ABSTRACT
Plants with diverse therapeutic properties are indigenous to Namibia. Concoctions of Colophospermum mopane and Acrotome inflata are widely used traditionally in the management of respiratory, gastrointestinal and wound infections. Limited studies have validated these traditional uses particularly against resistant bacteria strains such as Pseudomonas spp. This study aimed to determine the antimicrobial activity and phytochemistry of extracts of C. mopane and A. inflata, medicinal plants indigenous to Namibia. Phytochemical analysis and antimicrobial testing were done on leaves and barks of C. mopane and A. inflata whole plant. Voucher specimens were collected from Omugulugoonime village, Oshikoto region and validated at the National Botanical Research institute, Windhoek. Crude extraction of dried plants was done by maceration with ethanolic and aqueous solvents. Phytochemical screening was done using methods described by Harborne (1998) and/or Tiwari et al (2011). The antimicrobial activity against wild types of Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtillis and Candida albicans was done using the disc-agar-diffusion method. The mean diameters of the zones of inhibition (mm) for each extract were determined against each test organism. The antimicrobial activity (zones of inhibition) of ethanolic extracts (5 mg/ml) of C. mopane bark (10±0.6 mm) and leaves (10±1.2 mm) and A. inflata (8.7±0.6 mm) against P. aeruginosa is comparable to that of penicillin G (14±1.4 mm). Aqueous extracts leaves and bark of C. mopane showed activity against P. aeruginosa and S. aureus. The activity of the ethanolic extracts against B subtillis was: C. mopane leaves (7.3±0.6 mm), C. mopane bark (8.7±0.6 mm) and A. inflata (11.3±3.21 mm) and Penicillin G (26±1.4 mm). Both ethanolic and aqueous extracts did not have activity on C. albicans. Aqeous extracts of A. inflata had no activity on Pseudomonas and B. subtillis. Organic extracts of Colophospermum mopane and A. inflata exhibit antimicrobial potential against Pseudomonas and Bacillus species. The alkaloids, flavonoids and tannins should be further purified and characterized for antipseudomonal activity.
Key words: Antipseudomonal, Namibia, Colophospermum mopane, Acrotome inflate.
The sub-Saharan Africa is indigenous to over 50,000 species flora and ethno-medicines (Clarkson et al., 2004; Cowan, 1999; Hosttetmann et al., 1996; Iwu, 2014). Namibia is home to about 8159 plant species with therapeutic potential (Hedimbi and Chinsembu, 2012; Ilonga, 2012; Cunningham, 1993; Grote, 2003; Cheikhyoussef et al., 2011; Thomas, 2015). Over 80% of the world’s population, mostly in low and middle income countries (LMIC) rely on traditional medicine for primary health care of common ailments (Fabricant and Farnsworth, 2001; Ganatra et al., 2012; Thirumurugan, 2010; WHO, Traditional Medicines, 2012). Despite the wide practice of ethno-medicine the sub-Saharan Africa, the region bares highest global burden of infectious diseases such as HIV/AIDS, tuberculosis and malaria. Out of the 500 000 estimated plant species found in nature, a limited proportion undergone phytochemicals, biological and pharmacological characterization (Mahesh and Satish,2008; Dushimemaria, 2014).
The antimicrobial potential of higher medicinal plants as sources for new drugs in Namibia is still largely unexplored. Recently, there is a rise in patients living with non-communicable diseases and the WHO has estimated that 3 out of 4 patients with hypertension reside in sub-Sahara region (WHO, 2016). This calls for action for further research for efficacious and cost-effective medicines from the indigenous plants and/or ethno-medicines. In addition, the global surge in resistance against essential antimicrobials has had its greatest impact on health care in the sub-Saharan region and is a public health concern (Wagate et al., 2009; Parekh and Chanda, 2007). As a result, antimicrobial resistance has led to use of more expensive medicines and or treatments, further constraining health care systems (Kamaraj et al., 2012).
Despite the global efforts to increase access to essential medicines, access to essential and alternative antimicrobial remains a challenge, particularly in remote settings where indigenous systems are commonly practiced as part of the primary health care (WHO, Traditional Medicines, 2012; Carlet et al., 2012). In recent years, the clinical efficacy of essential antibiotics such as cotrimoxazole and penicillin used in the sub-Saharan region against common pathogens has reduced tremendously (Mutembei et al., 2011; Monroe and Polk, 2000). Namibia has one of the highest case notification rates for multi-drug resistant tuberculosis (MDR-TB) in the world (World Health Organization, 2013). There is a growing burden of multi-drug resistance to bacterial pathogens in the public health care in Namibia including strains of Escherichia coli, Enterococcal pneumoniae, Proteus mirabilis, Klebsiella (Khan and Musharraf, 2004). The emergence of multi-drug resistant pathogens threatens the clinical efficacy of many existing antibiotics (Mutembei et al., 2011). In particular, there are high rates of resistance to amoxicillin, cotrimoxazole and nitrofurantoin. The resistance against amoxicillin is rising among strains of E.coli (79.6%), Klebsiella (96.72) and Proteus (55.91%). Similar patterns of resistance against cotrimoxazole have been documented on isolates of E. coli (78.6%), Proteus (57.85%) and Klebsiella (56.52%). The resistance against nitrofurantoin on Proteus mirabilis isolates is reported at 77.37% (Mengistu et al., 2014) . The rising burden of antimicrobial resistance is a wake-up call for further research and development of cost-effective medicines, particularly from the indigenous ethno-medicines of Namibia.
In Namibia, concoctions of Colophospermum mopane and Acrotome inflata are widely used traditionally in the management of infectious diseases (Musvoto et al., 2007; Cheikhyoussef et al., 2011; Bainbridge H, 2012). The traditional uses include cure and/or prevention of wound infections and treatment of syphilis, diarrhea, coughs, inflammatory diseases and fevers (Cheikhyoussef et al., 2011). However, despite the wide use of C. mopane and A. inflata in Namibia, the safety and efficacy profiles in the treatment of diseases have not been validated. C. mopane and A. inflata belong to the Fabaceae and Lamiaceae families, respectively. Secondary metabolites of these families including tannins, alkaloids, and flavonoids have exhibit antimicrobial activity (Cheikhyoussef et al., 2011; Mahesh and Satish, 2008; Lewis and Ausubel, 2006). Thus, extracts of these ethno-medicines from C. mopane and A. inflata may be a potential source of phytochemicals with antimicrobial activity conceivably with better safety profile and/or novel mechanisms against resistant bacteria (Thirumurugan, 2010; Parekh and Chanda, 2007; Tiwari et al., 2011; Cowan, 1999) Phytochemicals such as alkaloids, tannins, saponins and flavonoids have been reported to have antimicrobial activity (Heneman and Zidenberg-Cherr, 2008; Suliman, 2010). C. mopane (Figure 1) is a shrub or a small tree of the family Fabaceae (Leguminosae) and in Namibia, it is popularly known as Mopane or Omusati. C. mopane covers about 9% of Namibia’s surface area and is more common in the Northern parts of Namibia (Dushimemaria, 2014). Decoctions of Mopane leaves, bark and gums are used by the Heikum Bushmen, Wambo, Damara and Himba communities for, management of gastrointestinal complaints, diarrhoea,coughs, wounds symptoms of inflammation, oral hygiene and diarrhoea (Von-Koenen, 2009; Van den Eynden et al., 1992). The roots of C. mopane are used to treat wounds; stomach problems, inflamed eye, and syphilis, and have been reported to contain tannins and resin (Von-Koenen E, 2009, 2001). A. inflata (Figure 2) belongs to Lamiaceae (Labiatae) family, it is a herb called tumbleweed and locally known as Etselyakuku in Ndonga (Sharma and Kumar, 2013). A. inflata is mostly distributed in northern part of Namibia including in Oshikoto, Oshana and Omusati regions (Van Rooyen, 1988; Walt and Riche, 1999; Nordenstam, 1970). The leaves of A. inflata inflate are used by the Damara communities as a tea to treat coughs and stomach upsets (Mukanganyama et al., 2011). The Wambo communities use the entire plant for treatment of malaria and as an indoor insecticide. In Kavango region, ashes of the dried and burnt tumbleweed flower are used for management of pain and scratches over the temples (Von-Koenen, 2009; Nafuka, 2014).
Limited studies provide scientific evidence of the antibacterial potential of these plants against commonly resistant bacteria such as Pseudomonas. Consequently, the aim of this study was to screen for phytochemicals and validate the use of C. mopane and A. inflata, in folk medicine in Namibia.
Laboratory analysis of the phytochemistry and antimicrobial activity of crude extracts of C.mopane and A. inflate was done. The
experiments were conducted at the laboratory of School of Pharmacy, University of Namibia.
Plant material preparation
Plant specimens of A. inflata and C. mopane were collected from the Omugulugoonime village, Omuntele constituency in the Oshikoto region with the help of a local traditional healer. These specimens were subsequently validated at the National Botanical Research institute, Windhoek (voucher numbers: C.mopane-----, A. inflata -----). The plant samples, that is, the bark and leaves of C. mopane and the whole plant of A. inflata were rinsed with tap water, chopped into small pieces and air-dried at room temperature (23 to 26°C). The dry plant samples were then pulverized into fine powder using a laboratory mechanical mill. Crude extraction of the powders of the whole plant of Ac. inflata as well as the leaves and bark of C. mopane was done by maceration using ethanolic and aqueous solvents as described by Mutembei et al., (2011). This extraction method mimics the traditional processes for preparing concoctions of C. mopane and A. inflata. Aqueous extraction was done by soaking 10 g of the crude plant powder in 200 mL of distilled water in a conical flask. The aqueous mixture was subsequently heated at 70°C for 2 h using a hot plate. The aqueous extraction was done in triplet so as to obtain the mean percentage yield. The resulting mixture was cooled at room temperature and was subsequently filtered with Whatman Filter Paper No. 1 to remove the solvent and was subsequently concentrated by heating at 70°C. Organic extraction was done by macerating 10 g of the crude plant powders of C. mopane and A. inflata in 200 mL of ethanol at room temperature for 4 days. The organic extraction was also done in triplicate. The resulting mixture were filtered using Whatman Filter Paper No. 1. The filtrate was concentrated using a Rota evaporator at 40°C. The aqueous and ethanolic extracts were stored in the fridge until phytochemical and antimicrobial activity tests.
Test microorganisms
Phytochemical analysis
Phytochemical analysis of the aqueous and ethanolic extracts of C. mopane and A. inflata were performed according to standard methods described by Harbone (1989) and Tiwari et al., (2011). A mixture of aqueous (2 ml) extract and Ferling’s solution (3 ml) was heated to near boiling, a color change indicated the presence of reducing sugars. To 2 ml of the aqueous extract were diluted with distilled water and 1-2 drops of 0.1% ferric chloride solution were added- a dark-green, blue-green or blue-black color indicated the presence of tannins.
Saponin test
3 ml of the aqueous extract was shaken vigorously in a stoppered test tube- the persistence of froth for at least 5 min indicated presence of saponins. To 3 ml of the aqueous extract, dilute ammonia solution (3 ml) and sulphuric acid (1 ml) were added- a yellow color that disappeared on standing indicated the presence of flavonoids.
Test for cardiac glycosides
To a test tube containing 5 ml of the aqueous extract, 2 ml of glacial acetic acid added followed by a drop wise addition of 1 ml concentrated sulphuric acid. A brown ring at the interface indicated the presence of deoxy-sugars.
Anthraquinones test
To a test tube containing 2 ml of the ethanolic extract, a drop wise addition of 1 ml of dilute ammonia was done. A reddish color in the upper layer indicated the presence of anthraquinones.
Alkaloids test
A thin layer chromatogram (TLC) separation was done on the ethanolic extract. The dried TLC spots were subsequently were sprayed with Dragendorff reagent. A pink color indicated the presence of alkaloids.
Salkowski testfor sterols and steroid
To the 2 ml of the ethanolic extract, a drop wise addition of 3 ml of concentrated sulphuric acid was done. A reddish brown color at the interface indicated the presence of phytosterols.
Test for coumarins
1 ml of the ethanolic extract was spotted on the TLC plate for separation. Also, a mixture of 0.5 ml of diluted ammonia solution and the ethanolic extract was spotted on the other end of the TLC plate. The two spots were observed under UV light. Intense fluorescence indicated the presence of coumarins.
Antimicrobial activity analysis
The antimicrobial activities of the extracts were tested on four human pathogenic organisms: P. aeruginosa, B. subtillis, S. aureus and C. albicans (yeast). The test organisms were obtained from an accredited national laboratory, the Namibia Institute of Pathology (NIP) laboratory. The bacterial strains- P. aeruginosa, B. subtillis and S. aureus were cultured in a nutrient broth at 37°C for 24 h. The fungal strain- C. albicans was cultured on the potato dextroxe agar at 25°C for 24 h.
The antimicrobial activity of the extracts were determined with the mean zones of inhibition using Filter-paper disc-agar diffusion procedure as described by Kirby-Bauer (Bauer et al., 1966). In the Kirby-Bauer method, Whatman’s filter papers were punched into discs of diameters of 6 mm and 10 μL of varying concentrations (20, 10 and 5 mg/ml) of aqueous and ethanolic extracts incorporated using a micropipette. The concentrations were prepared by dissolving the respective quantities of dried ethanolic extract of C. mopane and A. inflata in the dimethyl sulfoxide (DMSO). The discs were allowed to dry and were subsequently stored at room temperature. A volume of 25 ml of sterilized nutrient agar wad added on sterile Petri-plates and allowed to solidify. A volume of 100 μl of fresh culture of human pathogens- P. aeruginosa, B. subtillis and S. aureus were separately applied on the nutrient Agar using a sterile spreader. Whatman’s paper discs with varying concentration of the extracts (20, 10 and 5 mg/ml) were placed on separate petri-plates containing the cultured micro-organisms using sterile forceps. The plates were then incubated at 37°C for 24 h, except the C. albicans which was incubated at 25°C for 24 h.
The zones of inhibition of each extract on each test organism at the three different concentrations were measured for three replicates. Penicillin G and streptomycin were used as positive controls for the antimicrobial susceptibility tests for bacterial activity and antifungal activity, respectively.
DMSO was used as the negative control for all experiments. The mean zone of inhibition was determined as a mean ± standard deviation.
Data analysis
The qualitative and quantitative methods were used to analyze the data. The percentage yield was calculated by using the formula: percentage yield = amount of extract/amount of starting material. The mean for zone of inhibition was calculated. The plant extracts that inhibited the growth of bacterial, indicated that the plant was effective and can be synthesized further to produce the antimicrobial agent. The comparison between the medicinal plants extracts and synthetic antibiotics was investigated. Aqueous and ethanol extracts were used to screen for phytochemicals which indicated certain colour change if there was any kind of phytochemical present.
Ethical considerations
The study was approved by the Faculty of Health Sciences, University of Namibia ethics review board, UNAM laboratory management and local leadership of Omuntele constituency council.
Percentage yield of crude extracts
Aqueous extracts gave higher percentage yields (23.79 to 31.62%) as compared to ethanolic extracts (8.16 to 18.11%).
Leaves of C. mopane gave the highest yield for both the aqueous and ethanolic extracts, as compared to the bark of C. mopane and whole plant of A. inflata (Figure 3).
Phytochemical profile
Table 1 show the phytochemicals identified in extracts of C. mopane and A. inflata. Aqueous extracts of inflate leaves and bark C. mopane gave positive tests for tannins, saponins, flavonoids and cardiac glycosides. The test was more reactive with the bark as compared to the leaf extracts. Aqueous extract of A. inflata tested positive for tannins and cardiac glycosides. The test for reducing sugars in the C. mopane and A. inflata were negative. Only the ethanolic extracts of the bark of C. mopane were positive for alkaloids, sterols and steroidsand coumarins. The enthanolic extracts of leaves of C. mopane and the whole plant of A. inflata were also positive for coumarins (Table 1).
Antimicrobial potential of C. mopane and A. inflata
Table 2 and Figure 4 show the mean zone of inhibition (±SD) of the aqueous and ethanolic extracts of C. mopane and A. inflata. Generally, the ethanolic extracts showed higher antimicrobial activity than the aqueous extracts on all the bacterial test organisms. Antimicrobial activity against S. aureus was higher with the aqueous (10.7±1.3 mm) than the ethanolic leaf (9.7±2.1 mm) and bark (9.0±1.0 mm) extracts of C. mopane. This was however lower than the positive controls. The aqueous and ethanolic extracts of A. inflata had no activity against S. aureus. There was antimicrobial activity against strains of P. aeruginosa with the aqueous extract of the bark of C. mopane (10.7±3.1 mm), ethanolic extract of the leaves (12.7±0.6 mm) and bark (11.3±0.6 mm) of C. mopane, as well as the ethanolic extract of A. inflata (11.7±2.1 mm). This was however lower than the activity of the positive controls against Pseudomonas.
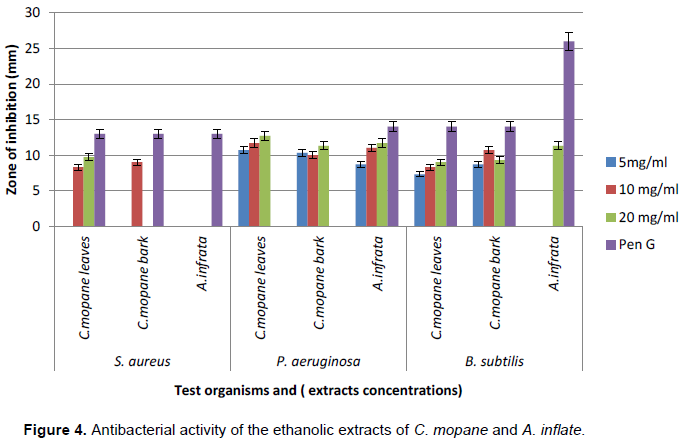
None of the aqueous extracts of the leaves and bark of C. mopane as well as A. inflata had activity against B. subtilis. All the ethanolic extracts of C. mopane leaves (9.0±1.0 mm) and bark (10.7±1.2 mm) as well as A. inflata (11.3±3.2 mm) had activity against strains of B. subitlis. C. albicans was not susceptible to any of the aqueous and ethanolic extracts.
Ethanolic extracts of C. mopane leaves and bark had significant antimicrobial activity against P. aeruginosa, S. aureus and B. subtilis (Table 2).
The antimicrobial activities of ethanolic extracts against Pseudomonas were comparable to the positive control penicillin G. The zone of inhibition for Penicillin G was highest with B. subtilis (26 mm) and lowest with S. aureus. Econazole had minimum zone of inhibition against C. albicans.
C. mopane and A. inflata gave significant yields of crude extracts. C. mopane and A. inflata are both higher plant and cover a wide area of Namibia and thus cultivation of these plants on large scale is commercially viable (Dushimemaria, 2014).
The phytochemical analysis revealed the presence of tannins, saponins, flavonoids and cardiac glycosides in the leaf and bark aqueous extracts of C. mopane. The aqueous extracts of A. inflata were positive for tannins and cardiac glycosides (Table 1). The findings in this study are similar to a study on Sphenostylis stenocarpa, both belonging to the Fabaceae family, also tested positive for presence of flavonoids, tannins and alkaloids (Nyananyo and Nyingifa, 2011). Similar studies have associated antimicrobial and/or activity of certain plants to the presence oftannins (Athanasiadou, 2001) and saponins (Avato et al., 2006) have been reported to have antimicrobial properties. The organic extract of the bark of C. mopane was positive for alkaloids, steroids and sterols and coumarins. Alkaloids have been documented to have several pharmacological properties including antimicrobial activity, antioxidant activity and analgesic properties. A study by Tiwari et al. (2011) has associated flavonoids, coumarins and tannins with antimicrobial activities in medicinal plants (Tiwari et al., 2011). This may explain why the bark of C. mopane is widely used as a folk medicine to manage inflammatory conditions as well as acute infections of the gastrointestinal, respiratory systems and well as wounds. However, the presence of coumarins may have safety implications as they have been documented to have anticoagulation effects that may aggravate bleeding. This calls for further investigation of the safety profile of preparations of C. mopane used in folk medicine in Namibia.
The extracts of C. mopane had antibacterial activity against S. aureus, Pseudomonas and B. subtillis. The plant extracts exhibited more antimicrobial activity against P. aeruginosa more than B. subtillis and S. aureus. The antimicrobial activity of the ethanolic extracts of C. mopane on Pseudomonas strains was comparable to penicillin G and streptomycin, the positive control. This result suggests that phytochemicals in C. mopane have activity against both aerobic Gram positive and facultative Gram negative bacteria. This may explain why preparations of C. mopane are widely used in the management of wounds, diarrhea, coughs and inflammatory diseases. Thus, further research is required to purify, characterize and test for antibacterial activity of the saponins, tannins and alkaloids in C. mopane that have been associated with antimicrobial activity (Table 1). Extracts of A. inflata showed antimicrobial activity against P. aeruginosa and B. subtillis but not S. aureus (Figure 4). This finding suggests that A. inflata has major activity on facultative Gram negative bacteria and anaerobic Gram positive bacteria limited activity on Gram positive bacteria. This explains the use of preparations of A. inflata in management of stomach upsets that are mainly attributed to Gram negative bacteria. The antimicrobial activity of extracts from C. mopane and A. inflata on all the test organisms was lower than the penicillin G, the positive controls (Figure 4) (this is attributed to the insufficient quantities of phytochemicals responsible for antimicrobial activity in the crude extracts, particularly, if the activity is dose dependent.
The organic extracts had more activity against the test organisms than the aqueous extracts (Table 2). Higher activity of organic extracts has been attributed to the ability of organic solvent systems to isolate a variety of bioactive compounds and activity (Fouche et al., 2008). However, the percentage yield was higher than the aqueous extraction than the ethanolic extract (Figure 3). The water preparations of C. mopane and A. inflata are widely used traditionally in Namibia. However, in traditional practice, C. mopane leaves are chewed and swallowed or applied on the wound and not boiled. Traditional therapies of A. inflata are prepared by boil in water for few minutes or the fresh plant material is soaked in hot water to make tea.
Thus, the methods used aqueous extraction using boiling may have had an effect on the antimicrobial activity of C. mopane (Pradeepa et al ., 2016; Clarkson et al., 2004). Further research is required to isolate most of the active phytochemicals using more efficient solvent systems with varying polarities and less thermolabile techniques.
The study concludes that aqueous and organic extracts of C. mopane and A. inflata contain phytochemicals with antimicrobial activity (Jäger et al., 2005). The extracts have antibacterial activity on P. aeruginosa, B. subtilis and S. aureus but lack antifungal. The lack of antifungal activity of A. inflata is contrary to the findings in Botswana that found antifungal activity (Mukanganyama etal., 2011). This is mainly because the study in Botswana used extracts from the leaf unlike our study that assessed the plant as a whole activity. Organic extracts have better antimicrobial activity as compared to the aqueous extracts for C. mopane and A. inflata and have a wide activity against aerobic Gram positive bacteria (S. aureus), Gram positive anaerobes (B. subtilis) and facultative Gram negative bacteria (Pseudomonas). The organic extracts of C. mopane have superior antibacterial activity against pseudomonas than other test organisms.
Consequently, this study provides evidence for further research on the antimicrobial potential against resistant bacterial strains including P. aeruginosa and B. subtilis of ethanolic extracts of C. mopane leaves and bark as well as A. inflata. Future studies should also focus on optimizing the extraction of phytochemicals using different solvent systems in order to achieve a greater yield of bioactive compounds. The phytochemicals with antimicrobial activity should be purified and characterized for antipseudomonal activity of these plant extracts. There is need to perform acute and chronic toxicity studies on the extracts of C. mopane and A. inflata so as to outline the safety of these plants.
Limitations
In this study, only aqueous and ethanol solvents were used to isolate phytoconstituents. In future, organic solvent systems such as methanol and chloroform should be used. The use of aqueous and ethanolic extracts was used in order to mimic the solvents and processes used to prepare the medicines in practice. Secondly, antimicrobial activity was not tested on E. coli, a common organism involved in gastrointestinal and urogenital infections. Specimens of E. coli were not accessible from the Namibia Institute of Pathology (NIP) at the time of the study. However, the use of strains of Pseudomonas represented the facultative Gram negative bacteria to which E. coli belongs.
The authors have not declared any conflict of interests.
REFERENCES
|
Athanasiadou S, Kyriazakis I, Jackson F, Coop RL (2001). Direct anthelmintic effects of condensed tannins towards different gastrointerstinal nematodes of sheep: in vitro and in vivo studies. Vet. Parasitol. 99:205-219.
Crossref
|
|
|
|
Avato P, Bucci R, Tava A, Vitali C, Rosato A, Bialy Z, Jurzysta M (2006). Antimicrobial Activity of Saponins from Medicago sp.:Structure-Activity Relationship. Phytother. Res. 20:454-457.
Crossref
|
|
|
|
|
Bainbridge H (2012). Desert Plants: Indigenous use of mopane (Colophospermum mopane) in northwestern Namibia. Available from URL:
View
|
|
|
|
|
Bauer A, Kirby W, Sherris J, Turck M (1966). Antibiotic susceptibility testing by a standardized single disk method. Am. J. clin. Pathol. 45(4):493.
|
|
|
|
|
Carlet J, Jarlier V, Harbarth S, Voss A, Goossens. (2012). Ready for a world without antibiotics? The pensières antibiotic resistance call to action. Antimicrob. Resist. infect. control 1(1):1.
Crossref
|
|
|
|
|
Cheikhyoussef A, Mapaure I, Shapi M (2011). The use of Some Indigenous Plants for Medicinal and other Purposes by Local Communities in Namibia with Emphasis on Oshikoto Region. 5(4):406-419.
|
|
|
|
|
Clarkson C, Maharaj VJ, Crouch NR, Grace OM, Pillay P, Matsabisa MG, Bhagwandin N, Smith PJ, Folb PI (2004). In vitro antiplasmodial activity of medicinal plants native or naturalized in South Africa. J. Ethnopharmacol. 92:177-191.
Crossref
|
|
|
|
|
Cowan MM (1999). Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12(4):564-582.
|
|
|
|
|
Cunningham A (1993). African medicinal plants: setting priorities at th einterface between conservation and primary ealth care. UNESCO: Paris, France: United Nations Educational, Scientific and Cultural Organization. Available from URL:
View
|
|
|
|
|
Dushimemaria F (2014). An Investigation into the Antineoplastic Properties of Schinziophyton rautonenii and Colophospermum
|
|
|
|
|
Fabricant DS, Farnsworth NR (2001). The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 109(1):69-75.
Crossref
|
|
|
|
|
Fouche G, Cragg GM, Pillay P, Kolesnikova N, Maharaj VJ, Senabe J (2008). In vitro anticancer screening of South African plants. J. Ethnopharmacol. 119:455-461.
Crossref
|
|
|
|
|
Ganatra SH, Durge SP, Patil SU (2012). Preliminary Phytochemicals Investigation and TLC Analysis of Ficus racemose Leaves. J. Chem. Pharm. Res. 4(5):2380-2384.
|
|
|
|
|
Grote K (2003). The increased harvest and trade of Devil's Claw (Harpagophytum procumbens) and its impacts on the peoples and environment of Namibia, Botswana and South Africa. Italy: Global Facilitation Unit for Underutilized Species.
|
|
|
|
|
Harborne AJ (1998). Phytochemical methods a guide to modern techniques of plant analysis. Springer Science & Business Media
|
|
|
|
|
Hedimbi M, Chinsembu KC (2012). Ethnomedicinal study of plants used to manage HIV/AIDS-related disease conditions in the Ohangwena region, Namibia. Int. J. Med. Plant Res. 1(1):004-011.
|
|
|
|
|
Hosttetmann K, Wolfender JL, Rodriguez S, Marston A (1996). Strategy in the search for bioactive plant constituents. In Chemistry, Biological and Pharmacological properties of African Medicinal Plants: Proceeding of the First International IOCD Symposium Victoria Falls, Zimbabwe. pp. 25-28.
|
|
|
|
|
Ilonga SK (2012). Anticancer, antioxidants and antimicrobial screening of extracts from Ziziphus mucronata, Heliotropium ciliatum and Gnidia polycephara from Oshikoto region of Namibia.
|
|
|
|
|
Iwu M (2014). Handbook of African medicinal plants. second edition CRC press Taylor and Francis group. Newyork United states of America.
Crossref
|
|
|
|
|
JägerA, Mohoto S, van Heerden F, Viljoen A (2005). Activity of a traditional South African epilepsy remedy in the GABA-benzodiazepine receptor assay. J ethnopharmacol, 96(3): 603-606.
Crossref
|
|
|
|
|
Kamaraj C, Rahuman A, Siva C, Iyappan M, Kirthi A (2012). Evaluation of antibacterial activity of selected medicinal plant extracts from south India against human pathogens. Asian Pacific J. Trop. Dis.2: S296-301.
Crossref
|
|
|
|
|
Khan A, Musharraf A (2004). Plasmid-mediated multiple antibiotic resistance in Proteus mirabilis isolated from patients with urinary tract infection. Medical science monitor: international medical journal of experimental and clinical research. 10(11):CR598-602.
|
|
|
|
|
Lewis K, Ausubel F (2006). Prospects for plant-derived antibacterials. Nat. biotechnol. 24(12): 1504-1507.
Crossref
|
|
|
|
|
Mahesh B, Satish S (2008). Antimicrobial activity of some important medicinal plant against plant and human pathogens. World j agric sci, 4(5): 839-843.
|
|
|
|
|
Mengistu A, Gaeseb J, Habimana G (2014). A Review of Empirical Treatment of Urinary Tract Infections Based on National Antimicrobial Sensitivity Data. Enliven: Pharmacovigil. Drug Saf. 1(1):2.
Crossref
|
|
|
|
|
Monroe S, Polk R (2000). Antimicrobial use and bacterial resistance. Current opinion in microbiology 3(5): 496-501. mopane. University of Namibia.
Crossref
|
|
|
|
|
Mukanganyama S, Ntumy A, Maher F, Muzila M, Andra (2011). Screening for anti-infective properties of selected medicinal plants from Botswana. Afr. J. Plant Sci. Biotechnol. 5(1):1-7.
|
|
|
|
|
Musvoto C, Mapaure I, Gondo T, Ndeinoma A, Mujawo (2007). Reality and references in community mopane (Colophospermum mopane) woodland management in Zimbabwe and Namibia. International Journal of Social Sciences., 1(3):173-177.
|
|
|
|
|
Mutembei J, NjongeF, Kutima H, Karanja J (2011). Antimicrobial and phytochemical evaluation of selected medicinal plants in Meru community of Kenya. Nairobi, Kenya: Jomo Kenyatta University of Agriculture and Technology. Available at:
View
|
|
|
|
|
Nafuka S (2014). In vitro Antiplasmodial activity and phytochemicals screening of ethnomedicinal plants used to treat Malaria associated symptoms. Windhoek, Namibia: University of Namibia. Available from:
View
|
|
|
|
|
Nordenstam B (1970). Notes on the flora and vegetation of Etosha Pan, South West Africa. Dintera, 5:3-6.
|
|
|
|
|
Nyananyo B, Nyingifa A (2011). Phytochemical investigation on the seed of Sphenostylis stenocarpa (Hochst ex A. Rich.) Harms (Family Fabaceae). JASEM 15 (3):419-423.
|
|
|
|
|
Parekh J, Chanda S (2007). Antibacterial and Phytochemical studies on Twelve Species of Indian Medicinal Plants. pp.175-181.
|
|
|
|
|
Pradeepa M, Kalidas V, Geetha N (2016). Qualitative and Quantitative Phytochemical Analysis and Bactericidal Activity of Pelargonium Graveolens L'her. Int. J. Appl. Pharm. 8(3):7-11.
|
|
|
|
|
Sharma M, Kumar A (2013). Leguminosae (Fabaceae) in tribal medicines. J. Pharmacogn. Phytochem. 2(1):8192.
|
|
|
|
|
Suliman A (2010). The antimicrobial activity and chemical profile of traditional medicinal plants indigenous to Southern Africa used to treat respiratory tract infections. MSc thesis. Witwatersrand, South Africa: University of Witwatersrand. South Africa. Available at : http://wiredspace.wits.ac.za/xmlui/bitstream/handle/10539/8849/6th%20draft_final%2026.01.10%20(Anisa%20Suliman%209602930y).pdf?sequence=1&isAllowed=y.
|
|
|
|
|
Thirumurugan K (2010). Antimicrobial activity and phytochemical analysis of selected Indian folk medicinal plants. Steroids (10):430-434.
|
|
|
|
|
Thomas J Elpel (2015). Web world portal, Wildflowers-and-Weeds.com. Plant Identification, Edible Plants, Weed Ecology, Mushrooms, and more; patterns of the lamiaceae (mint) family. Green University.Available at:
View
|
|
|
|
|
Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H (2011). Phytochemical screening and extraction: a review. Available at:
View
|
|
|
|
|
Van den Eynden V, Vernemmen P, Van Damme P (1992). The ethnobotany of the topnaar. Universiteit Gent.
|
|
|
|
|
Van Rooyen N, Van RensburgD, Theron G, Bothma J (1988). A check list of flowering plants of the Kalahari Gemsbok National Park. Koedoe, 31(1):115-135.
Crossref
|
|
|
|
|
Von-Koenen E (2009). Medicinal, poisonous, and edible plants in Namibia, 4th edition. Windhoek, Namibia. Klaus Hess Verlag. Gottingen: Germany
|
|
|
|
|
Wagate CG, Mbaria JM, Gakuya DW, Nanyingi MO, Kareru GP, Njuguna A, Gitahi N, Macharia JK, Njonge FK(2009). Screening of some Kenyan Medicinal Plants for Antibacterial Activity. Phytother. Res. 150-153.
|
|
|
|
|
Walt P van der, Riche E (1999). The Kalahari and its plants. 1st ed. Info Naturae. ifo Naturae. Pretoria: South Africa.
|
|
|
|
|
WHO (World Health Organisation) (2012). Traditional medicines. Geneva, Switzeland: World Health Organisation. Available from URL:
View
|
|
|
|
|
WHO (World Health Organisation) (2016). "A global brief on hypertension: silent killer, global public health crisis.". Geneva: World Health Organisation.
|
|
|
|
|
WHO (World Health Organization) (2013). Global tuberculosis report 2013. Geneva, Switzeland: World Health Organization.
|
|