ABSTRACT
High levels of cyclooxygenase (COX) enzymes and its metabolites, especially prostaglandin E2 (PGE2) are generated in inflammatory conditions and, eventually, can contribute to neoplasms growth, justifying, in certain malignancies. COX occurs in two major isoforms, physiologically as isoform COX-1 and during inflammation as COX-2. In certain neoplasms, benefits are reported with the use of the selective COX-2 inhibitor drug, meloxicam. This study aimed to evaluate the effect of meloxicam on the development of solid Ehrlich tumor. Fourteen male Swiss mice, 30-45 days old, 35-45 g of weight, were inoculated with 107 of the tumor cells, and divided in two groups according to the treatment: Phys) 0.1 mL intraperitoneal injection (IP) of sterile 0.9% saline solution, and Mel) treated with meloxicam, 2 mg/kg, 0.1 mL, IP, both once a day. After 21 days, all the animals were euthanized and the tumor removed, weighed and prepared for histomorphometric and immunohistochemical evaluation for COX-2. Mel group presented significantly reduction of the tumor weight and COX-2 production, as well as an increase in stromal component, but did not interfere in tumor growth, in comparison to Phys group. From these results it was concluded that meloxicam did not restrain tumor growth in this experimental model. Reduction in tumor weight alone cannot be taken as a reliable criterion for evaluation of tumor progression.
Key words: Ehrlich solid tumor, arachidonic acid, inflammation, Meloxicam.
Neoplasms represent an irreversible cell proliferation, associated with a high mortality rate in human species and in many species of domestic animals or of free life. Parallel to the establishment of the tumor mass, inflammatory process is triggered that ends up providing an environment that supports its progression. Several chemical mediators are produced, and among them the derivatives of arachidonic acid produced by the enzymes cyclooxygenases (COX). They occur in two predominant isoforms named COX-1 and COX-2.COX-1 is a constitutive enzyme,while COX-2 is induced during inflammation (Lewis and Smith, 2013). From both of them, prostaglandins (PG) are derived as an important metabolite,an dinneoplasms, PGE2 specific class of PG is highly produced (Rizzo, 2011). PGE2 acts as a modulator of the immune response, regulating the balance between Th1/Th2 subpopulations (Yoshida et al., 2000), and the change of the Th1 profile to Th2 affects the production and the action of cytotoxic and NK cells (Nakanishi and Rosemberg, 2013).
Several pro-tumor effects have been attributed to COX, especially to COX-2 and its metabolites, such as cell proliferation, motility, invasiveness, and apoptosis resistance (Banu et al., 2008; Rizzo, 2011). Inhibition of COX by non-selective or selective COX-2 inhibitors brings benefit as supportive therapy for various neoplasms, as colon carcinoma (Koehne and Dubois, 2004). However, the mechanism of action of COX-2 is related to its ability to generate PGs during anti-cancer defense response. An interesting in vitro study of HeLa, human rhabdomyosarcoma, mammary adenocarcinoma and rat embryo fibroblasts cell culture demonstrated an important role of COXs in controlling growth of this line of cancer cells, treated with aspirine and diclofenac. Similar effect on normal cells were observed especially with the use of diclofenac, drawing attention to the use of non-steroidal anti-inflammatory drugs (NSAIDs) under physiological conditions (Al-Nimer et al., 2015).
Meloxicam [4-hydroxy-2-methyl-N- (5-methyl-2-thiazolyl) -2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide] is a potent COX-2 inhibitor, able to induce apoptosis of hepatocellular carcinoma tumor cells, significantly reducing its growth (Li et al., 2016). Its preventive effect on recurrence of patients receiving conventional treatment is also reported (Takami et al., 2016). Little is known about its mechanism of action in reducing tumor growth, but it is probably able to modulate PGE2 action by blocking its EP2 receptor (Dong et al., 2014). This action, however, seems to be related to the period in which the drug is administered. The stage of tumor evolution has a direct influence on it, as it is demonstrated by Rodrigues et al. (2013) in a study where late administration of meloxican did not exert any effect on tumoral proliferation rate in reflux-induced gastric tumors in rats.
Another effect on the control of neoplastic growth associated with meloxicam is the induction of apoptosis. It is observed in some osteosarcoma lineages cells, as MG-63, in the HOS and U2-OS osteosarcoma cells, which do not produce high levels of COX-2 during their growth, such effect does not occur (Naruse et al., 2006).
Therefore, the selective inhibition of COX-2 may be dependent on the neoplasm and the stage of neoplasm evolution, which is the main question the present study aims to clarify in relation to Ehrlich solid tumor.
Study design
Fourteen male Swiss mice, 30-45 days old, 35-45 g of weight, were inoculated with 107 Ehrlich tumor cells in the subcutaneous tissue of the dorsal region. A higher than 95% cell viability suspension was used, evaluated by Tripan blue exclusion test. After 24 h of the implant, the animals were divided in two groups: Phys) 0.1 mL intraperitoneal injection (IP) of sterile 0.9% saline solution, and Mel) treated with meloxicam, 2 mg/kg, 0.1 mL, IP, both once a day. After 21 days, all the animals were euthanized with ketamine (100 mg/kg of animal weight) and xylazine (10 mg/kg of animal weight), for the removal of tumor mass, which was weighed in a semi-analytical balance. After that, the tumors were sectioned in their central portion following their larger axis, fixed in 10% buffered formalin and sent to histological and immunohistochemical processing. This protocol was evaluated and approved by Ethics Commission, protocol nº 15/13.
The histological material was processed, embedded in histological paraffin, and sections of 5 μ thickness were stained by hematoxylin-eosin (H & E) for posterior image capture and histomorphometric study.
Tumor cells
Ehrlich's ascitic tumor, maintained in vivo through weekly ticks, was used in this study. Through the Trypan Blue exclusion test, cell viability was analyzed and only cell suspensions with viability greater than 95% were used.
Treatments
The animals was treated whit sterile 0.9% saline solution – Phys, 0.1 mL intraperitoneal injection , IP, or meloxicam - Mel (M3935, Sigma Aldrich, purchased from commercial sources), 2mg/kg of animal weight, 0.1 mL, IP, both once a day.
Immunohistochemistry
For immunolabeling of COX-2, 3 μm sections were depafafinized in xylene and rehydrated in a graded series of etanol to distiled water, and then immersed in 0.01 M citrate-buffer at pH 6.0 to be heated in a steamer for 30 min. Histological slices were be treated with proteinase K for 30 min in room temperature. Endogenous peroxydase was be blocked with 2% peroxyde hydrogen for 10 min and washed with PBS (phosphate buffer solution). After this period, pimary polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, EUA) anti-COX-2 was incubated overnight at 4°C and washed for 30 min, three times. The slices were incubated with biotinylated secondary antibody for 30 min, washed in PBS and incubated with streptavidin-peroxidase conjugate (LSAB, Dakocytomation) for more 30 min. Amplification of the reaction signal was performed using the avidin/biotin-ABC (Vector Laboratories). Thereaafter, reaction was stained with 3,3’-diaminobenzidine tetrahydrochloride (Sigma Aldrich, St Louis, MO, USA) and conterstained with Harris hematoxylin. For negative control, primary antibody was ommited.
Histomorphometry
Histological sections stained by H & E or with immunohistochemical staining for COX-2 were captured and registered in light microscope (Nikon, Eclipse 80i, Tokyo, Japan). The images were analyzed using Image Pro-Plus software, version 5.1 (Media Cybernetics), observing the following parameters: total area, necrosis, parenchyma. Twenty non-coincident fields were captured from the immunolabeled slices, examined in 400x magnification, considering the stroma and parenchyma of the tumor.
Obtained data were treated by Student test for mean comparison of the analyzed parameters, considering p<0.05.
In order to follow Ehrlich solid tumor evolution, tumoral weight was used as comparison parameter, and it was noted that at day 21, Mel group presented a significant reduction of tumor weigh (Figure 1).
Selective inhibition of COX-2 was confirmed by analysis of the immunolabed slices in the parenchymal areas consisting of viable tumor cells and in the stroma areas. In Mel group, there is an evident reduction in immunohistochemical labeling for COX-2 (Figure 2) when compared to Phys group, it is noted that the area of greatest COX-2 inhibition is the tumor parenchyma (Figures 2 and 3).
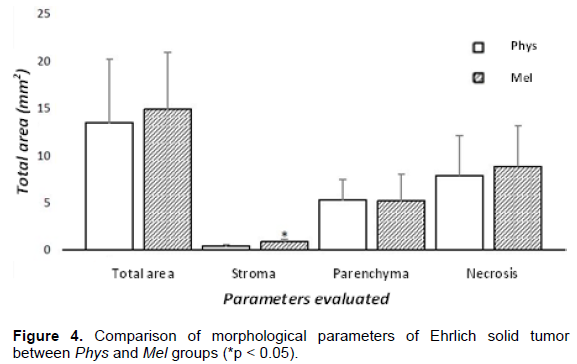
Overall analysis of the histomorphometric parameters demonstrate that there is no influence of meloxican on total area, parenchyma, or necrosis, in comparison to the non-treated group. Meloxican significantly increased tumor stroma (Figure 4), where vascularization and leukocytes can be found.
The evaluation of tumor weight is one of the most used techniques to verify the growth of solid tumors, like the Ehrlich tumor used as a model in this study. We found that treatment with meloxicam was able to significantly reduce this parameter, but the reduction in tumor weight may not be related to a reduction of tumor cells since the tumor mass consists of a central area of necrosis, a stromal full of blood vessels and immune system cells that can influence its weight.
In some neoplasms the therapy with COX inhibitors is beneficial, especially COX-2, as is the case of meloxicam. Inhibition of COXs results in the non-production of PGE2, a metabolite closely associated with the facilitation of tumor growth. Acetylsalicylic acid, a non-selective cyclooxygenase inhibitor (Vane, 2000), has been used as an adjunct drug for treatment rectal colon tumors ttreatment, increasing survival rates when used in low doses (Reimers et al., 2012). The effect of selective COX-2 inhibition has been investigated in some kind of tumors, oral cavity and lung carcinomas (Blackhall et al., 2005; Wang, 2005). The effects associated with treatment with NSAIDs indicate a reduction in tumor growth rate, since PGE2, besides being immuno-suppressive, is angiogenic (Bonanno et al., 2016). The reduction in angiogenesis hinders the nutrition of the tumor mass by slowing its growth, but not totally impeding it. Currently, it is suggested that COX-2 expression can be used for the prognosis of renal carcinoma (Tabriz et al., 2016).
Immunohistochemical labeling revealed that admini-stered dose was sufficient to significantly inhibit the production of COX-2. By image analysis, we found that the immunolabeling was prevalent in the parenchyma of the tumor, confirming that the main source of COX-2 was from the tumor cells (Salgado et al., 2016).
Regarding the histomorphometry of the total tumor area, we did not find differences between Phys and Mel groups, except in relation to the stroma. It is presumed that the total area corresponds to a series of constituents and that, although the treatment did not affect this parameter, it significantly affected tumor weight. Despite inhibition of COX-2 had occur, no reduction of the parenchyma areas was observed. In this way, the selective inhibition of COX-2 was not able to reduce tumor proliferation, although tumor weight has reduced.
Although emphasis is given to the role of COX-2 in relation to a wide variety of tumors, some types of neoplasia present a close correlation with COX-1, which this seems to be the case of the Ehrlich solid tumor. Osman and Youssef (2015) also reported this relationship in renal cell carcinoma and that there is an intimate association with the production of COX-1 and the production of vascular endothelial growth factor (VEGF). In ovarian carcinoma, COX-1 is produced at high levels in the early stages of the tumor, and is dependent on angiogenesis to evolute (Perrone et al., 2014). These data suggest that there is an alternance between the two COX isoforms during neoplastic development, assuming a more relevant role in the early, intermediate or final phase depending on the tumor.
Other studies suggest that non-selective inhibition of COX is also beneficial, as in endometrial cancer (Matsuo et al., 2016), and murine pancreatic carcinoma (Rao et al., 2014). In vitro study with myeloma cells demonstrated induction of apoptosis of tumor cells, and that it was able to reduce tumor proliferation in in vivo experiment, due to apoptosis induction and reduction in VEGF production (Ding et al., 2014).
Taken together, these data suggest a distinct action of COX depending on the type of the neoplasm and also depending on the developing phase in which the tumor is detected. Considering these characteristics is of vital importance not only for the treatment, but especially for monitoring and prognosis of a wide variety of human and animal neoplasms.
Treatment with meloxicam resulted in a significant reduction of COX-2 production by tumor cells, but selective inhibition of COX-2 was not able to control the growth of Ehrlich solid tumor. It is presumed that COX-2 was not involved in the modulation of inflammatory response in the developmental phase of the studied neoplasm. Further studies are needed to clarify whether there is an association between COX-1 and 2 in Ehrlich solid tumor, or whether there is a functional variation between the two isoforms during tumor development.
The authors have not declared any conflict of interests.
REFERENCES
|
Al-Nimer MS, Hameed HG, Mahmood MM (2015). Antiproliferative effects of aspirin and diclofenac against the growth of cancer and fibroblast cells: In vitro comparative study. Saudi Pharm. J. 23(5):483-486.
Crossref
|
|
|
|
Banu SK, Lee J, Speights VO Jr, Starzinski-Powitz A, Arash JA (2008) Cyclooxygenase-2 regulates survival, migration, and invasion of human endometriotic cells through multiple mechanisms. Endocrinology 149(3):1180-1189.
Crossref
|
|
|
|
|
Blackhall F, Papakotoulas PI, Danson S, Thatcher N (2005). Perspectives on novel therapies for bronchial carcinoma. Expert. Opin. Pharmacother. 28(2):103-113.
Crossref
|
|
|
|
|
Ding JH, Yuan LY, Huang RB, Chen GA (2014). Aspirin inhibits proliferation and induces apoptosis of multiple myeloma cells through regulation of Bcl-2 and Bax and suppression of VEGF. Eur. J. Haematol. 93(4):329-339.
Crossref
|
|
|
|
|
Dong X, Li R, Xiu P, Dong X, Xu Z, Zhai B, Liu F, Jiang H, Sun X, Li J, Qiao H (2014). Meloxicam executes its antitumor effects against hepatocellular carcinoma in COX-2 dependent and -independent pathways. PLoS One. 9(3):e92864.
Crossref
|
|
|
|
|
Koehne CH, Dubois RN (2004). COX-2 inhibition and colorectal cancer. Semin. Oncol. 31(2 Sup 7):12-21.
|
|
|
|
|
Lewis ML, Qi W, Smith FG (2013). Distribution of cyclooxygenase (COX) isoforms in the developing ovine kidney. FASEB J. 27(1):733-9.
|
|
|
|
|
Li T, Zhong J, Dong X, Xiu P, Wang F, Wei H, Wang X, Xu Z, Liu F, Sun X, Li J (2016). Meloxicam suppresses hepatocellular carcinoma cell proliferation and migration by targeting COX-2/PGE2-regulated activation of the β-catenin signaling pathway. Oncol. Rep. 35(6):3614-3622.
Crossref
|
|
|
|
|
Matsuo K, Cahoon SS, Yoshihara K, Shida M, Kakuda M, Adachi S, Moeini A, Machida H, Garcia-Sayre J, Ueda Y, Enomoto T, Mikami M, Roman LD, Sood AK (2016). Association of low-dose aspirin and survival of women with endometrial cancer. Obstet. Gynecol. 128(1):127-137.
Crossref
|
|
|
|
|
Nakanishi M, Rosenberg DW (2013). Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 35(2):123-37.
Crossref
|
|
|
|
|
Naruse T, Nishida Y, Hosono K, Ishiguro N (2006). Meloxicam inhibits osteosarcoma growth, invasiveness and metastasis by COX-2-dependent and independent routes. Carcinogenesis 27(3):584-92.
Crossref
|
|
|
|
|
Osman WM, Youssef NS (2015). Combined use of COX-1 and VEGF immunohistochemistry refines the histopathologic prognosis of renal cell carcinoma. Int. J. Clin. Exp. Pathol. 8(7):8165-8177.
|
|
|
|
|
Perrone MG, Malerba P, Uddin MJ, Vitale P, Panella A, Crews BC, Daniel CK, Ghebreselasie K, Nickels M, Tantawy MN, Manning HC, Marnett LJ, Scilimati A (2014). PET preliminary investigation. Euro. J. Med. Chem. 80:562-568.
Crossref
|
|
|
|
|
Rao CV, Mohammed A, Janakiram NB, Li Q, Ritchie RL, Lightfoot S, Vibhudutta A, Steele VE (2012). Inhibition of pancreatic intraepithelial neoplasia progression to carcinoma by nitric oxide-releasing aspirin in p48(Cre/+)-LSL-Kras(G12D/+) mice. Neoplasia 14(9):778-787.
Crossref
|
|
|
|
|
Reimers MS, Bastiaannet E, van Herk-Sukel MP, Lemmens VE, van den Broek CB, van den Velde CJ, de Craen AJ, Liefers GJ (2012). Aspirin use after diagnosis improves survival in older adults with colon cancer: a retrospective cohort study. J. Am. Ger. Soc. (12):2232-6.
|
|
|
|
|
Rizzo MT (2011). Cyclooxygenase-2 in oncogenesis. Clin. Chim. Acta 412(9-10):671-687.
Crossref
|
|
|
|
|
Rodrigues PA, Naresse LE, Rodrigues MA, Kobayasi S (2013). Late administration of a specific COX-2 inhibitor does not treat and/ or prevent progression of gastric tumors in rats submitted to duodenogastric reflux procedure. Acta Cir. Bras. 28(6):453-7.
Crossref
|
|
|
|
|
Salgado FLL, Artigiani-Neto R, Lopes-Filho GJ (2016). Growth factors and COX2 in wound healing: an experimental study with ehrlich tumors. ABCD Arq. Bras. Cir. Dig. 29(4):223-226.
Crossref
|
|
|
|
|
Tabriz HM, Mirzaalizadeh M, Gooran S, Niki, Jabri M (2016). COX-2 expression in renal cell carcinoma and correlations with tumor grade, stage and patient prognosis. Asian Pac. J. Cancer Prev. 17(2):535-538.
Crossref
|
|
|
|
|
Takami Y,Eguchi S, Tateishi M, Ryu T, Mikagi K, Wada Y, Saitsu H (2016). A randomised controlled trial of meloxicam, a Cox-2 inhibitor, to prevent hepatocellular carcinoma recurrence after initial curative treatment. Hepatol. Int. 10(5):799-806.
Crossref
|
|
|
|
|
Vane JR (2000). The mechanism of Action of Anti-Inflammatory Drugs. Ernst Schering Res. Found. Workshop 31:1-23.
Crossref
|
|
|
|
|
Wang Z (2005). The role of COX-2 in oral cancer development, and chemoprevention/ treatment of oral cancer by selective COX-2 inhibitors. Curr. Pharm. Des. 11(14):1771-1777.
Crossref
|
|
|
|
|
Yoshida Y, Matsumura H, Nakajima T, Mandai M, Urakami T, Kuroda K, Yoneda H (2000). Prostaglandin E (EP) receptor subtypes and sleep: promotion by EP4 and inhibition by EP1/EP2. Neuroreport. 11(10):2127-2131.
Crossref
|
|