Full Length Research Paper
ABSTRACT
The aerial parts of Capsella bursa-pastoris (L.) Medic are used to treat nephritis, edema and enteritis. Every barber knows that C. bursa-pastoris had a good anti-inflammatory effect. This suggests that the extracted components from C. bursa-pastoris could potentially treat inflammatory disease. For discovering of the anti-inflammatory effects and chemical composition of C. bursa-pastoris, EtOAc extract was extracted from C. bursa-pastoris (EECB) and researched on EECB’s anti-inflammatory effects. On the carrageenan-induced paw oedema experiment, the EECB used at the doses (100, 200 and 300 mg/kg) after 10 h (p < 0.01), 5 h (p < 0.01) and 3 h (p < 0.01), respectively, showed significant anti-inflammatory effects. Moreover, on the egg-albumin-induced inflammation experiment, the EECB used at the doses 200 and 300 mg/kg after 4 h (p < 0.01) and 2 h (p < 0.01), respectively, showed significant anti-inflammatory effects. In accordance with the HPLC isolation of the EECB, there are four major compounds, namely, apigenin-7-O-β-D-glucopyranoside (S1), luteolin-7-O-β-D-glucopyranoside (S2), α-adenosine (S3), and uridine (S4), which may explain the activity.
Key words: Capsella bursa-pastoris (L.) Medic, anti-inflammatory, high performance liquid chromatography (HPLC) isolation, flavonoids.
INTRODUCTION
MATERIALS AND METHODS

RESULTS
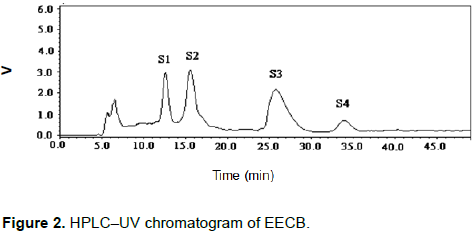
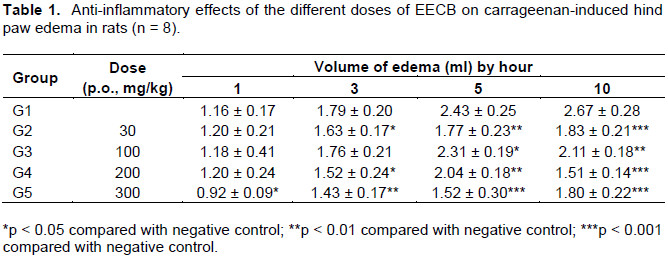
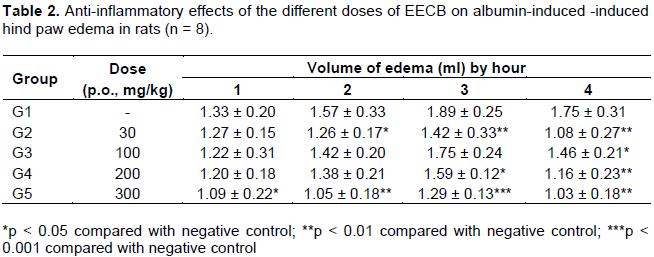
DISCUSSION
CONFLICT OF INTERESTS
REFERENCES
|
Bai YJ, Yu M, Zhao SW, Chen YX, Chang KL (2013). Studies on the pharmacological effects and mechanism of alkaloids. J. Harb. Univ. Com. 29:8-11. |
|
|
Da Silva G, Taniça M, Rocha J, Serrano R, Gomes ET, Sepodes B (2010). In vivo anti-inflammatory effect and toxicological screening of Maytenus heterophylla and Maytenus senegalensis extracts. Hum. Exp. Toxicol. 30: 693-700. |
|
|
Dash PR, Nasrin M, Saha MR (2011). Evaluation of analgesic and neuropharmacological activities of methanolic rhizome extract of Hedychium coronarium, Int. J. Pharm. Sci. Res. 2:979-984. |
|
|
Deng YR, Ding L, Wu SX (2005). Studies on Chemical Constituents in Herb of Lamium maculatum L. var Kansuense. J. Chin. Mater. Med. 30: 2721-2724. |
|
|
El-Shenawy SM, Abdel-Salam OM, Baiuomy AR, El-Batran S, Arbid MS (2002). Studies on the anti-inflammatory and antinociceptive effects of melatonin in the rat. Pharm. Res. 46:235-243. |
|
|
Gomase PV, Shire PS, Nazim S, Choudhari AB, Shaikh S, Khairnar (2011). Phytochemical evaluation and analgesic activity of fresh juice of young stem (tender) bark of Azadirachta indica A. Juss. Pharm. Lett. 3: 407-415. |
|
|
Han J, Guo Y, Wan HT (2006). Study on pharmacological action and pharmacokinetics of alkaloids in traditional Chinese Medicine. Zhongyiyao Xuekan 24: 2326-2327. |
|
|
Hasan K, Etil A, Milena M (2012). Iridoid, phenylethanoid and flavonoid glycosides from Sideritis trojana. Fitoterapia 83:130-136. |
|
|
Huang N, Rizshsky L, Hauck C, Nikolau BJ, Murphy PA, Birt DF (2011). Identification of anti-inflammatory constituents in Hypericum perforatum and Hypericum gentianoides extracts using RAW 264.7 mouse macrophages. Phytochemistry 72:2015-2023. |
|
|
Julião LDS, Leitão SG, Lotti C (2010). Flavones and phenylpropanoids from a sedative extract of Lantana trifolia L. Phytochemistry 71:294-300. |
|
|
Kang XR, Wang XL, Sa CL (2012). Research progress of Capsella bursa-pastoris. Chin. J. Nat. Med. 6:43-46. |
|
|
Liu L, Chen YP, Wan Z (2007). Studies on chemical constituents of Luticao. Chin. Pharm. J. 32:1762-1765. |
|
|
Meng QM, Liang J, Wu GF, Lu H (2003). Research progress on the pharmacological effects of alkaloids. Lish. Med. Mater. Med. Res. 14:700-702. |
|
|
Morimoto A, Nakamori T, Watanabe T, Ono T, Murakami N (1988). Pattern differences in experimental fevers induced by endotoxin, endogenous pyrogen, and prostaglandins. Am. J. Physiol. 254:633-640. |
|
|
Rahman H, Vakati K, Eswaraiah MC (2012). In invo and in intro anti-inflammatory activity of Aquilaria agallocha oil. Int. J. Basic. Med. Sci. Pharm. 2:7-10. |
|
|
Ren YL, Yang J (2001). Studies on chemical constituents of Saussurea tridac-tyla Sch-Bip. Chin. Pharm. J. 36:590-593. |
|
|
Song N, Xu W, Guan H (2007). Several flavonoids from Capsella Bursa-pastoris (L). Medic. Asian J. Tadit. Med. 2:218-222. |
|
|
Vázquez AI, Sánchez CM, Delgado NG, Alfonso AM, Ortega YS, Sánchez HC (2011). Anti-inflammatory and analgesic activities of red seaweed Dichotomaria obtusata. Braz. J. Pharm. Sci. 47:111-118. |
|
|
Wang QH, Jin M, Dai NYT, Han NRCGT (2016). Anti-inflammatory effects, nuclear magnetic resonance identification, and high-performance liquid chromatography isolation of the total flavonoids from Artemisia frigida. J. Food Drug Anal. 24:385-391. |
|
|
Wang QH, Wu EQ, Dai NYT (2014). Study on Chemical Constituents of Capsella bursa-pastoris. Nat. Prod. Res. Dev. 26:50-52. |
|
|
Wang XF, Wang XJ (2007). Study on chemical constituents from Ixeris chinensis. Chin. Tradit. Herb. Drug 38:1151-1153. |
|
|
Winter CA, Risley EA, Nuss GW (1962). Carrageenan-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 11:544-548. |
|
|
Woo JC, Seong KK, Hee KP, Uy DS, Wonyong K (2014). Anti-Inflammatory and Anti-Superbacterial Properties of Sulforaphane from Shepherd's Purse. Korean J. Physiol. Pharm. 18:33-39. |
|
|
Xu RB, Liu WW, Wang MY, Zhan YC, Yan M (2007). Studies on the extracting technology of flavones from Capsella bursa-pastoris (L.) added with ultra-wave technique. Food Sci. Dev. 10:149-151. |
|
|
Zhang J, Jing ZM (2012). Identification of Capsella bursa-pastoris (L.). J. Chin. Med. Mater. 35:1241-1243. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0