ABSTRACT
Malawian public hospitals have reportedly been experiencing a lot of shortages in medicines and medical supplies in recent years. This was at least in part, attributed to the traditional placement of the drug supply system under the Ministry of Health, and therefore a change in the organizational set-up was implemented in 2011. This study aimed at finding out if the availability of medicines in central hospitals in Malawi improved after the change of Central Medical Stores (CMS) to a Trust (CMST). A retrospective cohort study was done to quantify the availability of selected essential medicines before and after the change of CMS from stock cards. A questionnaire was filled by 23 health professionals to assess their views on whether the change of CMST resulted in improved availability of medicines. The study was done at Queen Elizabeth Central Hospital (QECH), Kamuzu Central Hospital (KCH) and Central Medical Store Trust. The targeted study period was before the change of CMS (2010/2011) and after (2013/2014) the change to CMST. The results of the study showed considerable reduction in stock-out days for both KCH and the CMST (from an average of 80 and 16 days to 42 and 9 days, respectively), with CMST results being statistically significant (p=0.023). However, in QECH, there was no improvement (from 22 to 24 days). The view of most respondents was that there was no improvement in medicine availability after the change of CMS, which represented a certain contradiction to the results of the quantitative part. This may be attributed to the fact that the questionnaire targeted only participants from QECH and KCH and left out participants from CMST. The study indicated that the radical shift in the management of CMS was followed by an improvement of drug availability in CMST itself, and in one of the two investigated hospitals. The non-improvement in drug availability in hospitals calls for further investigation in the future to understand the reasons for this.
Key words: Central medical stores, Malawi, medicine availability, essential medicine, autonomous supply agency, pharmaceutical logistics, supply chain.
The lack of access to essential medicines in developing countries is one of the most pressing global health issues and had an effect on the achievement of Millennium Development Goals (MDG). Also, the change of MDG to the Sustainable Development Goals (SDG) would be meaningless if lack of essential medicines in low income countries is not solved (UN Millennium Project, 2005; Ministry of Health-Malawi, 2009). Many low-income countries are still facing acute shortages of essential medicines because of the limited supply of affordable medicines and inadequate logistical systems to deliver them, and a continuing shortage of new products to meet developing country’s health needs. As such, efficient medicine logistic and supply management is viewed as the key strategy in reducing costs of drugs and ensuring their availability in the healthcare facilities (WHO June, 2004).
Essential medicines are those medicines that satisfy the priority health care needs of the majority of the population (Baumgarten et al., 2011; Gyimah et al., 2009). They are selected with due regard to public health relevance, evidence on efficacy and safety, and comparative cost-effectiveness ("WHO Model List of Essential Medicines," 2013). Since 1977, the World Health Organization (WHO) has published a model list of essential medicines, and Malawi created its own Essential Medicines List in 1987. Since then, Malawi has revised its list of essential medicines, approximately every five years.
Essential medicines are provided to the people of Malawi free of charge at all public health facilities, and at a heavily subsidized fee in Christian affiliated hospitals. This is due to the fact that majority of Malawians are poor, and the World Bank has consistently ranked Malawi as one of the ten poorest countries in the world in the last ten years (International Fund for Agricultural Development, 2011; Index Mundi, 2015). Because of the extreme poverty in the country, thus 53% of the population is below poverty line (Index Mundi, 2015); it becomes very difficult for majority of households to pay for the health services. Thus, the government of Malawi provided the free essential medical service to the public which includes free medicines.
In trying to provide free medicines and medical supplies, the Malawi government established in 1968 a centralized facility (Central Medical Stores-CMS) to procure medicines and medical supplies for the country’s public sector, for the Christian Health Association of Malawi (CHAM) institutions and some private non-profit health institutions (Supply Chain Management Assistance in Malawi, 2011; Department for International Development-DfID UK, 2011). At that time, this was the most common approach to getting medicines at a fair price for the population, and is sometimes referred to as a traditional CMS.
The main objectives of a traditional CMS are warehousing, procurement, and distribution operations of pharmaceuticals which are to be done under full government control (as owners and executors). In contrast, an autonomous supply agency emerged due to the failures of the traditional CMS, and this differs from the traditional CMS in that the management responsibility for the CMS rests on an autonomous or semi-autonomous board (Govindaraj and Herbst, 2010; Ministry of Health Tanzania, 2008; Watson and McCord 2013; Wright et al, 2013). Until August 2011, the procurement of drugs in Malawi was done by CMS using the ‘traditional CMS model’ approach. However, there have been widespread documented evidence of stock-outs of drugs in public hospitals, often being attributed to the failure of the traditional CMS approach (Chandani et al., 2012; Chirwa et al, 2013; Lufesi et al., 2007; Wright et al., 2013). Because of the failure of the traditional role, the CMS in Malawi was converted to a Central Medical Stores Trust (CMST) in 2011. This was done to reduce the government control of CMS which was seen as being a cause of inefficiencies. It was hoped that an autonomous CMST would improve drug availability to the public health system of Malawi.
The organizational change from central medical stores to a trust meant that management responsibility for the CMS now began to rests on an autonomous or semi-autonomous board (Alternative Public Health Supply Chains, 2013). The system which Malawi adopted in the pharmaceutical supply chain is also used in other countries for example, Tanzania, Cameroon, Burkina Faso and Senegal with mixed results (Govindaraj and Herbst, 2010; Gyimah et al., 2009; Tanzania, 2008). The procurement system in Tanzania has improved greatly as compared to the traditional CMS that was used before the change to the semi-autonomous approach of CMS.
So far, no research has been conducted to assess the performance of CMST in Malawi. However, also after the change of CMS to a Trust (CMST), there have been newspaper reports on frequent stock-outs of medicines in the country (Malawi News Agency, 2013a,b; 2014; Masina, 2013; Mphande, 2014; Department for International Development-DfID UK,, 2011). Thus, the present study was aimed at assessing whether drug availability improved in the health facilities in Malawi after the change of CMS to a Trust, and assessing the perceptions of health professionals on the performance of CMST. Further, the study investigated whether the media reports on stock-out of medicine (Malawi News Agencies of, August 2013, November 2013 and September 2014) were true or an exaggeration.
Study sites
The study was conducted at the two largest tertiary hospitals in Malawi and the Central Medical Stores Trust which supplies the hospitals, QECH and KCH, which are also teaching hospitals for both undergraduate and postgraduate medical and allied health professional. The study population and area included CMST, QECH and KCH’s drug stock records/cards, and health care workers from QECH and KCH.
Study design
A retrospective cohort study, for a period of 1st July 2010-30th June 2011 and 1st July 2013-30th June 2014, was used to describe whether the change of CMS to CMST was followed by an improvement of drug availability, by looking at the stock card of selected essential medicines, and to get the perceptions of health personnel towards the performance of the CMST.
Sampling
A total of 24 essential drugs were selected from the Malawi Essential Medicine List (MEML) 2009 and grouped into antibiotics/antimicrobial drugs (amoxicillin capsule/tablet 250 mg, ceftriaxone PFR-injection 2 g, ciprofloxacin tablet 250 mg, erythromycin tablet 250 mg, gentamicin ampoules 80 mg, and metronidazole tablet 200 mg); analgesics (aspirin tablet 300 mg, carbamazepine tablet 200 mg, paracetamol tablet 500 mg, ketamine vial and pethidineampoules); antidiabetic drugs (glibenclamide tablet 5 mg, Insulin Lente vials 10 mL, insulin soluble vials 10 ml, and metformin tablet 500 mg); antihypertensive drugs (hydrochlorothiazide tablet 25 mg, furosemide tablet 40 mg, nifedipine tablet 10 mg and propranolol tablet 40 mg); and others (chlorpheniramine tablet 4 mg, cimetidine tablet 200 mg, phenobarbitone tablet 30 mg, promethazine tablet 25 mg and Salbutamol inhaler). All stock cards of a selected list of drugs years before CMST changed (2010/2011) to a trust and after it changed (2013/2014) were included. For self-administered questionnaire, a total of 58 participants were selected from the staff of QECH and KCH using inclusion criterion. The inclusion criterion for this part was all health professionals that have worked for 5 years or more in the Malawian government health service, specifically at the facility (either KCH or QECH). This ensured that the respondents had personal experience on pharmaceutical supply services before the change from CMS to CMST.
Data collection
The study was conducted from January 2014 to May 2015. Stock cards of 24 selected medicines were used to obtain the information on the availability of essential medicines for quantitative part. The period of stock-outs was determined from the stock cards by checking the number of days the medicines were out for the period 1st July 2010-30th June 2011 and 1st July 2013-30th June 2014.
Data analysis
Data was entered into excel and analysed using SPSS version 22, paired sample correlations and statistics was used to measure associations.
Ethical consideration
College of Medicine Research and Ethics Committee (COMREC) approved the study protocol. Further permission was obtained from the medical directors in the hospitals and the responsible authorities at CMST before conducting the study. For the sake of confidentiality, names were not recorded but codes were used for identification of interviewed persons.
The number of days at which a particular drug was out of stock were calculated from the stock cards of the selected by drugs counting the number of days when the drug was out of stock for the entire period. Table 1 shows the number of days when individual drugs were out of stock before and after the change (Table 1). The overall results showed an improvement of drug availability at both the CMST and KCH after the change from CMS to a Trust. In contrast, for QECH there was no improvement for the time period. Detailed analysis of the different categories of drugs for QECH showed an improvement in availability for analgesics (from 85 stock-out days to 3 days) and anti-diabetics (from 29 stock-out days to 3 days) while there was a decrease in the availability of antimicrobials (from 102 stock-out days to 284) and anti-hypertensives (from 62 stock-out days to 127). Notably, in QECH no stock-outs were recorded for ciprofloxacin and metronidazole before the change, while after the change, the recorded stock-out days were 230 and 42, respectively.
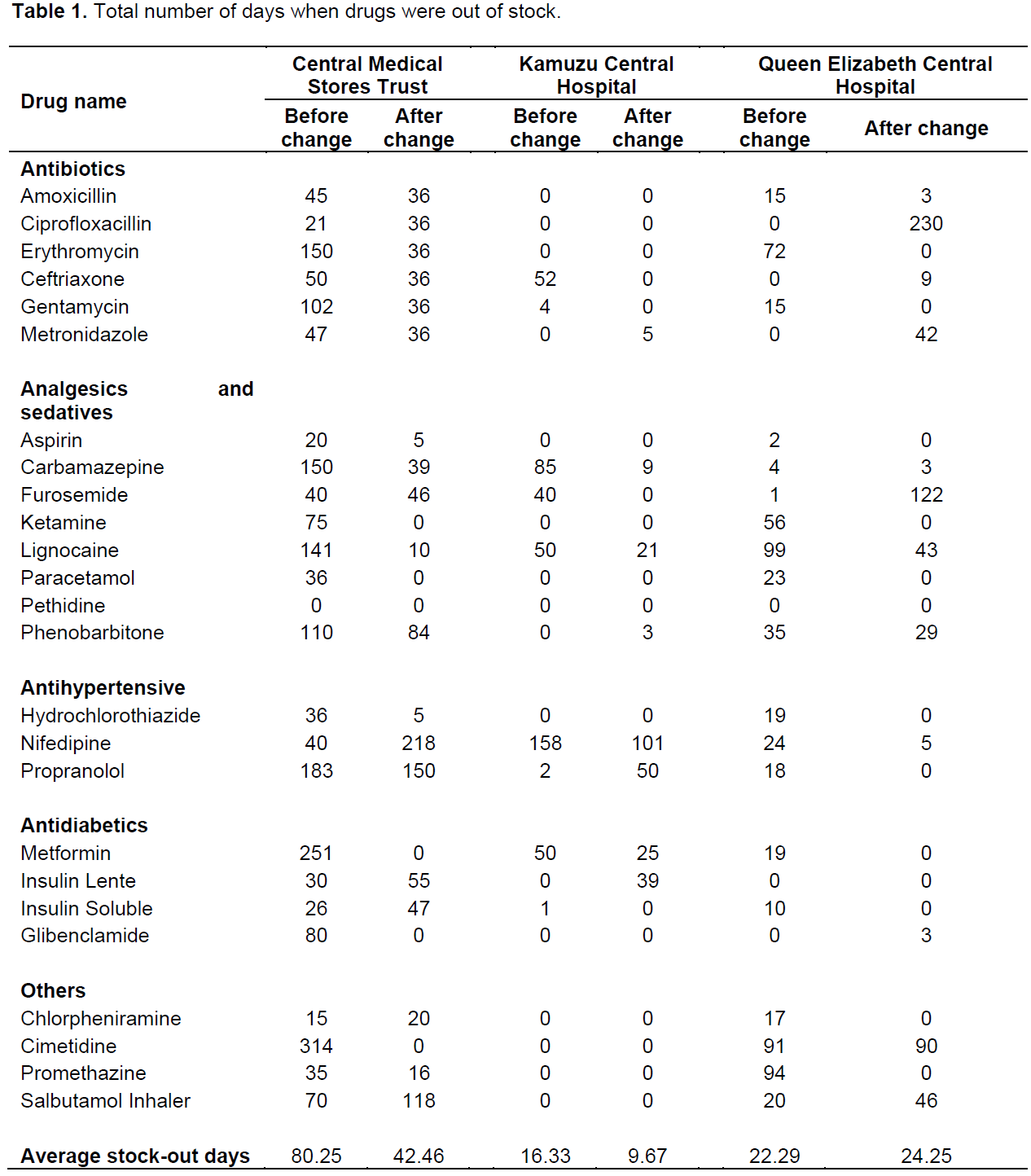
On average, stock-out days in CMST decreased from 80 to 42 days, corresponding to a reduction of 47% and was statistically significant (p=0.029). Stock-out days in KCH reduced from 16 to 10 days, corresponding to a reduction of 41% but was not statistically significant (p-value=0.173). In contrast, stock-out days in QECH increased from 22 to 24 days, with an increase of 9% p-value of 0.733.
Perceptions of health professionals on the performance of CMST
Out of 58 study participants whom were asked to fill a questionnaire, only 23 (representing 40%) filled and returned the questionnaire. There was equal distribution of responses from both hospital (12 from KCH and 11 from QECH). The number of years the health professionals had worked at each health facility ranged from 5 to 30 with most of them having worked for at least 7 years in their respective hospitals.
All 23 respondents knew the functions and mandates of Central Medical Stores Trust. On the change of CMS to a Trust (CMST), 87% knew about the change unlike the 13% who still thought it was fully under government control. 26.1% of respondent believed that there was an improvement in drug supply to the hospitals from CMST, 69.6% saw no improvement and 4.3% was neutral. With reference to different categories of drugs, the majority of the health professionals were of the opinion that the availability of TB drugs, anti-malarial and anti-retroviral drugs (ART drugs) had improved. However, they said that the availability of analgesics, anti-hypertensives and antibiotics has not improved.
The results showed a strong decrease in stock-out days for CMST after the change (from an average of 82 days to an average of 42 days stock out days per drug per year) which was statistically significant. The results are similar to findings by Wiedenmayer (2000) which showed an improvement in drug availability and performance of CMS in Tanzania after changing the management, supply monitoring and documentation of the institution. Using multidisciplinary training and procurement policy changes, the Association of Central Medical Stores for Essential Drugs (French acronym, ACAME) improved the performance of national CMSs in many Francophone African countries (Govindaraj et al., 2010). A study by Millot (2006) on three countries- Senegal, Cameroon and Burundi also demonstrated improvements in efficiency-related outcomes, especially service quality and inventory availability after strengthening the CMS (Millot, 2006). Improvements service quality and inventory availability was attributed to the autonomous nature of the CMS which resulted in improved management decision-making, increased accountability and transparency (Millot, 2006). It is suggest that the improvement in availability of drugs (shown by reduction in stock-out days) at CMST, Malawi, may at least in part be attributable to the change of control from solely government control to an autonomous agent-trust. This gives credence to published literature which supports the beneficiary effect of changing the roles of a ‘traditional’ CMS to improve its performance (Govindaraj and Herbst, 2010; Gyimah et al., 2009; Millot, 2006).
However, the improvement in drug supply at CMST did not translate to massive improvement in drug availability at the two referral/central hospitals. The results from the two central hospitals were mixed. The results from KCH showed an improvement in drug availability (reduction of stock out days from an average of 16.33 to 9.67 days) while for QECH, there was no improvement. Of special interest is that while data from CMST showed an improvement in drug availability especially for the antimicrobial agents, the results of QECH showed the opposite.
The results may be partly attributed to insufficient number of trained personnel as the pharmacist training in Malawi started in 2006, with few intake of graduates. The baseline drug stock-out at QECH was higher than at KCH as such, may have contributed to the disparity in drug availability between the two hospitals. 70% of the interviewed health workers stated that there was no improvement in the operation of CMST. This is in accordance with data from QECH, but not from KCH. The improvement of drug availability in CMST, recorded in our study, cannot be directly noticed by the health workers, who received their medicines through the hospital pharmacies. This may be attributed to the nature of prepared self-administered questionnaire. If the study had used an interview guide and directly interviewed the participants, the findings could have been different. At the same time, using collecting data on drug availability and questionnaire at the same time, will strength this study as the respondents were not influenced by prior results of drug availability, and hence the inconsistency in their response.
Majority of the health professionals stated that, especially, the availability TB drugs, anti-malarial and the ARV’s had improved, unlike some other drug categories. However, this improvement may not be attributable to the change from CMS to CMST. In Malawi, there are vertical supply programs for donor funded drugs and ART, anti-TB and antimalarial falls in this category. The categories in which the respondents stated less improvement is solely funded and supplied by government through CMS.
Limitation to this study includes cases of missing stock cards at the hospitals and the CMST, and refusal by many health workers to participate (sometimes demanding money for participation). Due to limited funding, district hospitals and health centres could not be included in this study, which would have given a more complete representation.
In conclusion, the study indicated that the radical shift in the management of CMS was followed by an improvement of drug availability in CMST itself, and in one of the two investigated hospitals. However, most interviewed health professionals stated that they did not observe any effect of the change of CMS to CMST on drug availability in their workplaces. Future research need to be conducted to study the frequency and the reasons for drug shortages in the hospital of Malawi.
The authors have not declared any conflict of interests.
FK is a PhD candidate and CARTA fellow (Consortium for Advanced Research Training in Africa). Funding for the research was provided by College of Medicine, University of Malawi.The funding agency did not influence the design and carrying out of the research. Many thanks are due to Professor Lutz Heide for proofreading and making constructive comments to the study and final manuscript.
REFERENCES
|
Baumgarten I, Frerick B, Kuper M, Tiba MR (2011). Availability and Management of Medicines and Medical Supplies: Findings from an Assessment of 87 Health Facilities in Four Regions in Tanzania. Dar es Salaam, Tanzania: Tanzanian German Programme to Support Health (TGPSH). Available at:
View.
|
|
|
|
Chandani Y, Noel M, Pomeroy A, Andersson S, Pahl MK, Williams T (2012). Factors Affecting Availability of Essential Medicines among Community Health Workers in Ethiopia, Malawi, and Rwanda: Solving the Last Mile Puzzle. Am. Society Trop. Med. Hyg. P 87.
Crossref
|
|
|
|
|
Chirwa ML, Kazanga I, Faedo G, Thomas S (2013). Promoting universal financial protection: contracting faith-based health facilities to expand access – lessons learned from Malawi. Health Res. Policy Syst. 11(1):27.
Crossref
|
|
|
|
|
Department for International Development-DfID UK (2011). Essential Medicine Project 2011-2013: Business Case. Department for Internation Development, United Kingdom.
|
|
|
|
|
Govindaraj R, Herbst CH (2010). Applying Market Mechanisms to Central Medical Stores: Experiences from Burkina Faso, Cameroon, and Senegal. Washington DC: The International Bank for Reconstruction and Development/The World Bank. Available at: View
|
|
|
|
|
Gyimah PE, Yellu FD, Andrew-Annan E, Gyansa-Lutterodt M, Koduah A (2009). Assessment of Medicines Procurement and Supply Management Systems in the Public Health Sector-Ghana. Accra, Ghana: Ministry of Health, Ghana National Drugs Programme. Available at:
View
|
|
|
|
|
Index Mundi (2015). Malawi Population below poverty line. Available at:
View.
|
|
|
|
|
International Fund for Agricultural Development (2011). Enabling poor rural people to overcome poverty in Malawi. Rome, Italy.
|
|
|
|
|
Lufesi NN, Andrew M, Aursnes I (2007). Deficient supplies of drugs for life threatening diseases in an African community. BMC Health Serv. Res. 7(1):86.
Crossref
|
|
|
|
|
Malawi News Agency (2014). Zero aid budget MoH. Nyasatimes. Available at:
View
|
|
|
|
|
Malawi News Agency (2013). Malawi Public Hospitals Run out of dental anaesthesia. Nyasatimes. Available at:
View
|
|
|
|
|
Malawi News Agency (2013). Poor quality local drug supplies worry Malawi medical stores. Nyasatimes. Available at:
View
|
|
|
|
|
Masina L (2013). Malawi Public hospitals Face Acute Drug Shortages. Voice of America. Available at:
View
|
|
|
|
|
Millot G (2006). Access to Essential Medicines in Africa: a Global Approach. Med. Trop. 66(7).
|
|
|
|
|
Ministry of Health-Malawi (2009). National Medicine Policy-Malawi. Lilongwe, Malawi.
|
|
|
|
|
Ministry of Heath-Tanzania (2008). In-depth Assessment of the Medicines Supply System in Tanzania. Dar es Salaam, Tanzania.
|
|
|
|
|
Mphande H (2014). KCH suspends eletive surgeries: Malawi drug shortage "crisis" Nyasatimes. Available at:
View
|
|
|
|
|
Supply Chain Management Assistance in Malawi (2011). Technical Assistance to Central Medical Stores-Trust.
|
|
|
|
|
UN Millennium Project (2005). Prescription for Healthy Development: Increasing Access to Medicine. Report of the Task Force on HIV/AIDS, Malaria, TB, and Access to Essential Medicines, Working Group on Access to Essential Medicines. Sterling, VA: Earthcscan
|
|
|
|
|
Watson N, McCord J (2013). Alternative Public Health Supply Chains: Reconsidering the Role of the Central Medical Store. Arlington, Va.: USAID | DELIVER PROJECT, USA.
|
|
|
|
|
WHO (2013). Model List of Essential Medicines Available at:
View
|
|
|
|
|
WHO (2004). Increasing access to essentia lmedicines in the developing world. Geneva, Switzerland WHO.
|
|
|
|
|
Wiedenmayer K (2000). Access to Medicines; Medicine Supply: Lessons Learnt in Tanzania and Mozambique. Basel: Basel: Swiss Tropical Institute for the Swiss Agency for Development and Cooperation.
|
|
|
|
|
Wright, Christopher, David P, Wiklund M (2013). Malawi: Assessment of the Integrated Logistics Management Information System: Review of the Processes and Software Tools. . Arlington, Va, USA. Available at:
View
|
|