The non-vitamin K antagonist oral anticoagulants have been approved since 2008 for the prevention of systemic embolism and stroke in patients with non-valvular atrial fibrillation with prior stroke, transient ischemic attack, or CHA2DS2-VASc score ≥2. According to the latest guidelines of 2016, if CHA2DS2-VASc score ≥2 for all male patients or if CHA2DS2-VASc score ≥3 for all female patients, oral anticoagulants are recommended, and considering shared decision between doctor and patient. However, they are contraindicated in patients with mechanical prosthetic valve and in patients with moderate-to-severe mitral stenosis and atrial fibrillation. They have also been approved for prevention and treatment of deep vein thrombosis and pulmonary embolism. Dabigatran, a direct thrombin inhibitor, was the first to be approved, followed by the factor Xa inhibitors, rivaroxaban, apixaban, and recently, in 2015, edoxaban. They have advantages such as the use of fixed-dose, with infrequent drug interaction, rapid onset of action and do not require monitoring of their anticoagulant action. Nonetheless, they have different half-lives, the need for dose adjustment according to renal function, weight and age of the patient. Its use in pregnant women and children or adolescents is not well established. There are also peculiarities about the risks of bleeding, effects on coagulation tests and specific antidotes. Through this review we discuss the pharmacokinetics and pharmacodynamics characteristics of direct oral anticoagulants, its indications, interactions and contraindications. Analysis of its efficacy, safety and risk-benefit ratio will also be addressed.
Dabigatran
Historical
Dabigatran etexilate mesilate is a pro-drug of an orally-direct thrombin inhibitor, dabigatran, which binds to thrombin with high affinity and specificity. This pro-drug was in phase II clinical trials for thromboembolism in 1999. It had shown excellent potential as an antithrombotic agent in preclinical studies and it demonstrated an acceptable safety profile for prevention deep vein thrombosis after total hip replacement (Mungall, 2002; Eriksson et al., 2005; Di Nisio et al., 2005). It was the first NOAC; U.S approved in many countries worldwide (in 2010 by the Food and Drug Administration - FDA – and in 2008 by European Medicines Agency - EMA) for the prevention of stroke and blood clots from AF based on the results of the RE-LY (Randomized Evaluation of Long-term Anticoagulant Therapy, Warfarin, compared with Dabigatran) trial (Connolly et al., 2009; Beasley et al., 2011).
Pharmacokinetic properties
The chemical name of pro-drug is ethyl N-{[2-({[4-((E)-amino {[(hexyloxy) carbonyl] imino} methyl) phenyl] amino} methyl)-1-methyl-1H-benzimidazol-5-yl] carbonyl}-
Npyridin-2-yl-β-alaninate methane sulfonate. Its molecular formula is C35H45N7O8S. The molecular formula of active
substance (dabigatran) is C25H25N7O3 (EMA, 2016; FDA, 2016).
Drug absorption: Dabigatran etexilate mesylate contains a tartaric acid pellet that creates an acid microenvironment, which permits the absorption from the gut, since it features low solubility at pH ≥ 3. Thus, it is absorbed rapidly (up 1 h in fasted state) and hydrolyzed completely to the active molecule, dabigatran, by ubiquitous non-specific esterases in the gut, plasma, and liver. Dabigatran is not well absorbed from the gut due to being highly polar and lipophobic. Absorption is not influenced by food intake (FDA, 2016; Gallego et al., 2014;
Harder and Graff, 2013).
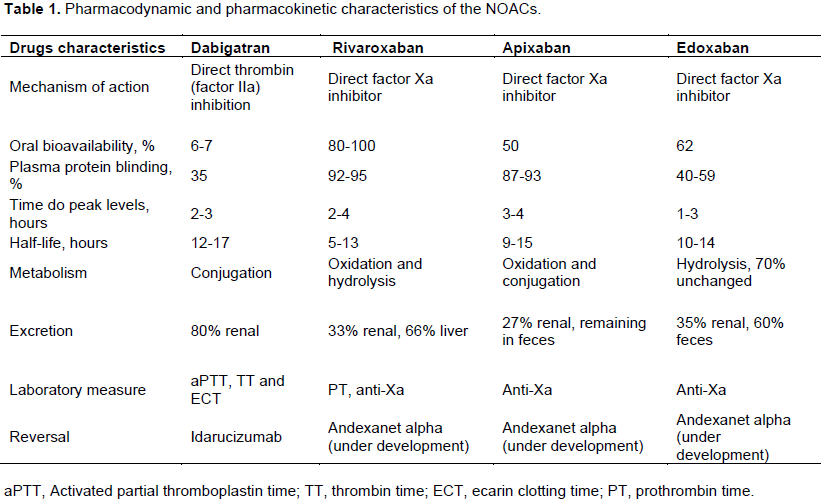
In healthy male subjects, peak plasma concentrations of dabigatran are reached within 2 h of administration and its half-lives are 8 to 10 and 12 to 17 h with single and multiple dose administrations (three times daily), respectively (FDA, 2016;
Stangier et al., 2007; Blech et al., 2008). In the elderly, its peak plasma concentration is 3 h (range 2 to 4 h), and half-life of 12 to 14 h, after administration twice daily (Stangier et al., 2008).
Distribution, metabolism and excretion: Following oral administration, its peak plasma concentrations is within 2 to 3 h after administration. Dabigatran is metabolized primarily by esterases and is independent of the P450 cytochrome. It is a substrate of P-glycoprotein (Gallego et al., 2014;
Harder and Graff, 2013). The integrity of capsule of dabigatran must be preserved during administration. Its bioavailability is increased by 75% without capsule intact (Gong et al., 2013). There is low (35%) concentration binding to human plasma proteins (EMA, 2016; Blech et al., 2008). The steady-state is reached within 48 h of administration in a subject with normal renal function (EMA, 2016; FDA, 2016).
The dominant elimination pathway is renal excretion, which accounts for approximately 80%. The remainder of the drug that undergoes conjugation to form acyl glucuronides is eliminated by the liver. Its oral bioavailability is low (6 to 7%) and is independent of the dose of the pro-drug and pH-dependent. It has a moderate volume of distribution of 50 to 70 L. Steady state concentrations are achieved after 4 to 7 days of dosing, with no evidence of significant accumulation (EMA, 2016; FDA, 2016; Gallego et al., 2014;
Harder and Graff, 2013; Blech et al., 2008).
Pharmacodynamics
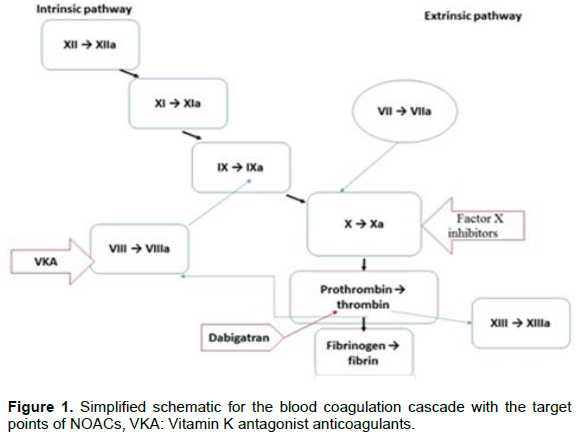
Mechanism of action: Dabigatran is a synthetic drug, non-peptide, competitive inhibitor of thrombin, and quick action. This oral direct reversible thrombin inhibitor connects to thrombin, with high specificity and affinity (Figure 1), inactivating both fibrin-bound as well as unbound thrombin. Thrombin is the final enzyme in the coagulation cascade and it is the mediator in the formation of fibrin and a potential platelet activator (EMA,2016; FDA, 2016). Therefore, dabigatran potentially also inhibits platelet aggregation (Stangier et al., 2009). The specificity of dabigatran provides a linear dose response, wide therapeutic index, and fixed dosing.
Therapeutic indications: Dabigatran is indicated for prevention of systemic embolism and stroke in patients with non-valvular AF taking into account CHA2DS2-VASc score [Congestive Heart failure, hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled), Vascular disease, Age 65 to 74, and Sex (female)]. With prior stroke, transient ischemic attack, or CHA2DS2-VASc score ≥2, oral anticoagulants are recommended, considering the peculiarities and preferences of patients (January et al., 2014). According to the latest guidelines of 2016, if CHA2DS2-VASc score ≥2 for all male patients or if CHA2DS2-VASc score ≥3 for all female patients, oral anticoagulants are recommended (Kirchhof et al., 2016). It is also indicated for treatment of acute venous thromboembolism, pulmonary embolism and to prevent its recurrence (Konstantinides et al., 2014; Schulman et al., 2009; Schulman et al., 2014; Burnett et al., 2016). Other indication is primary prevention of venous thromboembolic events in adult patients who have undergone elective total hip replacement surgery or total knee replacement surgery (EMA, 2016).
Posology and method of administration: For prevention of embolism in patients with AF, the dose of 150 mg twice a day of dabigatran by the oral route has been approved for those with creatinine clearance (CrCl – calculated by Cockcroft-Gault Equation) > 50 mL/minute. Low doses (110 mg twice daily) may be considered in patients aged over 75 years, patients with moderately reduced kidney function and other patients who are at increased risk of bleeding. Dose of 75 mg twice daily is approved only in the United States. Dabigatran is not approved for patients with a CrCl less than 30 mL/min. Renal function should be monitored at least once a year if their kidney function is mildly to moderately reduced or if they are over 75 years old (EMA, 2016; FDA, 2016; January et al., 2014). Dabigatran exposure is about 40 to 50% higher among women than among men (EMA, 2016), reflecting sex-related differences in CrCl rates.
At the moment, after parenteral anticoagulation, dabigatran is approved for treatment of venous thromboembolism and pulmonary embolism as an alternative to treatment with vitamin K antagonists. The dose is 150 mg twice daily or 110 mg twice daily for patients ≥ 80 years age or those under concomitant verapamil use and treatment should be done for 6 months or at least 3 months (Konstantinides et al., 2014). For primary prevention of venous thromboembolism in elective knee replacement surgery, the treatment should be initiated within 1 to 4 h of completed surgery with a single of 110 mg capsule and continuing with 2 capsules once daily thereafter for a total of 10 days. Following elective hip replacement surgery, the dose is the same, but the treatment time is 28 to 35 days (EMA, 2016). Dose adjustment is needed for patients with moderate renal impairment or over the age of 75.
Interactions: Interactions between dabigatran and drugs metabolized by cytochrome P450 are not clinically relevant (Blech et al., 2008). Moderate hepatic impairment did not affect the pharmacokinetics and pharmacodynamics or dabigatran safety profile. This NOAC does not induce changes in relation to liver aminotransferases. Thus, no dose adjustment is needed (FDA, 2016; Stangier et al., 2008). However, dabigatran etexilate, but not dabigatran, is a substrate of the efflux transporter P-glycoprotein (P-gp) and drugs that affect this system will influence its pharmacokinetics (EMA, 2016; FDA, 2016; Gallego et al., 2014;
Harder and Graff, 2013; Blech et al., 2008; Heidbuchel et al., 2016).
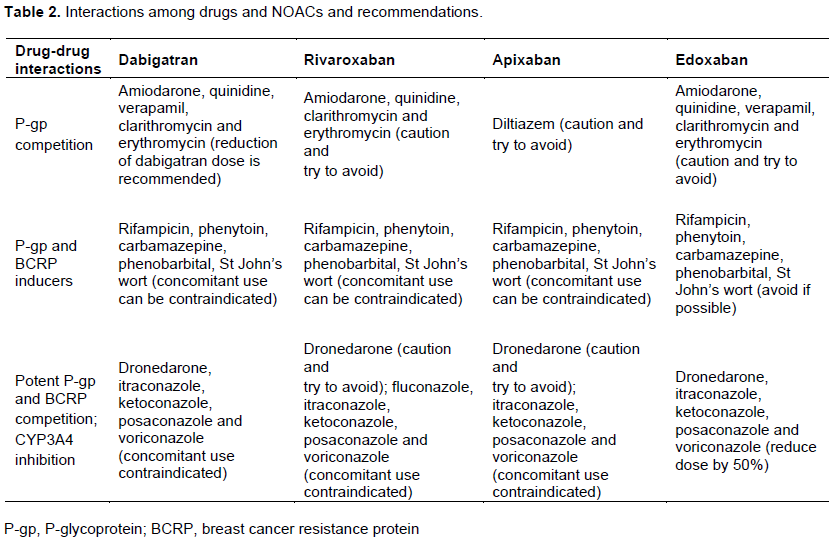
The reduction in dabigatran dose (110 mg twice daily) should be considered if concomitant use of these drugs due to moderate competition with P-gp: Amiodarone, quinidine, verapamil, clarithromycin and erythromycin. There is an increased dabigatran plasma levels of up to 20% with concomitant use those macrolides, up to 53% with quinidine, up to 60% with amiodarone and up to 180% with verapamil. Some interactions lead to the reduction NOAC plasma levels of 66% and this may also constitute a contraindication for simultaneous use. This occurs with concomitant administration of P-gp inducers (rifampicin, phenytoin, carbamazepine, phenobarbital, St John’s wort - Hypericum perforatum) (Heidbuchel et al., 2016).
There are drugs that should not be co-administered because they were potent inhibitors of P-gp. These drugs are dronedarone (100% increase of the plasma level), and fungostatics itraconazole, ketoconazole, posaconazole and voriconazole (up 150% increase dabigatran plasma level). Co-administration with cyclosporine, tacrolimus and HIV protease inhibitors is not recommended. There are no data yet about concomitant use of fluconazole or naproxen with dabigatran. And there is no effect between digoxin or diltiazem and dabigatran (Heidbuchel et al., 2016).
The most adverse event associated with both dabigatran doses is dyspepsia, which is probably related to the tartaric acid within the capsule (Gallego et al., 2014;
Harder and Graff, 2013; Stangier et al., 2007; Blech et al., 2008). Nevertheless, antacids and proton pump inhibitors decrease the plasma concentration of dabigatran by 20 to 25%. Therefore, dabigatran should be ingested 2 h before those medications (Stangier et al., 2009; Potpara and Lip, 2013).
Contraindications: In addition to the contraindications because of interactions among drugs, severe renal impairment is another contraindication to the use of dabigatran. Among patients with severe renal impairment (CrCl 15 to 30 mL/min), there is a 6.3-fold increase of dabigatran plasma level and its half-live increases to almost 28 h (Stangier et al., 2010). Its use in dialysis patients is contraindicated because this NOAC is mainly eliminated by the kidneys and its bioaccumulation may precipitate bleeding (Chan et al., 2015).Dabigatran well as the other NOACs are contraindicated in patients with mechanical prosthetic valve, moderate-to-severe mitral stenosis (usually of rheumatic origin) due to severe valve complications and death (January et al., 2014; Kirchhof et al., 2016; Heidbuchel et al., 2016).
Characteristics in specific groups
The particularities of the use of dabigatran in relation to age, weight, gender, renal impairment have been discussed previously. The pharmacokinetics of dabigatran in children has not been studied (FDA, 2016). There is direct evidence of the transfer of dabigatran and its pro-drug across the term human placenta from the mother to the fetus. Therefore, its use in pregnant women is not recommended (Bapat et al., 2014).
Laboratory monitoring and antidote
Laboratory monitoring of NOACs is not necessary, but may be important in the management in cases of hemorrhage or during the perioperative period. Thus, in these circumstances, the anticoagulant effect of dabigatran can be measured using the activated partial thromboplastin time (aPTT), thrombin time (TT), ecarin clotting time (ECT), and the diluted version of TT (Hemoclot). Despite the fact that the measurements of TT, Hemoclot and ECT presented higher sensitivity and accurate, TT is sensitive to check the efficacy and Hemoclot is more appropriate to quantify the concentrations of dabigatran (Gong et al., 2013).
In the case of bleeding in addition to the general measures such as discontinuation of NOAC, hydration, maintenance of diuresis, transfusing blood products, activated charcoal, dialysis (January et al., 2014; Kirchhof et al., 2016), there is the antidote. Idarucizumab, a humanized, monoclonal antibody fragment that binds both free and thrombin-bound dabigatran, can be use in dose of IV 5 g for immediate reversal of dabigatran. Their safety and efficacy were subsequently established in phase III study (Tummala et al., 2016). It is approved under accelerated approval in the USA and in the European Union at the end of 2015 for patients in use of dabigatran that need emergency surgery or urgent procedures and in life-threatening or uncontrolled bleeding (EMA, 2016; FDA, 2016).
Rivaroxaban
Historical
Rivaroxaban is the second NOAC approved in September 2008 by EMA and by the FDA based on the results of the ROCKET AF (Rivaroxaban Versus Warfarin in Nonvalvular Atrial Fibrillation) for the prevention of systemic embolism and stroke in patients with AF (Ferns and Naccarelli, 2015; Verheugt and Granger, 2015; January et al., 2014; Kirchhof et al., 2016; Patel et al., 2011). It was the first oral factor Xa inhibitor available for the management of venous thromboembolism and pulmonary embolism (Bauersachs et al., 2010; Büller et al., 2012).
Pharmacokinetic properties
The chemical name of Rivaroxaban is 5-Chloro-N-({(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl) phenyl]-1,3-oxzolidin-5-yl}methyl)-2-thiophene-carboxamide. This active substance presents low solubility pH-independent in water and high permeability (EMA, 2016; Mueck et al., 2014).
Drug absorption, distribution, metabolism and excretion: Rivaroxaban is rapidly absorbed and its peak is within 2 to 4 h after a single oral dose and maximum factor Xa inhibition reached 3 h after administration. After multiple doses, there was no significant accumulation of the drug (Mueck et al., 2014; Kubitza et al., 2005). There are no significant differences in maximal inhibition of factor Xa activity between the first and second daily doses (Kubitza et al., 2005). Its oral absorption is almost complete and oral bioavailability is high (80-100%). Under fasting conditions, its bioavailability is 66% (EMA, 2016; Mueck et al., 2014). Intake with food and types of food (high-fat or high-carbohydrate meal) does not affect the plasma concentrations of Rivaroxaban (doses of 2.5 and 10 mg), that is, area under the plasma concentration-time curve. However, there is 39% increase in its plasma concentration when rivaroxaban 20 mg tablets are taken together with food (EMA, 2016; Mueck et al., 2014). Its half-lives are 5 to 9 h in young, healthy subjects and 11 to 13 hours in elderly subjects (EMA, 2016). There is high (92-95% in vitro) concentration binding to human plasma proteins, which is reversible (Table 1). Serum albumin is the main binding component. Therefore, this NOAC is not dialyzed. The volume of distribution at steady-state is about 50 L, indicating its low to moderate affinity to peripheral tissues (EMA, 2016; Mueck et al., 2014).
It has a dual mode of elimination; two-thirds of the dose undergoes metabolic degradation and one third (36%) of the dose is eliminated as unchanged drug in the urine. Half of the dose undergoes metabolic degradation are excreted by the kidney and half by the hepatobiliary route is metabolized by cytochrome P450 and mechanisms CYP independent (CYP3A4 accounts for approximately 18% and CYP2J2 for approximately 14% of total rivaroxaban elimination) (Ferns and Naccarelli, 2015; Verheugt and Granger, 2015; EMA, 2016; January et al., 2014; Mueck et al., 2014).
Pharmacodynamics
Mechanism of action: Factor Xa is an integral component of the coagulation cascade and is generated via both the intrinsic and extrinsic pathways. Rivaroxaban is a direct factor Xa inhibitor, which connects reversibly to the active site of factor Xa, with high specificity, and acts independently of endogenous antithrombin (Figure 1). So, it suppresses the production of new molecules and plasma thrombin has no significant effect on the activity of the existing thrombin (Gallego et al., 2014; Harder and Graff, 2013; Potpara and Lip, 2013).
Therapeutic indications:
1
1.1
1.2
1.3
1.3.1
Rivaroxaban is indicated for prevention of systemic embolism and stroke in patients with non-valvular AF in the likeness of dabigatran (Ferns and Naccarelli, 2015; Verheugt and Granger, 2015; January et al., 2014; Kirchhof et al., 2016), treatment of deep vein thrombosis and pulmonary embolism and to prevention of venous thromboembolism in patients who are undergoing surgery to replace a hip or knee (Konstantinides et al., 2014; Schulman et al., 2009; Schulman et al., 2014; Burnett et al., 2016). The 2.5 mg twice-daily dose (co-administrated with acetylsalicylic acid alone or with acetylsalicylic acid plus clopidogrel or ticlopidine) has been approved for prevention of atherothrombotic events in patients with acute coronary syndrome with elevated cardiac biomarkers (EMA, 2016; Mega et al., 2012).
Posology and method of administration: A dose of 20 mg orally per day is indicated for patients with non-valvular AF with CrCl >50 mL/min. For patients with a CrCl between 15 and 50 mL/min, the recommended dose is 15 mg per day. Rivaroxaban is not approved for use in subjects with a CrCl <15 mL/min (EMA, 2016; FDA, 2016; January et al., 2014). Thus, periodic assessment of renal function should be made.
For the treatment of deep vein thrombosis and pulmonary thromboembolism and for reducing the risk of recurrence of such emboli, oral treatment with rivaroxaban should be started directly or after a 1 to 2 days administration of unfractionated heparin or low molecular weight heparin or fondaparinux. The dose is 15 mg twice daily for 21 days. After this period, the dose is 20 mg once daily for 3 to 6 months or even indefinitely for secondary prevention. For prophylaxis of deep vein thrombosis following hip or knee replacement surgery, the dose is 10 mg orally once daily (Konstantinides et al., 2014; EMA, 2016; FDA, 2016). It is recommended taking doses of 15 or 20 mg with food (once daily with the evening meal) (FDA, 2016). The dose of 2.5 mg twice a day is recommended for patients with acute coronary syndromes, as previously described (EMA, 2016; Mega
et al., 2012).
No dose adjustment is necessary for gender and/or body weight. Extremes in body weight (less than or equal to 50 kg or above 120 kg) showed little influence on the concentrations of rivaroxaban (less than 25%) compared to individuals with abodyweightof70to80kg.There was also no significant inter-individual variability among ethnic groups (Caucasian, African-American, Hispanic, Chinese, Japanese) (Kubitza et al., 2007).
Interactions: Rivaroxaban is substrate of efflux transporters P-gp and breast cancer resistance protein (BRCP). Thus, this NOAC is affected by medications with P-gp inhibition or induction. It is also affected strongly by cytochrome P450 3A4 (CYP3A4) inducers and inhibitors (Gallego et al., 2014; Harder and Graff, 2013; Heidbuchel et al., 2016).
Nevertheless, there are fewer interactions among drugs in relation to rivaroxaban. There is an increased rivaroxaban plasma levels of up to 54% with concomitant use those macrolides (clarithromycin and erythromycin), up to 160% with fungostatics (itraconazole, ketoconazole, posaconazole and voriconazole) and up to 153% with HIV protease inhibitors. Co-administration with systemic fluconazole should be done with caution because there is a 42% increase in plasma levels of rivaroxaban. The concomitant use of rivaroxaban and amiodarone or diltiazem or verapamil should be with caution if renal impairment and its dose reduction should be considered. Some interactions lead to the reduction NOAC plasma levels of 50% and this may also constitute a contraindication for simultaneous use. This occurs with concomitant administration of P-gp inducers (rifampicin, phenytoin, carbamazepine, phenobarbital, St John’s wort - Hypericum perforatum). There are no data about concomitant use of dronedarone, quinidine, cyclosporine, tacrolimus or naproxen with rivaroxaban. Thus, its use should be done with caution or avoided (Table 2). And there is no effect between digoxin or antacids and rivaroxaban (Heidbuchel et al., 2016).
Contraindications: Besides the contraindications as severe renal impairment, drug-drug interactions and active pathological bleeding, another contraindication is severe hepatic impairment. Rivaroxaban is contraindicated in patients with hepatic disease, including cirrhotic patients classified as Child-Pugh B and C (EMA, 2016; FDA, 2016).
Characteristics in specific groups
As described with respect to dabigatran there are also no clinical data on the use of rivaroxaban in the population under 18 years of age. There is an ongoing study of children and adolescents treated with rivaroxaban for venous thromboembolism (Mueck et al., 2014; ClinicalTrials.gov identifier: NCT02234843, EINSTEIN Junior Phase III, 2014).
The use of rivaroxaban in pregnant women has not been established, because there are no adequate or well-controlled studies. Its use is not recommended for patients with mechanical prosthetic heart valves or with moderate-to-severe mitral stenosis (FDA, 2016; Kirchhof et al., 2016).
Laboratory monitoring and antidote
1.1
2.2
The anticoagulant effect of rivaroxaban can be measured using the anti-factor Xa activity assay which is most sensitive. Other more readily available tests are the prothrombin time and HepTest (Gong et al., 2013). Unlike dabigatran, rivaroxaban is not dialyzable because of its plasma protein binding. Besides the usual measures previously described for management of bleeding, it can be made administration of prothrombin complex concentrates, which results in a partial reversal of prolongation in prothrombin time in healthy volunteers (FDA, 2016). Until the present time, a specific antidote for rivaroxaban was not approved. Andexanet alpha, a recombinant human factor Xa analogue that competes for the factor Xa inhibitors with factor Xa, and ciraparantag (Aripazine), a synthetic molecule that seems to have more widespread antagonistic effects, are under investigation (Kirchhof et al., 2016; Heidbuchel et al., 2016; Tummala et al., 2016).
Apixaban
Historical
Apixaban is the third new oral anticoagulant approved by the FDA and by EMA in May 2011, based on the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) for the prevention of systemic embolism and stroke in patients with AF (
Verheugt and Granger, 2015; January et al., 2014; Kirchhof et al., 2016; Granger et al., 2011). It has been approved by the FDA in August 2014, also for the treatment of deep vein thrombosis and pulmonary embolism (FDA, 2016; Agnelli et al., 2013).
Pharmacokinetic properties
The chemical name of apixaban is 1-(4- methoxyphenyl)-
7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4c]pyridine-3-carboxamide (FDA, 2016).
Drug absorption, distribution, metabolism and excretion
It is another oral reversible direct factor Xa antagonist. It
has rapid absorption with of up to 15-h half-life and food does not affect its bioavailability. Its absolute bioavailability is approximately 50% for doses up to 10 mg. Its peak is within 3 to 4 h after oral dose (FDA, 2016; Gallego et al., 2014;
Harder and Graff, 2013; Potpara and Lip, 2013). Plasma protein binding is 86.8 to 93.2% and distribution volume is 0.30 L/kg after oral dose (Wong et al., 2011). The metabolic pathways are
O-Demethylation and hydroxylation at the 3-oxopiperidinyl moiety. Apixaban is primarily metabolized through CYP3A4. There are minor contributions from CYP1A2, 2C8, 2C9, 2C19, and 2J2 (EMA, 2016; FDA, 2016; Wong et al., 2011).
Its elimination involves multiple pathways, each responsible for one third of the dose excreted, including hepatic metabolism and renal excretion and intestinal-biliary secretion. Twenty-seven percent (range 24.5 to 28.8%) of the drug is excreted by the kidneys (Table 1). The majority of the recovered dose is in feces and biliary clearance is a minor apixaban elimination pathway (<5% of dose). Intestinal secretion of this NOAC also occurs (Wong et al., 2011; Raghavan et al., 2009).
Pharmacodynamics
Mechanism of action: Apixaban has high affinity and selectivity for human factor Xa and relatively low affinity for thrombin and trypsin. Thus, for its antithrombotic action, it does not require antithrombin III. It inhibits free and clot-bound factor Xa, and prothrombinase activity. For factor Xa inhibition, apixaban decreases thrombin production and the development of thrombi (Figure 1). It has no direct effect on platelet aggregation, nonetheless indirectly inhibits aggregation of platelet induced by thrombin (FDA, 2016; Raghavan et al., 2009).
Therapeutic indications: Apixaban is indicated for prevention of systemic embolism and stroke in patients with non-valvular AF (Ferns and Naccarelli, 2015; Verheugt and Granger, 2015; January et al., 2014; Kirchhof et al., 2016), for prevention of venous thromboembolic events in adult patients who have undergone elective hip or knee replacement surgery, and for treatment of deep vein thrombosis and pulmonary embolism (EMA, 2016; FDA, 2016).
Posology and method of administration: The approved dose is 5 mg bid with a reduction in dose to 2.5 mg orally twice daily for patients with at least 80 years of age or body weight not exceeding 60 kg or serum creatinine ≥ 1.5 mg/dL (133 micromole/L). The drug was not approved for patients with severe or end stage chronic kidney disease and in patients with severe hepatic impairment (Verheugt and Granger, 2015; EMA, 2016; FDA, 2016; January et al., 2014). For prevention of venous thromboembolic events, the initial dose is 2.5 mg twice daily 12 to 24 h after elective hip or knee replacement surgery. The duration of treatment is 32 to 38 days for hip surgery and for 10 to 14 days for knee replacement surgery (EMA, 2016).
Interactions: Apixaban has low potential to inhibit or induce cytochrome P450, or to form reactive metabolites. Therefore, the drug interaction potential is low. Current recommendations include a reduction in dose when co-administered with drugs that are potent dual inhibitors of cytochrome P450 3A4 and P-glycoprotein (Heidbuchel et al., 2016; DeWald and Becker, 2014). There is an increased apixaban plasma levels of up to 40% with concomitant use diltiazem and of 55% with naproxen. Thus, the co-administration with these drugs should be undertaken with caution.
Despite the lack of data, this also applies for use with amiodarone and dronedarone. There is a 54% reduction in plasma levels of apixaban with the concomitant use of rifampicin, phenytoin, carbamazepine, phenobarbital, St John’s wort. Because of the 100% increase of the plasma level of apixaban with fungostatics (itraconazole, ketoconazole, posaconazole and voriconazole) or its strong increase with HIV protease inhibitors, there is contraindication to the concomitant use of these drugs (Table 2). There are no data on the concomitant use with digoxin, quinidine, verapamil, atorvastatin, clarithromycin, erythromycin, fluconazole, cyclosporine and tacrolimus. There is no interaction with antacids (Burnett et al., 2016).
Contraindications: The contraindications to the use of apixaban are severe renal impairment and those already discussed due to interaction with other drugs, as well as significant active bleeding and hypersensitivity to the drug (EMA, 2016; FDA, 2016; Burnett et al., 2016).
Characteristics in specific groups
There is no data on the use of apixaban in children and adolescents (EMA, 2016; FDA, 2016), but there is an ongoing study (ClinicalTrials.gov identifier: NCT02464969, 2015). There is evidence that apixaban reaches the fetal circulation, detecting total concentrations of 35 to 90% in cord blood relative to maternal levels. Thus, its use is not recommended during pregnancy (Bapat et al., 2016).
Laboratory monitoring and antidote
Anti-Xa may be used for apixaban quantification, but the prothrombin time is less sensitive. Andexanet alpha reverses over 90% of anti-factor Xa activity for all factor Xa inhibitors (rivaroxaban, apixaban, edoxaban) in healthy volunteers. The drug acts within 2 to 5 min after infusion (bolus and maintained for 2 h), and has a half-life of approximately 45 min (Reiffel et al., 2016). However, this antidote is still under investigation (Kirchhof et al., 2016; Heidbuchel et al., 2016; Tummala et al. 2016).
Edoxaban
Historical
Edoxaban is the latest NOAC to be approved in 2015, based on the ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation Thrombolysis in Myocardial Infarction 48 trial), for the prevention of systemic embolism and stroke in patients with AF (Giugliano et al., 2013). It has been approved for the treatment of deep vein thrombosis and pulmonary embolism based on the Hokusai-VTE studies (Raskob et al., 2013; Büller et al., 2013).
Pharmacokinetic properties
The chemical name of edoxaban is N-(5-Chloropyridin-2-yl)-N′-[(1S,2R,4S)-4-(N,N-dimethylcarbamoyl)-2-(5 methyl4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxamido)cyclohexyl]oxamide mono 4methylbenzenesulfonate) monohydrate (FDA, 2016).
Drug absorption, distribution, metabolism and excretion: Edoxaban displays rapid absorption between 1 and 3 h with peak plasma levels at 1.5 h. Its absorption is mostly performed in the upper gastrointestinal tract, and about 13% of the dose is absorbed in the colon. It has a relatively low protein binding, which ranges from 40 to 59%. The bioavailability is 62%. Its volume of distribution is 170 L and its half-life ranges from 10 to 14 h (EMA, 2016; FDA, 2016; Ogata et al., 2010; Lip and Agnelli, 2014; Parasrampuria and Truitt, 2016). The absorption, elimination, peak concentration and half-life of a single 60-mg dose of edoxaban are not affected by food (Ogata et al., 2010).
About 70% of unchanged drug is the predominant form in the plasma. The remainder is biotransformed to several metabolites, the most abundant (M-4) of which is formed by hydrolysis (mediated by esterases), conjugation and oxidation by CYP3A4 (FDA, 2016; Lip and Agnelli, 2014).
Renal excretion of edoxaban is 35% (from 34.7 to 38.8%) and the elimination of 60% of the drug is by feces (Table 1). There is a minimum clinical effect of gender on the pharmacokinetics of edoxaban (Ogata et al., 2010; Lip and Agnelli, 2014).
Pharmacodynamics
Mechanism of action: Edoxaban is a highly selective, reversible and direct inhibitor of activated factor Xa (Figure 1). It binds to both the free factor Xa as the prothrombinase activity. This NOAC does not require antithrombin III for antithrombotic activity. Thus, its action reduces thrombin generation and thrombus formation. It also inhibits thrombin-induced platelet aggregation (EMA, 2016; FDA, 2016).
Therapeutic indications: Edoxaban is indicated for prevention of systemic embolism and stroke in patients with non-valvular AF, but it should not be used by patients with CrCL > 95 mL/min because of an increased risk of ischemic stroke compared to warfarin. This NOAC is also indicated for treatment and prevention of deep vein thrombosis and pulmonary embolism (EMA, 2016; FDA, 2016).
Posology and method of administration: For patients with non-valvular AF, the recommended dose is 60 mg once daily. If CrCl 15-50 mL/min or body weight ≤ 60 Kg, the dose is 30 mg once daily (EMA, 2016; FDA, 2016; January et al., 2014; Kirchhof et al., 2016).
For treatment of venous thromboembolic events and pulmonary embolism, the initial dose is 60 mg after initial use of parenteral anticoagulant for 5 to 10 days. The duration of use of edoxaban should be at least 3 months (Konstantinides et al., 2014; EMA, 2016; FDA, 2016).
Interactions: Edoxaban is a substrate of P-gp transporter. Thus, there is an increased its plasma levels of 40% with concomitant use amiodarone, 77% with quinidine, 53% with verapamil and 73% with cyclosporine and tacrolimus. The 50% reduction of edoxaban dose is
recommended with concomitant use of dronedarone, as well as for co-administration with fungostatics, clarithromycin and erythromycin. There is a 35% reduction in plasma levels of edoxaban with the concomitant use of rifampicin, phenytoin, carbamazepine, phenobarbital, St John’s wort (Table 2). Concomitant use of HIV protease inhibitors is contraindicated. There are no data on the concomitant use with diltiazem, atorvastatin and fluconazole. There is no interaction with digoxin and antacids (Burnett et al., 2016).
Contraindications: Contraindications to the use of edoxaban were discussed previously, such as renal failure, interactions among drugs, and active bleeding or risk of major bleeding (EMA, 2016).
Characteristics in specific groups
The use of edoxaban has not been established in pregnant and breast-feeding women. Therefore, these clinical conditions are contraindications to the use of this NOAC (EMA, 2016).
Laboratory monitoring and antidote
The anticoagulant effect of edoxaban can be measured using the anti-factor Xa activity. The oral 60 mg dose produces a 2-fold increase in prothrombin time (Ogata et al., 2010). The antidote Andexanet alpha is still under investigation (Heidbuchel et al., 2016; Tummala et al., 2016).
Effectiveness and safety of NOACs
Randomized and controlled studies have demonstrated safety, efficacy and favorable risk-benefit ratio of NOACs to prevent both stroke and systemic embolism in patients with non-valvular AF compared with warfarin therapy (Connolly et al., 2009; Beasley et al., 2011; Patel et al., 2011; Granger et al., 2011; Giugliano et al., 2013). These studies included patients with strict selection criteria, which might not reflect the real world. However, meta-analyzes, real-world studies, and post-marketing studies have demonstrated comparable results to phase III studies. Meta-analyzes with up to 50,578 patients using NOACs showed reduction of stroke, intracranial hemorrhage and mortality, but there was a higher gastrointestinal bleeding rate compared to the group using warfarin (Miller et al., 2012; Capodanno et al., 2013; Ruff et al., 2014). Recent systematic review, including meta-analyzes and observational studies, with overlapping studies, comparing patients on NOACs and those using warfarin, for the consolidated indications (non-valvular AF and deep venous thrombosis/pulmonary thromboembolism), showed results similar to those described above (Raschi et al., 2016). Post-marketing studies are non-randomized, retrospective studies, but reflect the real-world clinical practice. These studies have also demonstrated similar findings (Romanelli et al., 2016; Villines and Peacok, 2016). In unselected population, the first prospective, observational, international study showed low stroke rates and bleeding in patients with non-valvular AF in use of rivaroxaban (Camm et al., 2015).
A current issue is the comparison between NOACs regarding the prevention of embolism and important adverse effects such as major bleeding. Observational and retrospective study evaluating the risk of severe hemorrhage among patients newly initiated with warfarin, dabigatran, rivaroxaban and apixaban demonstrated some differences between NOACs (Lip et al., 2016). Patients with rivaroxaban had a significantly increased risk of bleeding compared with patients newly started with apixaban. Recently, a large study with three propensity-scoring-matching cohorts of patients with non-valvular AF was published (Noseworthy et al., 2016). Regarding the prevention of stroke and systemic embolism, there was no difference between NOACs. Meantime, there was an increased risk of major bleeding and intracranial bleeding among patients in use of rivaroxaban compared to those in use of dabigatran.
Direct comparison of apixaban, dabigatran and rivaroxaban showed a low risk of bleeding among patients using apixaban. However, there is still no independent confirmation in randomized clinical trials with head-to-head NOACs (Lip, 2016). Considerations for choosing NOACs should be made such as patient's age, stroke risk, bleeding risk, renal function, drug interactions, cost, besides adhesion and preference of patient.