ABSTRACT
Entamoeba histolytica and Giardia lamblia are parasitic protozoa that cause gastrointestinal disorders such as diarrhea and dysentery. Naegleria fowleri is a free living amoeba that causes primary amoebic meningoencephalitis. However, there are limited treatments for these parasitic diseases. Extracts, fractions from extracts and some isolated compounds from selected Ghanaian medicinal plants were screened against Naegleria fowleri, Giardia lamblia and Entamoeba histolytica in the search for newer and safer agents for the treatment of infections caused by these parasites. Of all the extracts and compounds tested for the activity against E. histolytica, only xylopic acid and geraniin were active with IC50 values of 4.80 μg/mL (13.30 μM) and 34.71 μg/mL (36.44 μM), respectively. Metronidazole, the positive control had an IC50 of 1.287 μM. All other extracts and fractions exhibited IC50 values >100 µg/mL. For G. lamblia, extracts of Albizia glaberrima, Margaritaria nobilis, Maerua angolensis and Ulva fasciata, the ethyl acetate fraction of Erythrophleum ivorense bark extract, and the isolated compound, xylopic acid exhibited IC50 values of 15.91, 44.25, 20.00, 35.86, 13.76 and 11.45 µg/mL, respectively. The IC50 of the positive control agent metronidazole, was 10.47 µM. The extract of A. glaberrima and xylopic acid exhibited IC50 values of 38.70 and 16.06 µg/mL, respectively, against N. fowleri. The IC50 of the reference drug, amphotericin B, was 0.2 µM. Thus, Ghanaian medicinal plant extracts, their fractions and isolated compounds possess anti-parasitic activity.
Key words: Naegleria fowleri, Giardia lamblia, Entamoeba histolytica, medicinal plants, geraniin, xylopic acid, Albizia glaberrima, Margaritaria nobilis, Maerua angolensis, Ulva fasciata
Plants have been exploited for their medicinal use Since 1500 BC (Chopra and Doiphode, 2002). They serve as a source of medicine and are used to treat and prevent several infections, diseases and other ailments. The use of plant medicines is widely accepted in the culture and traditions of indigenous Africans and other nationalities such as India, China and Sri Lanka (Calixto, 2005; Ayyanar and Ignacimuthu, 2011).
There have been several reports on the use of medicinal plants in Ghana for wound infections and other diseases; the use of Erythrophelum ivorense (A. Chev.) in treating wounds (Adu-Amoah et al., 2014) and the use of Myrianthus arboreus and Alchornea cordifolia for treating wounds and other infections in Ghana (Agyare et al., 2014).There have also been reports on the use of Hilleria latifolia as an antinociceptive agent (Woode and Abotsi, 2011). The analgesic and anti-inflammatory activities of Xylopia aethiopica have been reported (Woode et al., 2012) and Phyllanthus muellerianus has been reported to possess anti-inflammatory activities (Boakye et al., 2016).
Entamoeba histolytica and Giardia lamblia are parasitic protozoa that cause gastrointestinal disorders such as diarrhea and dysentery. Metronidazole, the first line drug for the treatment is reported to have unpleasant side-effects such as a metallic taste, headache and dry mouth, and to a lesser extent nausea, glossitis, urticaria, pruritus and dark colored urine. In addition, carcinogenic, teratogenic and embryogenic properties have been documented when metronidazole is administered (Upcroft et al., 1999; Upcroft and Upcroft, 2001).
Naegleria fowleri is a free-living amoeba that causes primary amoebic meningoencephalitis (PAM). PAM is mainly managed with amphotericin B which has a very narrow therapeutic index making it toxic for use. The toxicity and adverse effects associated with the drugs used for the treatment of these parasitic infections underscore the need for newer medicines that are safe and effective for treating infections caused by these parasites. In this light, some Ghanaian medicinal plants were selected and screened against these parasites. The plants investigated were selected due to their antimicrobial and anti-inflammatory properties, and their ethnopharmacological uses in Ghana.
Plant materials
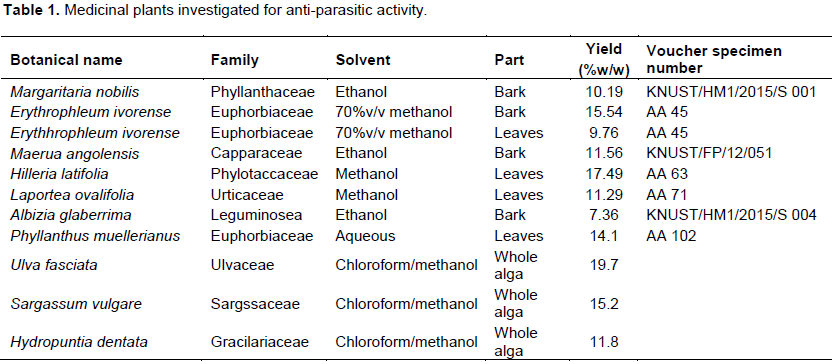
Plants materials (Table 1) were obtained from the Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana. Xylopic acid and geraniin were isolated from X. aethiopica and Phyllanthus muellerianus, respectively. Marine algae were obtained from the beaches at Prampram, Sakumono, Osu and James town in the Greater Accra region of Ghana. The plant samples were authenticated by Prof. Alex Asaase of the Department of Botany, University of Ghana, Legon, Ghana. Voucher specimens are kept at the Ghana Herbarium, University of Ghana, Legon, Ghana. The algae samples were authenticated by Mr. Emmanuel Klubi of the Department of Marine Science and Fisheries, University of Ghana, Legon, Ghana (Table 1).
Preparation of plant and algae materials
The extracts were prepared by cold maceration of 300 g of powdered dry plant material in stoppered flasks containing 700 mL of the respective solvent (acetone, ethyl acetate, pet ether and methanol (Sigma-Aldrich, MO, USA) for 1 week at room temperature (28°C). After filtration using Whatmann filter paper No. 1 (Whatmann, London, UK), the solvent was evaporated under reduced pressure in a rotary evaporator at 40°C until a solid mass was obtained. The percentage yield of the various extracts related to the dried powdered plant material was determined (Table 1). The different extracts were tightly sealed in glass vials and stored in the refrigerator at 4°C. Exhaustive successive extraction was performed on Erythrophleum ivorense bark and leaf to obtain acetone, ethyl acetate, pet ether and methanol fractions from the extracts. This was to obtain fractions of different polarities and find the most active fraction. This would also help in activity guided isolation of the active ingredient(s).
Source of compounds
Geraniin (96% w/w HPLC grade) was kindly provided by Prof. Dr. Andreas Hensel, Institute of Pharmaceutical Biology and Phytochemistry, University of Muenster, Muenster, Germany and had been isolated from the aqueous extract of the aerial parts of P. muellerianus and it was found to be the major compound (4.3% w/w, related to the dried plant material) (Agyare et al., 2011).
Xylopic acid (95% w/w) was obtained from Prof. Dr. David Obiri Danso, Department of Pharmacology, Faculty of Pharmacy and Pharmaceutical Sciences, KNUST, Kumasi, Ghana. Xylopic acid (1.47% w/w, related to plant extract) was isolated from the fruits of X. aethiopica (Woode et al., 2012).
Test parasites
N. fowleri strain KUL, E. histolytica strain HM1:IMSS and G. lamblia WB strain used in all the experiments were maintained at the Center for Discovery and Innovation in Parasitic Diseases, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California, San Diego, USA (CDIPD, SSPPS, UCSD, USA). E. histolytica was maintained in TYI-S-33 medium (Diamond et al., 1978) supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL) and 10% heat inactivated adult bovine serum (Sigma-Aldrich, MO, USA). G. lamblia trophozoites were cultured in TYI-S-33 modified medium supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL) and 10% v/v heat inactivated fetal bovine serum. N. fowleri was maintained axenically in Nelson’s culture medium supplemented with 10% heat inactivated FBS and 1x penicillin (100 U/mL), streptomycin (100 μg/mL) in vented flasks.
The trophozoites were axenically maintained at 37°C and 5% CO2 and the assays were performed with trophozoites maintained in the log phase of growth.
In vitro susceptibility assay
In vitro susceptibility assays were performed using a modified CellTiter Glo® (Promega, Madison, WI, USA) method as described by Debnath et al. (2014). Briefly, 5 x 103 trophozoites of G. lamblia and E. histylotica and 10 x 103 trophozoites of N. fowleri were incubated for 48 h at 37°C in the presence of the various extracts with concentrations within the range of 0.78 to 100 µg/mL. DMSO (Sigma-Aldrich, MO, USA) was used as solvent for preparing the concentrations of the extract. Metronidazole was used as positive control for G. lamblia and E. histylotica and amphotericin B for N. fowleri. A negative control (culture medium plus trophozoites and DMSO), and a blank (culture medium) were also included in the experimental setup. After incubation, the assay plates were equilibrated at room temperature (25°C) for 30 min and 50 μL of CellTiter-Glo® was added to each well. The plates were placed on an orbital shaker for 10 min to induce cell lysis and equilibrated at room temperature (25°C) for another 10 min to stabilize the signal. The procedure was performed in triplicates. The luminescent signal, resulting from the lysis of the cells was measured with an EnVision luminometer (Software version 1.13.3009.1401) (PerkinElmer, USA), and converted into the percentage of inhibition of the cell growth relative to maximum and minimum reference signal controls using the following equation:

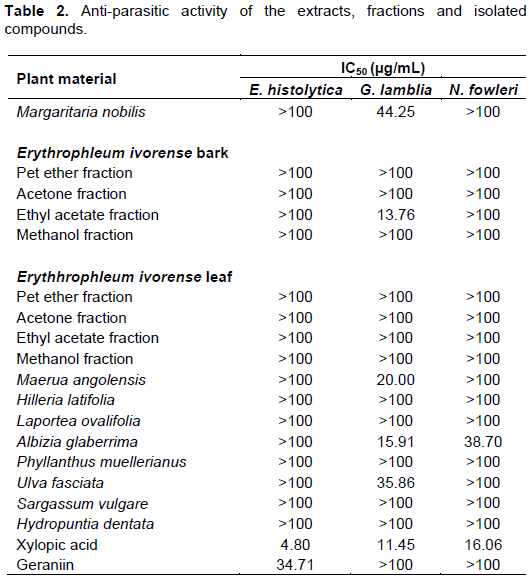
For E. histolytica, only xylopic acid and geraniin were active with IC50 values of 4.80 (13.30 μM) and 34.71 μg/mL (36.44 μM), respectively (Table 2). Metronidazole, the positive control had an IC50 of 1.287 μM. All other extracts and fractions exhibited activity more than 100 µg/mL.
Extracts of A. glaberrima, M. nobilis, M. angolensis, U. fasciata, ethyl acetate fraction of the extract of E. ivorense bark and xylopic acid had IC50 values of 15.91, 44.25, 20.00, 35.86, 13.76 and 11.45 µg/mL, respectively, against G. lamblia (Table 2). The IC50 of the control metronidazole was 10.47 µM.
The extract of A. glaberrima and xylopic acid exhibited IC50 of 38.70 and 16.06 µg/mL (44.55 µM), respectively, against N. fowleri (Table 2). The IC50 of the reference drug amphotericin B was 0.2 µM. The IC50 of the extracts and fractions were calculated using the mean and standard deviations of the percentage inhibition. The IC50 signifies the amount or concentration of the extracts and fractions that kills 50% of the parasites. The lower the IC50, the more active the said extract or fraction and vice versa.
Several studies have been carried out over the years and it has been proven that plants and their isolates can be a source of anti-parasitic agents. Barbosa et al. (2007) also reported that epicatechin, a flavonoid isolated from the Geranium mexicanum exhibited potent activity against G. lamblia more than metronidazole which is widely used as the main therapy. From our results, it was observed that xylopic acid exhibited anti-parasitic activity against all the three parasites at concentrations of less than 50 µg/mL. All the parasites tested cause inflammatory conditions and xylopic acid has been shown to possess anti-inflammatory properties (Obiri et al., 2014). Terpenes are well known to be active against protozoan parasites (Phillipson and Wright, 1991). This could be the reason for the activity exhibited by xylopic acid. McGaw et al. (2000) reported that plant extracts and compounds containing tannins and alkaloids possess activity against diarrhea-causing parasites such as G. lamblia and E. histolytica.
U. fasciata and A. glaberrima (Jato, 2015), E. ivorense (Adu-Amoah et al., 2014) and M. nobilis (Mothana et al., 2009) contain alkaloids and tannins. These bioactive constituents could have been responsible for the activity exhibited by these extracts and fractions. For all these activities observed, it could be attributed to the nature of phytochemical constituents present in the extracts and fractions. Geraniin is known to have anti-inflammatory activity (Boakye et al., 2016) and this could be the reason for its activity against E. histolytica, which causes inflammatory conditions such as amoebic colitis (Stanley, 2003). Xylopic acid could be said to be more active against E. histolytica than G. lamblia and N. fowleri since the IC50 values for the parasites increased in that order. Ethyl acetate fraction of bark extract of E. ivorense exhibited activity against G. lamblia, whereas the fractions from the leaf had no activity. The extracts from A. glaberrima, M. nobilis, M. angolensis, the bark of E. ivorense and U. fasciata exhibited some activity against at least one of the parasites and therefore could be said to possess anti-parasitic activity. The extracts from the plants: H. latifolia, L. ovalifolia, P. muellerianus, and the algae H. dentata and S. vulgare, exhibited no activity against any of the parasites. Geraniin, which is an isolate from the aqueous leaf extract of P. muelleranus, exhibited activity against E. histolytica. It is possible that in the aqueous extract, the amount of the geraniin was not enough to elicit an anti-parasitic effect.
Xylopic acid was active against E. histolytica, G. lamblia and N. fowleri. A. glaberrima exhibited activity against N. fowleri and G. lamblia. The ethyl acetate fraction of the methanol bark of E. ivorense, extracts of M. angolensis, M. nobilis and U. fasciata exhibited activity against G. lamblia.
The authors declare that there is no conflict of interest.
REFERENCES
|
Adu-Amoah L, Agyare C, Kisseih E, Ayande PG, Mensah KB (2014). Toxicity assessment of Erythrophleum ivorense and Parquetina nigrescens. Toxicol. Reports. 1:411-420.
Crossref
|
|
|
|
Agyare C, Owusu-Ansah A, Ossei PPS, Apenteng, JA, Boakye YD (2014). Wound healing and anti-infective properties of Myrianthus arboreus and Alchornea cordifolia. Med. Chem. 4(7):533-539.
Crossref
|
|
|
|
Agyare C, Lechtenberg M, Deters A, Petereit F, Hensel A (2011). Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell.: Geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine 18:617-624.
Crossref
|
|
|
|
Ayyanar M, Ignacimuthu S (2011). Ethnobotanical survey of medicinal plants commonly used by Kani tribals in Tirunelveli hills of Western Ghats, India. J. Ethnopharmacol. 134(3):851-864.
Crossref
|
|
|
|
Barbosa E, Calzada F, Campos R (2007). In vivo antigiardial activity of three flavonoids isolated of some medicinal plants used in Mexican traditional medicine for the treatment of diarrhea. J. Ethnopharmacol. 109(3):552-554.
Crossref
|
|
|
|
Boakye YD, Agyare C, Abotsi WKM, Ayande PG, Ossei PPS (2016). Anti-inflammatory activity of aqueous leaf extract of Phyllanthus muellerianus (Kuntze) Exell. and its major constituent, geraniin. J. Ethnopharmacol. 187:17-27.
Crossref
|
|
|
|
Calixto JB (2005). Twenty-five years of research on medicinal plants in Latin America: a personal view. J. Ethnopharmacol. 100(1):131–134.
Crossref
|
|
|
|
Chopra A, Doiphode VV (2002). Ayurvedic medicine: core concept, therapeutic principles and current relevance. Med. Clin. North Am. 86(1):75-89.
Crossref
|
|
|
|
Debnath A, Shahinas D, Bryant C, Hirata K, Miyamoto Y, Hwang G, Gut J, Renslo AR, Pillai D R, Eckmann L, Reed SL, McKerrow JH (2014). Hsp90 inhibitors as new leads to target parasitic diarrheal diseases, Antimicrob. Agents Chemother. 58(7):4138-44.
Crossref
|
|
|
|
Diamond LS, Harlow DR, Cunnick CC (1978). A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72(4):431-32.
Crossref
|
|
|
|
Jato J. (2015). Anti-inflammatory, antimicrobial and antioxidant properties of Margaritaria nobilis, Stylochiton lancifolius, Drypetes principum, Crescentia cujete and Albizia glaberrima (MPhil Thesis, Kwame Nkrumah University of Science and Technology, Kumasi).
|
|
|
|
McGaw LJ, Jäger AK, Van Staden J (2000). Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. J. Ethnopharmacol. 72(1):247-263.
Crossref
|
|
|
|
Mothana RA, Lindequist U, Gruenert R, Bednarski PJ (2009). Studies of the in vitro anticancer, antimicrobial and antioxidant potentials of selected Yemeni medicinal plants from the Island Soqotra. BMC Complement. Altern. Med. 9(1):7.
Crossref
|
|
|
|
Obiri DD, Osafo N, Ayande PG, Antwi AO (2014). Xylopia aethiopica (Annonaceae) fruit extract suppresses Freund׳ s adjuvant-induced arthritis in Sprague-Dawley rats. J. Ethnopharmacol. 152(3):522-531.
Crossref
|
|
|
|
Phillipson JD, Wright CW (1991). Antiprotozoal agents from plant sources. Planta Med. 57(S 1):S53-S59.
|
|
|
|
Stanley SL (2003). Amoebiasis. The Lancet. 361(9362): 1025-1034
Crossref
|
|
|
|
Upcroft J, Campbell RW, Benakli K, Upcroft P, Vanelle P (1999). Efficacy of new 5-nitroimidazoles against metronidazole-susceptible and -resistant Giardia, Trichomonas, and Entamoeba spp. Antimicrob. Agents Chemother. 43(1):73-76.
|
|
|
|
Upcroft P, Upcroft J (2001). Drugs targets mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 14(1):150-164.
Crossref
|
|
|
|
Woode E, Abotsi WKM (2011). Antinociceptive effect of an ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H. Walt. (Phytolaccaceae). J. Pharm Bioall Sci. 3(3):384-96.
Crossref
|
|
|
|
Woode E, Ameyaw EO, Boakye-Gyasi E, Abotsi WK (2012). Analgesic effects of an ethanol extract of the fruits of Xylopia aethiopica (Dunal) A. Rich (Annonaceae) and the major constituent, xylopic acid in murine models. J. Pharm Bioall. Sci. 4(4):291-301.
Crossref
|