant capacity by phosphomolybdenum method
The antioxidant capacity of flowers and fruits was evaluated according to
Prieto et al. (1999). An aliquot mixture of 0.3 mL of extract sample solution (200 μg mL
-1) was mixed with 3 mL of mixture reagent solution (0.6 M sulfuric acid, 30 mM sodium phosphate and 4 mM ammonium molybdate). Sample tubes were sealed and incubated in a water bath at 95°C for 90 min. When reactant samples cooled to room temperature, sample absorbance was measured at 695 nm. Antioxidant activity of samples was expressed in relative antioxidant activity (AAR%), as compared to ascorbic acid and rutin standards (Equation 1).
Where, Asample: absorbance of sample; Ablank: absorbance of sample blank; Astandard: absorbance of standard.
Radical scavenging assay
DPPH radical scavenging activity was determined following Mensor et al. (2001), with modifications. Samples (71 µL) at various dilutions were added to 29 µL of 0.3 mmol mL-1 DPPH solution in a 96-well microplate. The solution was incubated in the dark at room temperature and absorbance was measured at 540 nm employing a microplate reader. The antioxidant capacity was calculated by Equation 2.
where, Acontrol: absorbance of control; Asample: absorbance of sample; Ablank: absorbance of sample blank. Antioxidant activity was expressed as IC50 (µg mL-1), or rather, the concentration of the sample which was required to cause a 50% decrease in absorbance at 540 nm. IC50 was calculated by linear regression and the linear range was established by equation y = ax + b.
Determination of thiobarbituric acid reactive substances (TBARS)
Reactive species to thiobarbituric acid was determined according to methodology by Morais et al. (2006), with adaptations. Filtered egg yolk solution in SDS was employed as lipid source and butylhydroxidetoluene (BHT) as standard. Extracts and its fractions (100 μL of 1000 mg L-1 solution) were added to filtered water (400 μL), yolk solution 5% (500 μL) and TBA solution 0.4% (1500 μL) and placed in a water bath (95°C) for one hour. After cooling, 1500 μL n-butanol were added to enhance lipid extraction. Supernatants were collected after tube centrifugation (3000 rpm, 3 min) and their absorbance was determined at 532 nm, with results as antioxidant content (Equation 3).
where, Asample: sample absorbance, Acontrol: control absorbance.
Antimicrobial assay
Extracts and fractions were evaluated against six microorganisms, including two Gram-positive [Staphylococcus aureus ATCC 25923 (S. aureus), Enterococcus faecalis ATCC 29212 (E. faecalis)], three Gram-negative [Escherichia coli ATCC 25922 (E. coli), Pseudomonas aeruginosa ATCC 27853 (P. aeruginosa), Klebsiella pneumonia ATCC 700603 (K. pneumonia)] and one yeast [Candida albicans ATCC 10231 (C. albicans)]. Minimum inhibitory concentration (MIC) rates were determined by the broth microdilution method (CLSI, 2008a). The analysis for antifungal activity comprised serial dilutions of extracts and fractions within a concentration range between 1000 and 7.81 µg mL-1. Dilutions were prepared with liquid medium RPMI 1640 in 96-well U-shaped bottom sterile microplates (CLSI, 2008b).
Brine shrimp lethality bioassay
The brine shrimp toxicity assay was adapted from method described by Meyer et al. (1982). Cysts of A. salina (0.5 g ml-1) were added to aired saline water and incubated in an environmental chamber at 27 ± 2°C and relative humidity 80 ± 5%, for 48 h. Nauplii were collected and immediately used. Samples were tested in 10, 100 and 1000 µg mL-1 concentrations (0.5% of dimethyl sulfoxide (DMSO) in saline water). The test was performed in triplicate, with 10 organisms per replicate. Saline water and dodecyl sulfate (SDS) were respectively employed as negative and positive controls.
Statistical analysis
Statistical analyses were performed by one-way ANOVA, followed by Dunnett’s and Tukey´s post-hoc tests. Differences were statistically significant when p < 0.05. Total phenolic content data and antioxidant activities were analyzed by Pearson´s correlation and analysis of main components (PCA). Microsoft Office Excel 2010, GrahPad Prism 5.Ink and Matlab R2015a softwares were used for calculations.
Total phenolic content of samples
Analysis results for total phenolic content demonstrated that G. uruguensis is a good source of phenolic compounds (Table 1). Total phenolic contents (expressed in mg GAE g-1) for flowers ranged between 109.8 ± 3.44 and 406.8 ± 20.47, and were higher than those from fruits (between 57.98 ± 1.34 mg and 86.72 ± 2.961). Among the samples analyzed, the lowest phenolics concentrations (<100 mg L-1) revealed FRR < FRC fractions. FLA (406.8 ± 20.47 mg GAE g-1) and FLB (339.3 ± 20.31 mg GAE g-1) showed the highest phenol contents. Phenolic content rates in FLA were approximately two times higher than FRA (184.1 ± 11.27 mg GAE g-1).

Phenolic compounds are the secondary products of plant metabolism that constitute a large and complex group. These molecules are essential for plants’ growth and reproduction, and their synthesis is induced under biotic and abiotic stress conditions, such as infections, injury, UV radiation, ozone, salinity, water stress and heat. They are partially responsible for color, astringency, aroma, and oxidative stability in food (Manach et al., 2004).
According to
Singleton and Rossi (1965), phenolic compounds have different responses to Folin-Ciocaulteu assay, depending on their chemical structure. Phenolic compounds contribute to multiple biological effects, including antioxidant activity. This activity is believed to be mainly due to their redox properties, which can play an important role in absorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides (
Osawa, 1994).
Plant phenols are widely distributed in the plant kingdom and they are sometimes present in surprisingly high concentrations (
Harborne, 1993). The high content of phenolic compounds reported in current study has also been registered for different
Guettarda species. Lima et al. (2009) also observed a higher content of phenolic compounds in
G. grazielae with rates ranging from 347.75 ± 0.02 GAE g
-1 in MeOH-H
2O fraction of stems and 298.03 ± 0.002 mg GAE g
-1 in EtOAc fraction of leaves. Revathi and Rajeswari (2015) reported a lower content for
G. speciosa (115.81 ± 0.67 mg TAE g
-1). Total phenolic content for
G. uruguensis was higher than that registered by other authors for different
Guettarda species.
HPLC fingerprint of flower and fruit ethyl acetate fractions
Flower and fruit ethyl acetate fractions fingerprint revealed chromatographic profiles (Figures 1 and 2, respectively). The two fractions showed chromatographic peaks with retention time 06.09 and 15.75 min, although peaks in the flower fraction had a higher concentration. Peaks that differentiated fractions were: peak at 13.75 min in flower and peak at 14:33 min in the fruit, both presenting different intensities and different ultraviolet profiles. Profile of the fruit´s ethyl acetate fraction is characteristic of flavonoids. Caffeic acid standard at 6:09 min revealed the same UV profile. However, flower fraction had higher concentrations as compared to those from fruit fractions.
Antioxidant capacity
Several techniques have been used to determine in vitro antioxidant activity for a rapid screening of medicinal plants. Free radicals are known to play a definite role in a wide variety of pathological manifestations. Antioxidants combat free radicals and protect people from various diseases either by scavenging the reactive oxygen species or by protecting the antioxidant defense mechanisms.
The phosphomolybdenum assay has been routinely used to evaluate the antioxidant capacity of extracts (Prieto et al., 1999). Although, some extracts and fractions from flower and fruit demonstrated a higher relative antioxidant activity as compared to rutin standard,all samples showed a lower antioxidant activity than ascorbic acid (Table 2 and Figure 3). In the ranking of antioxidant capacity obtained by this method, the remaining fraction of G. uruguensis flower showed higher phosphomolybdenum reduction (26.01 ± 0.01% of ascorbic acid and 121.59 ± 0.01% of rutin), followed by remaining fraction of G. uruguensis fruit (27.1 ± 0.03% of ascorbic acid and 128.14 ± 0.03% of rutin).


Assessment of antioxidant activity by the DPPH method revealed a large variation among extracts and fractions from flower and fruit (Table 2 and Figure 3). The lowest IC50 rate, or rather, the highest scavenging activity of DPPH radicals, was obtained from ethyl acetate and butanol fractions of flowers. FLA (IC50 = 13.21 µg mL-1) showed a rate (p < 0.05) which was statistically similar to that of ascorbic acid (IC50 = 4.78 µg mL-1) and rutin (IC50 = 6.19 µg mL-1). FLB also demonstrated a strong scavenging activity with IC50= 22.73 ± 0.29 µg mL-1. In the case of fruit samples, the extracts’ scavenger capacity was comparatively lower than extract and fractions of flowers. FRA (IC50 = 68.8 ± 0.06 µg mL-1) showed the highest scavenger capacity among other fruit extracts (Table 2 and Figure 3).
Specialized literature has scanty information on the antioxidant activity of the species Guettarda. DPPH assay was performed on G. viburnoides leaves and on the G. uruguensis stem bark. Naressi et al. (2015) showed higher antioxidant activity in G. viburnoides, with IC50 rates for crude extract (24.69 µg mL-1), ethyl acetate (18.92 µg mL-1), aqueous-methanol (26.47 µg mL-1) fractions from leaves, and also for grandifloroside (20.52 µg mL-1), a compound isolated from leaves. In a previous paper, Duarte et al. (2014) reported antioxidant activity of crude extract and fractions from the stem bark of G. uruguensis and proved that ethyl acetate fractions (IC50 = 10.91 µg mL-1) were greatly capable of quenching the DPPH radical.
Lipid peroxides are likely involved in many pathological events, including inflammation, metabolic disorders, oxidative stress and cellular aging. Table 2 and Figure 3 summarize the effects of the flower and fruit extracts and fractions of G. uruguensis. FLB (IA = 53.42 ± 4.29%) and FLA (IA = 52.08 ± 2.21%) obtained the highest antioxidant index from other samples analyzed, although both are not significantly different (p > 0.05) from BHT (IA = 54.6%).
The antioxidant activity of phenolic compounds was correlated to their chemical structures. The relationship of the structure activity of several phenolic compounds has been studied (Rice-Evans et al., 1996; Lien et al., 1999; Son and Lewis, 2002). Free radical scavenging and antioxidant activity of phenolics mainly depend on the number and position of hydrogen-donating hydroxyl groups on the aromatic ring of the phenolic molecules. However, it is also affected by other factors, such as glycosylation of aglycones, H-donating groups (-NH, -SH) and others. There are currently several reports on antioxidant components, generally focusing on flavonoids and phenolic acids (Rice-Evans et al., 1996; Nakatani, 2000; Zheng and Wang, 2001).
The flavonoids, namely, quercetin-3-O-B-D-galactopyranoside, quercetin-3-O-B-D-glucopyranoside and grandifloroside (Naressi et al., 2015) and the phenolic acids, namely, 5-caffeoylquinic acid, 4,5-dicaffeoylquinic acid (Capasso et al., 1998, Oliveira et al., 2008, Testa et al., 2012) and shickimic acid (Capasso et al., 1998) were identified in the genus Guettarda. The literature also shows that 3,5- and 4,5-O-dicaffeoylquinic acids and 3-O-glucosilates derived from quercetin have significant scavenging abilities of free radicals, with IC50 rates close to those for FLA in the DPPH assay.Antioxidant capacity and total phenolicrates may also enhance other investigations and co-relate such activity with other important ones, such as the anti-inflammatory activity which is directly related to the popular use of several species of the genus Guettarda. Specialized literature suggests the co-relationship between antioxidant and anti-inflammatory activities. In other words, several vegetal extracts decrease inflammation by eliminating superoxides known to participate in the recruitment of polymorphonuclear cells (PMN) occurring in inflamed tissues (Thambi et al., 2009; Ródenas et al., 2000).
Current analysis registered that flowers and fruits of G. uruguensis proved to have high antioxidant activity and elevated levels of phenolic compounds.It is a widely grown plant, possessing fruits used as food and aromatic flowers. Since there is an inverse relationship between dietary intake of antioxidant-rich foods and the occurrence of several human diseases, above results are interesting and research on the determination of antioxidant-rich foods is highly relevant and rewarding.
Correlations between total phenolic contents and antioxidant activities
Antioxidant activities of medicinal plant extracts are often associated with redox proprieties when they function as reducing agents. Phenolic compounds constitute one of the major groups of secondary metabolites acting as free radical scavengers and antioxidants. The antioxidant activities of flower and fruit extracts and fractions of G. uruguensis were measured by the phosphomolybdenum, DPPH and TBARS assays. FLA and FLB registered a high level of antioxidant activity in different assays (Table 3). FLA (13.21 ± 0.66 µg mL-1) provided a statistical result (p < 0.05) with DPPH similar to ascorbic acid (4.78 ± 0.04 µg mL-1) and rutin (6.19 ± 0.06 µg mL-1) controls. In the case of the TBARS method, FLA (IA = 52.08 ± 2.20%) and FLB (IA = 53.42 ± 4.29%) presented statistical antioxidant indexes similar (p < 0.05) to BHT control (IA = 54.6%).

Results of antioxidant assays were consistent and correlated with the polyphenolic contents assessed by linear regression analysis. Table 3 shows Pearson’s correlation coefficients. An important correlation of flower and fruit extracts and respective fractions may be observed between the phosphomolybdenum: ascorbic-acid and rutin tests (

. Pearson´s coefficient indicated a strong association (

of phenolic compounds and antioxidant activity TBARS, whereas negative correlation (

revealed an inverse relationship between phenolic compounds and IC50. In fact, IC50 is the necessary concentration to inhibit 50% of free radicals, in other words, lower IC50 rates point to better antioxidant activity.
Figure 4 shows two main principal components (PCs) characterizing total phenolic content and antioxidant capacity (phosphomolybdenum, DPPH and TBARS) of flowers and fruit fractions. The first principal component (PC1) accounted for 60.03% of variability in the data set, whilst the second PC (PC2) accounted for 32.78% of variance in the data.
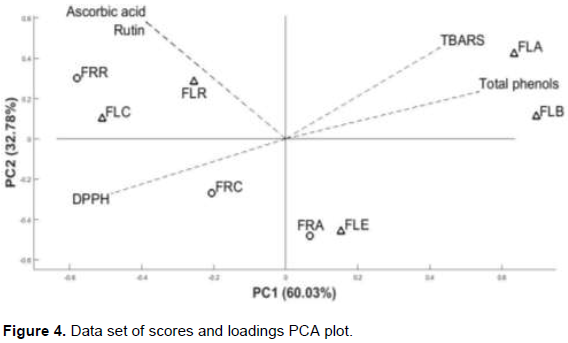
PC1 distinguishes two groups: (i) ethyl acetate fractions (FLA and FRA), FLE and FLB from (ii) remaining fractions (FRR and FLR), FRC and FLC. The fractions obtained from the chloroform solvent (FLC and FRC) constitute a distinct group from the remaining fractions (FRR and FLR). The proximity of FLC to the FRC quadrant may be explained by the different extraction mode between flower and fruit. Consequently, chloroform and remaining fractions could be discriminated by the polarity scale in PC2. Figure 4 shows the five assays represented by vectors. Since they are inversely proportional, the coefficients for total phenol, TBARS and DPPH, demonstrate that the higher rates for the two tests were FLA and FLB. The best rates with the greatest contribution in the phosphomolybdenum tests were from
FRR and FLR.
Antimicrobial activity
There is no consensus at an acceptable inhibition level for the use of natural antimicrobial products when compared with known antibiotics (Aligianis et al., 2001). According to Ayres et al. (2008), MIC rates obtained were classified as having good inhibitory potential (<100 µg mL-1); moderate activity (500-1000 µg mL-1) and absence of inhibitory activity (>1000 µg mL-1).
Assessment of antimicrobial susceptibility profile indicated that samples presented moderate activity, or rather, the flower´s remaining fraction (500 µg mL-1) and fruit extract (500 µg mL-1), against Enterococcus faecalis. Extracts and fractions were classified as having low activity (CIM=1000) or as inactive (CIM > 1000) in the remaining microorganisms.
In previous studies on G. uruguensis, Kelmer et al. (2011) demonstrated the stem bark´s activity (CIM=500 µg mL-1) against S. aureus and that of the chloroform fraction (CIM=125 µg mL-1) against S. aureus. The best performance of chloroform fraction was attributed to ursolic acid. Duarte et al. (2014) showed that crude extract and stem bark fractions of G. uruguensis had antimicrobial activity against S. epidermidis and C. albicans. Divergences among studies may be due to the fact that the biosynthesis of secondary metabolites was affected by environmental factors, such as seasonality, circadian rhythm and development. The collection period may be highlighted since the quantity and even the nature of chemical constituents are not constant throughout the year. Another explanation may comprise the difference between plant organs and the characteristics of extraction process (Gobbo-Neto and
Lopes, 2007).
Brine shrimp lethality bioassay
The lethal concentration (LC50) obtained from regression and probit analysis (Bliss, 1934) in 24 h are presented in Table 4. Except FRR (LC50>1000 µg mL-1), other samples were also considered toxic. In fact, highest toxicity was presented by FLC (LC50 < 10 µg mL-1), significantly (p < 0.05) similar to SDS (16.27 µg mL-1).

In previously studies, current research team reported toxicity for chloroform fraction (LC50 = 80.31 µg mL-1) obtained from the stem bark of G. uruguensis. Toxicity may have been related to alkaloids in the chloroform fraction (Duarte, 2012). Alkaloids have also been described in other Guettarda species (Husson et al., 1977; Kan-Fan and Husson, 1979; Brillanceau et al., 1984; Kan-Fan et al., 1985; Ferrari et al., 1986; Montagnac et al., 1997; Capasso et al., 1998). In the wake of such results, an alkaloid extract was assessed to verify whether toxicity could be reproduced. The alkaloid extract actually provided a second high toxicity score (LC50 < 21.54 µg mL-1) and suggested that high toxicity by FLC may have been due to alkaloids.
From a pharmacological point of view, the brine shrimp lethality test was employed to detect general toxicity. It proved to be a good detector of compounds with antiviral, insecticidal, anti-parasitic and anti-tumoral activities (Siqueira et al, 1998; McLaughlin et al., 1998). With the exception of FRR, extracts and fractions obtained from the flower and fruit of G. uruguensis displayed a strong activity against brine shrimp, which is highly suggestive of bioactivity and its pharmacology potential. Since G. platypoda and G. pohliana revealed antitumoral activities in human cancer cell strains (Oliveira, 2013; Pina et al., 2012), the antitumoral capacity of G. uruguensis should be evaluated.