ABSTRACT
Gentamicin is widely used as an effective antibiotic. A study was conducted to investigate the effects of intramuscular administration of gentamicin on 20, 5 to 6 weeks male Swiss albino mice weighing 25 to 30 g. The mice were divided into 4 groups: Group A (kept as control); groups B, C and D (treated with gentamicin intramuscularly daily at the dose rate of 5 mg/kg for 7 days, 5 mg/kg for 30 days, and 10 mg/kg for 30 days, respectively). The mice of treated groups showed specific clinical signs such as dullness, roughness of the body coat, anorexia and weakness. Blood was collected by cardiac puncture for estimation of various blood chemical parameters, such as total erythrocyte count (TEC), total leucocyte count (TLC), hemoglobin percentage (Hb%), alanine amino transferase (ALT), and serum creatinine. Kidneys were collected for gross and histological study. Body weight (P<0.01) and kidney weight (P<0.05) decreased significantly in gentamicin treated group. In hematological study, TEC, TLC, and Hb% values decreased significantly (P<0.01), whereas in biochemical study, serum creatinine and ALT values increased significantly (P<0.01) in treated group when compared with control group. Gross study of kidneys showed abnormal characteristics, such as, soft, flabby, brownish color with decreased size of left kidney in treated group. Histological study revealed desquamation of glomerulus, loss of glomerular architecture, distortion of renal tubules and hemorrhage in tubules of treated group. These data supports the view that gentamicin has a toxic effect on the morphology of kidney after long term treatment with higher dose.
Key words: Gentamicin, kidney, toxic effect, morphology, mice.
The kidney is a vital organ for animal and it regulates the water and salt concentration of the body. It plays an important role to remove foreign substances from the blood. Many environmental contaminants and chemical variables including drugs alter the function of kidney (Mahmood and Waters, 1994).
To date, among the antibiotics, gentamicin is the most widely studied aminoglycoside antibiotic. The aminoglycoside antibiotic, gentamicin, synthesized by Micromonospora purpurea, is used for the treatment of various bacterial infections including both gram-negative and gram-positive bacteria (Gilbert et .al., 2000). The action on bacteria is bactericidal and gentamicin has increased activity at alkaline pH. In case of gentamicin, oral absorption is minimal and for systemic use, gentamicin must be given by the parenteral route. Uptake is rapid after intramuscular injection and has a serum half- life of 75 to 110 min (Gonzelman, 1980). Whole animal isolated perfused rat kidney and human renal clearance and micropuncture studies have clearly demonstrated that aminoglycosides are eliminated in nonmetabolized form from the body in all animal species, primarily by renal glomerular filtration (Chiu et al., 1976). The inhibition of protein synthesis is mediated through aminoglycosides energy-dependent, sometimes irreversible binding to the cytocolic, and membrane-associated bacterial ribosome (Levison, 2012).
The use of gentamicin has tremendously increased in human and veterinary practice due to their greater effectiveness against human, livestock, and poultry diseases (Craig et al., 1998) but, most of the people of Bangladesh are inexperienced about the taking of antibiotic. They purchase antibiotics without any prescription from physician or even when the practice is not legal. For treatment purposes, they use overdose of antibiotic for a long time which may cause adverse effects in human beings. In rural Bangladesh, 95% of the people consume drugs without any prescription and purchase drugs from local pharmacies; only 8% of them consume drugs according to the prescription from physicians (Hossain et al., 1982). Drug takers usually have little or no knowledge of the required dosage, regimen, indications or contraindications (Dua et al., 1994).
Like many other antibiotics, gentamicin is not free from toxic effects both in human beings and livestock. Gentamicin can induce ototoxicity and nephrotoxicity, because both organs have higher than normal concentration of phospholipids in their cellular matrices (Ali et al., 1992). Nephrotoxic effects are found in 10 to 15% of cases due to over dosage or accumulation of gentamicin in renal cortical tubular epithelial cells. Necrosis of cells in the proximal tubule occurred due to over dose of gentamicin, leading to acute renal failure ( Leehey et al., 1993). Gentamicin acts by binding to anionic phospholipids of plasma lemma and decreasing the permeability of the glycerol moiety of phosphatidylinositol, membrane fluidity and promoting membrane aggregation. For that reason, renal proximal tubules take up gentamicin and concentration in the renal cortex is far greater than those observed concurrently in the serum and other tissues (Lopez et al., 2011). Additionally, blood chemical investigation was conducted for more elucidation of the effects of tissue damage which could be provoked by gentamicin.
Therefore, in the toxicity of gentamicin problems relating to their hazardous effects upon human beings, animals, and birds must be taken into account. In the present study, the short and long term effects of gentamicin on kidney in mice were investigated histochemically.
Chemicals
Inj. Gentaren 10% (Reneta, Bangladesh Ltd.) 100 ml bottle is a broad spectrum aminoglycoside antibiotic preparation which was purchased from the local market. Buffered neutral formalin, ethanol, xylene, hematoxylin, eosin, acetic acid, glycerin, and DPX were purchased from Merck Company, India.
Animals and treatments
The experimental male Swiss albino mice were collected from International Center for Diarrheal Disease Research (icddr’b), Mohakhali, Dhaka. All the mice possessed good health and devoid of any external deformities certified by the registered veterinarian from icddr’b. After procurement, all the mice were kept under close observation in order to acclimatize to the new environment for a period of one week prior to commencement of the experiment. All mice were raised under confinement as an intensive system. Twenty male mice aged 5 to 6 weeks old, weighing 25 to 30 g were used for this experiment. The rats were housed five per one plastic cage, maintained on a 12 h light/dark cycle at a constant temperature (70 to 74°F) and humidity (45 to 60%) and provided water and rodent pellets ad libitum. For each individual under study a record sheet with full details of each parameter were maintained. For the experimental purpose, the mice were randomly divided into four groups and each group contained five mice. Group A was kept as control, group B was treated with 5 mg/kg for 7 days, group C was treated with 5 mg/kg for 30 days and group D was treated with 10 mg/kg for 30 days.
Body weights of all mice were recorded before starting the treatment. Among the four groups, group A was kept as control without giving any treatment. The other 3 groups of mice (B, C, D) were treated with gentamicin (Gentaren 10%) intramuscularly. After administration of gentamicin, all the mice were kept under close observation for the entire 35 days (30 days of treatment period and 5 days of post treatment). The body weight of each animal was recorded twice weekly.
Clinical examination
All the mice were kept under close observation up to 7 and 35 days for investigation of any clinical signs.
Blood chemical
Each animal was euthanized under chloroform before 2 ml of blood was taken in 5 ml disposable syringe using anticoagulant (sodium citrate 3.8% w/v, Merck, India) by cardiac puncture for estimation of various blood chemical parameters, such as total erythrocyte count (TEC), total leucocyte count (TLC), hemoglobin percentage (Hb%), alanine amino transferase (ALT) and serum creatinine. The blood sample was allowed to stand for 1 h and centrifuged at 3000 rpm for 15 min. Eppendorf tubes were used for collection of serum and stored in freeze at -20°C. Serum creatinine and ALT were measured by using auto-analyzer machine (ERBA-Smart lab SL-10304) and commercially available kits.
Gross and histology
After sacrifice of each animal sequentially, kidney was collected from each animal and examined for gross study. For gross study, color, weight and size of kidney were taken into consideration.
For histological observation, 5 mm2 pieces were collected from different side of kidney and immersed in 10% formalin for 48 h. Then, the sample was washed in 10% phosphate buffer solution for 3 h, dehydration was done by passing the tissue in the ascending grade of alcohol, such as 70, 80, 90, 95, 100 (1), and 100% (2) each for 2 h and finally 100% (3) for overnight, cleared in xylene and embedded in paraffin. Sections from the paraffin blocks were cut in 5 µm in thickness by using rotatory microtome. Then, the sections were stained with Meyer’s Hematoxylin and Eosin (H&E). The sections were protected by a thin cover slip attached to the slide with a mounting medium ‘DPX’ (Luna, 1968). The samples were studied with the aid of light microscope.
Data analysis
A statistical software package (SPSS, version 20) was used for data analysis. The descriptive data is given as mean ± standard deviation (SD). Chi-squared test was used for the analytical assessment. The differences were considered statistically significant when P values were less than 0.05 and 0.01.
All the mice of group A were healthy and active without any abnormal signs during the whole experimental period. Mice of group B were apparently normal without any abnormal sign up to 7 days of intramuscular administration of gentamicin at a recommended dose (5 mg/kg). Mice of group C (5 mg/kg for 30 days) showed fear with less appetite, roughness of the body, apathy and weakness. However, in group D (10 mg/kg for 30 days), all the mice produced irritable behavior, roughness of the hair coat, dullness, less appetite and weakness. Mortality of the animals was found in groups C and D, but the highest concentration was found in group D treated with 10 mg/kg for 30 days.
The mean body weight of the mice of group A at the start and at the end of the experiment was 27.18±0.217 and 28.50±0.274 g, respectively. Total body weight was not significantly affected due to short duration of administration of gentamicin in group B. However, after the end of the experimental period, the body weight was more significantly (P<0.01) decreased in group D (10 mg/kg for 30 days) in comparison to control group (Figure 1).
In group A (Control), the mean value of TEC, TLC and Hb% was 891.80±1.304 ml/m3, 8.14±0.018 thousand/m3 and 8.99±0.013. The value of TEC, TLC and Hb% decreased significantly (P<0.01) in group C (5 mg/kg for 30 days) and group D (10 mg/kg for 30 days) in comparison to the control group (Table 1). In group A (control), the mean value of serum creatinine and ALT were 0.54±0.035 mg/dl and 17.28±0.130 U/L. These values increased significantly (P<0.01) in group D (10 mg/kg for 30 days).
Gross examination of kidney
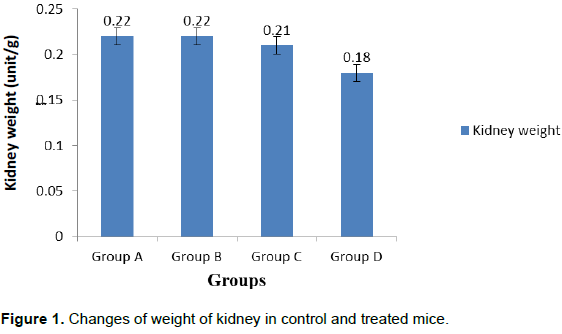
Reddish brown with smooth and shiny surface kidney was found in group A (Control) whereas, brownish color, soft and flabby kidneys were found in group C (5 mg/kg for 35 days) and group D (10 mg/kg for 35 days). The mean weight of kidney of control group was 0.22±0.001 g. The weight of kidney decreased (0.18±0.001**) significantly (P<0.01) in group D.
Histological examination of kidney
Long term administration of gentamicin with higher dose induced marked glomerular, tubular and interstitial alterations in treated mice.
Kidney of group A (control), renal corpuscles appeared as dense rounded structure with glomeruli, surrounded by double walled epithelial Bowman’s capsule and lined by simple squamous cells, having an outer parietal and inner visceral layers with a urinary space in between two layers (Figure 2). No deviation was found in glomerulus as well as tubules of the control group. In the experimental group B, there was no histological alterations observed in glomerulus and tubules of kidney treated with 5 mg/kg for 7 days. Desquamation of glomerulus, loss of glomerular architecture and marked lymphocytic infiltration were found in tubules of kidney in group C (5 mg/kg for 30 days) (Figure 3). Distortion of renal tubules (Figure 4), dilatation of tubule and tubular heammorhage were found in group D (10 mg/kg for 30 days) (Figure 5).
In the present study, behavioral changes, blood chemical and morphological alteration of kidney were observed after gentamicin administration.
Gentamicin in recommended (5 mg/kg for 30 days) and >recommended (10 mg/kg for 30 days) doses showed roughness of the body, apathy, loss of appetite and weakness. Similar findings were observed by Dantas et al. (1997) and Aguiar et al. (1997) when 10 dogs received gentamicin 10 mg/kg intramuscular (IM) 3 times a day for 14 days. However, they also found diarrhea and vomiting following administration of gentamicin in dog. In the current study, mortality was found in the highest concentration in group D (10 mg/kg for 30 days) whereas, Lichthorn (1985) reported death of new born rabbit following low dose (20 mg/kg) of intramuscular injection of gentamicin during gestation period. Gentamicin is known to generate reactive oxygen species associated with an increase in lipid peroxidation and decrease in antioxidant enzyme activity in the kidney (Banday et al., 2008). Gentamicin treatment for long term produced statistically significant (p<0.01) loss of body weight and kidney weight in treated group as compared to control group. Houghton and Ali (1997) observed that renal failure due to gentamicin treatment in rats resulted in acidosis associated with anorexia and leading to decrease in body weight and kidney weight. Various blood chemical parameters were tested for the evaluation of the function of organs such as serum creatinine and ALT. In the present study, a significant increase of serum creatinine was observed and an increased serum creatinine indicates that kidney function was affected by gentamicin treatment. The presently recorded significant increase in blood creatinine was associated with distinct renal structural damage in rats (Chaware et al., 2011). Blood level of ALT increased significantly in the treated group. The blood level of ALT is indicative of the functional efficacy of liver and kidney. The level of these enzymes is very sensitive to any disease conditions of such organs (Tietz, 1996). Increased level of serum creatinine and ALT due to gentamicin treatment induced oxidative injury causing tubular damage and renal impairment. This finding is in accordance with that of Lipsky et al (1980) who also reported similar results. The presently observed necrotic changes of the renal tubules confirm the concept that significant structural changes of the kidney led to significant increase in the blood level of ALT (Smith et al., 1988). In the present study, intramuscular administration of gentamicin in 3 different doses (5 mg/kg for 7 days, 5 mg/kg for 35 days and 10 mg/kg for 35 days) significantly reduced the TEC, TLC and Hb%. Similar findings were reported by Smith et al. (1988) that long term exposure of gentamicin in high dose affects the haemopoietic cells in the bone marrow and decrease erythrocyte production. In the present study, soft with brownish colored kidney was found in treated group (5 mg/kg for 30 days and 10 mg/kg for 30 days). Intramuscular administration of 10 mg gentamicin/ kg in 10 dogs, 3 times a day for 14 days, showed paled and soft kidney (Dantas et al., 1997) which is similar to the findings of the present study. For histological study, mice treated with gentamicin with high doses for long term showed progressive tubular, glomerular and interstitial alterations. The tubular damage and degenerative changes seen in the present work confirm with the findings of previous work (Ali et al., 2011; Dehghani et al., 2011; Kalayarasan et al., 2009).
The results of the present work show that the cortex of the kidney was more affected than the medulla as a result of long term administration of gentamicin. This might indicate that a relatively higher concentration of gentamicin reaches the cortex via the bloodstream than that entering the medulla. This is in agreement with the findings of Houghton et al. (1975). Kacew (1989) reported that gentamicin caused tubular necrosis, loss of brush borders and accumulation in renal cortex due to its reabsorption in proximal convoluted tubules causing necrosis and degenerative changes.
It is concluded that long term treatment with gentamicin in Swiss albino mice showed a fair degree of reduced food intake and body weight, increased mortality, induced significant blood chemical changes and caused derangement of kidney function with concomitant changes in the histological structures of that organ, which occurred mostly at highly gentamicin exposed group. The findings of the present study also revealed that treatment with gentamicin for 7 days is safe for human and animals. So, a physician prescription is needed before taking antibiotic to avoid the hazardous effects on kidney. People of Bangladesh should be conscious about taking antibiotic in major or minor issues. The present study may be considered as an experimental base of the relevant human studies.
The authors have not declared any conflict of interests.
REFERENCES
|
Aguiar HCR, Silva CF, Schoenau W, Kommers GD, Silva PA, Leitzka MRM, DE-Aguir HCR, De-Silva PA (1997). Urinary gamma glutamyl transpeptidase activity, urinalysis, BUN and Creatinine serum dosage as an auxiliary diagnostic means in dog nephrotoxicity induced by aminoglycosides. Ciencia- Rural. 27(2):237-244.
|
|
|
|
Ali BH, Abdel Gayoum AA, Bashirb AA (1992). Gentamicin nephrotoxicity in rats; some biochemical correlates. Pharmacol. Toxicol. 70:419-423.
Crossref
|
|
|
|
|
Ali BH, Ziada A, Al Husseni I, Beegam S, Al-Ruqaishi B, Nemmar A (2011). Effect of Acacia gum on blood pressure in rats with adenine-induced chronic renal failure. Phytomedicine 18(13):1176-80.
Crossref
|
|
|
|
|
Banday AA, Farooq, Neelam, Ahad NK, Khan F (2008). Time dependent effects of Gentamicin on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in rat kidney tissue. Life Sci. 82(9-10):450-459.
hCrossref
|
|
|
|
|
Chaware VJ, Chaudhary BP, Vaishnav MK, Biyani KR (2011). Protective effect of the aqueous extract of Momordica charantia leaves on Gentamicin induced nephrotoxicity in rats. Int. J. Pharmacol. 3(1):553-555.
|
|
|
|
|
Chiu PTS, Brown A, Miller G (1976). Renal extraction Gentamicin in anesthetized dogs. Antimicrob. Agents Chemother. 10:227-282.
Crossref
|
|
|
|
|
Craig W (1998). Pharmacokinetic/pharmacodynamics parameters; rational for antibacterial dosing of mice and men. Clin. Infect. Dis. 26(1):1-10.
Crossref
|
|
|
|
|
Dantas AF, Kommers GD, Hennemann CR (1997). Ciencia-Rural 27(3):451-456.
Crossref
|
|
|
|
|
Dehghani F, Namavar MR, Noorafshan A, Karbalay-Doust S, Esmaeilpour T (2011). Evaluation of the kidney extraction gentamicin- induced nephrotoxicity in rat. Kidney Res. J. 1(1):24-32.
Crossref
|
|
|
|
|
Dua V, Kunin CM, White LV (1994). The use of antimicrobial drugs in Nagpur, India. A window on medical care in a developing country. Soc. Sci. Med. 38:717-24.
Crossref
|
|
|
|
|
Gilbert DN, Mandell GL, Bennett, Dolin R (2000). Aminoglycosides in principles and practice of infectious diseases. 5th edition. New York, pp. 307-36.
|
|
|
|
|
Gonzelman GM (1980). Pharmacotherapeutics of aminoglycosides antibiotics. Am. J. Renal Med. 5:1076-1078.
|
|
|
|
|
Hossain MM, Glass RI, Khan MR (1982). Antibiotic use in a rural community in Bangladesh. Int. J. Epidemiol. 11:402-5.
Crossref
|
|
|
|
|
Houghton DC, Harnet M, Cambellm M, Porter G, Bennet W (1975). A light and electron microscopic analysis of Gentamicin nephrotoxicity. Am. J. Pathol. 82:589-612.
|
|
|
|
|
Kacew S (1989). Inability of nitrendipine to protect against Gentamicin nephrotoxicity in rats. Biol. Med. Environ. Sci. 2:160-6.
|
|
|
|
|
Kalayarasan S, Prabhu PN, Sriram N, Manikandan R, Arumugam M, Sudhandiran G (2009). Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of NrF2 against gentamicin–induced nephrotoxicity in wister rats. Eur. J. Pharmacol. 606:162-171.
Crossref
|
|
|
|
|
Leehey DJ, Braun BI, Tholl DA (1993). Can pharmacokinetic dosing decrease nephrotoxicity associated with aminoglycoside therapy. J. Am. Soc. Nephrol. 4:81-90.
|
|
|
|
|
Levison ME (2012). Aminoglycosides, The Merck Manual, accessed 22 February 2014.
|
|
|
|
|
Lichthorn M (1985). Clinical study on the safety of parental antibiotic treatment for growing, pregnant and lactating rabbits. P 163.
|
|
|
|
|
Lipsky JJ, Cheng L, Sacktor B, Leitman PS (1980). Gentamicin uptake by renal brush border membrane vesicles. J. Pharmacol. Clin. Ther. 215: 390-3
|
|
|
|
|
Lopez N, Jose M, Quiros Y, Vicente L, Morales AI, Lopez H, Francisco J (2011). New insights into the mechanism of aminoglycosides nephrotoxicity; An integrative point of view. Kidney Int. 79(1):33-45.
Crossref
|
|
|
|
|
Luna LG (1968). Manuals of histologic staining methods of the armed forces institute of pathology, 3rd edition, McGraw Hill Book Company, New York.
|
|
|
|
|
Mahmood DH, Waters A (1994). Comparative study of uranyl nitrate and cisplatin induced renal failure in rat. Eur. J. Drug Metab. Pharmacol. 91:327-336.
Crossref
|
|
|
|
|
Smith RL, Hill LR, Ilehman RJ, Lefkwitz P, Handler A, White (1988). Principles of biochemistry. Mammalian Biochemistry, 7th edition. McGraw-Hill, New York, USA.
|
|
|
|
|
Tietz NW (1996). Fundamentals of Clinical Chemistry, 4th edition. W.B. Saunders Company, USA.
|
|