ABSTRACT
Previously, we demonstrated that Hibiscus rosa-sinensis leaf contains various secondary metabolites including saponins, tannins and oxalates, which are known to possess documented pharmacological activities. In this present study, we investigated the effects of the leaf products on haematological indices, lipid profile and hepatic parameters using Wistar albino rat bioassay. Data suggested that though the haemoglobin counts, packed cell volume and red blood count were not significantly (P > 0.05) affected by the leaf products, lipid profile tests results showed that the blood total cholesterol (TC) and low-density lipoprotein (LDL) of rats increased on feeding with high fatty diet (HFD). Administration of the leaf products dose-dependently resulted in significant decreases (P < 0.05) in the TC and LDL levels while the high-density lipoprotein level was further increased. Liver function test (LFT) showed no evidence of hepatotoxicity on the administration of the products as assayed liver enzymes and proteins did not vary between HFD administered animals, treated with or without leaf products. Comparatively, dried leaf products had more potent biological activities than the extracted leaf products. These findings suggested that H. rosa-sinensis leaves possess pharmacological potentials for treatment of metabolic syndrome related disease conditions.
Key words: Liver function test, hematology, lipid profile, hepatic parameters, cholesterolemia, metabolic diseases.
Extended hyperlipidemia, including hypercholesterolemia, characterized by high circulating blood triglyceride (TG) and low HDL cholesterol levels, is a permissive factor interlinked with diverse metabolic syndrome, including atherosclerosis (Bhatnagar et al., 2008), hepatic dysfunctions (Semple et al., 2009), and diabetes mellitus (Dixit et al., 2014; Abbate and Brunzell, 1990). Between 2009 to 2012, of people aged 18 years or older with diagnosed diabetes in USA, 65% had serum low density lipoprotein cholesterol greater than or equal to 100 mg/dL or have used anti-hyperlipidemia medications (NDS, 2014). Of various causative factors of hyperlipidemia; sedentary life, poor dieting and genetic factors are the most prominent. In humans, continuous in-take of high amounts of fat ostensibly appears to contribute to hyperlipidemia.
In laboratory settings, hyperlipidemia induction is a well-known tool for investigation of the cholesterol metabolism-related disorders on one hand, as well as testing possible treatments for the reduction of circulating cholesterol levels on the other. Different models exist for hyperlipidemia induction in laboratory animals comparable to what obtains in human patients suffering from various metabolic disturbances (Li et al., 2010; Matos et al., 2005). Considering the prevalence of high cholesterol related metabolic disorders diseases world wide, effective and affordable treatments are urgently needed. In rural areas and as in most developing countries; reliance on natural sources for medications (traditional medicine) is common. Of these sources, plants (sometimes specific parts) are critical components. In therapeutic medicine, plants are also veritable sources of therapies as most of the currently available drugs are derived there off. Unlike primary metabolites which are central metabolites with intrinsic functions in plant growth, development and reproduction; secondary metabolites are not directly involved in those processes but play other critical roles in plant physiology and in its medicinal activity.
Saponin, for instance, is a widely known secondary metabolite of plant origin and has been reported to possess lytic actions against red blood cell membranes because of the affinity of saponin-aglycone moiety for membrane sterols, especially cholesterol, leading to formation of insoluble complexes (Glauert et al., 1962; Bang-ham and Horne, 1962); thus making saponins atypical cholesterol absorption inhibitor. Plant polyphenols are also known to prevent low-density lipoprotein oxidation and thrombocytes aggregation (Hayes et al., 2009; Singh et al., 2008), which are critical conditions that preclude atherosclerosis and coronary obstruction (Parthasarathy et al., 2010). Like saponins, fatty acids, vitamins, tannins and oxalates have also been implicated in several other biological mechanisms and therapeutic applications.
Hibiscus rosa-sinesis is an ornamental plant popularly grown in the tropics. Traditionally, it is a medicinal herb, with high anti-oxidant and vitamins contents, and documented potencies in decreasing chances of developing pyrexia, liver and cardiovascular disease (Bruneton, 1999; Chen, 2003; Imafidon and Okunrobo, 2009). The extracts are also known to block adipogenesis by suppression of the expression of adipogenic transcription factors (Kim et al., 2003). Similarly, other research groups have implicated extracts from diverse genera of Hibiscus plant as having potentials suitable for various metabolism-related therapeutic interventions (Hirunpanich et al., 2006; El-Sadaany et al., 1991; Lin et al., 2007; Hernández-Pérez and Herrera-Arellano., 2010; Hainida et al., 2008; Gurrola-Diaz et al., 2010; Alarcon-Aguilar et al., 2007; Mohd-Esa et al., 2010). Previously, we demonstrated that H. rosa-sinensis leaf products contain diverse secondary metabolites including saponins, fatty acids, vitamins, tannins and oxalates. Aware of the documented therapeutic potentials of these metabolites against metabolic dysfunctions, this current study undertook an experimental approach firstly to evaluate the effect of High fat diet feeding on lipid metabolism and liver function, and further examine the protective roles of H. rosa-sinensis leaf, using various forms of the leaf products, against damages caused by hyperlipidemia induction in rats.
Animal, treatment and biological assessment
Forty-four Wistar albino rats (both sexes) weighing 150 to 250 g purchased from the Animal house of the Department of Veterinary Pathology, The University of Nigeria, Nsukka were employed for this study. The animals were housed in metabolic cages and acclimatized for 7 days, then divided into eleven groups of four each, marked A1-D1, A2-D2, N, P and Q while been maintained with standard grower’s mash and tap water ad albitum prior to experimentation. They were maintained under normal room temperature with approximately normal 12:12 h dark/light cycle. The weight of each animal was taken pre-test samples administration. Within the first two weeks, rats in groups A1-D1, were fed with high fat diet HFD (composition of HFD is as described in supplementary material). They were treated two weeks later with 500 mg dose of the processed leaf samples while those in groups A2-D2 which were also fed with high fat diet and were treated two weeks later with 300 mg dose of the processed leaf sample. All doses were given to the animal once daily by oral administration. Group N was also fed with high fatty diet and later fed normal rat growers feed (No treatment). This was to investigate the effects of short-term HFD feeding; while group Q (Negative Control) was also fed with high fat diet throughout the period of the experiment. Neither changes in diet nor remedial treatment were administered.
Group P (Positive Control) was fed throughout the thirty days with normal growers rat ration (described in supplementary material). We adopted the Li et al. (2010) model to induce hyperlipidemia as our diet composition and other experimental conditions were similar. Effects of the processed leaf samples on haematology, lipid profile and liver function of the experimental animals were determined according to previous protocols of Burtis and Ashwood (1999). Animals were observed throughout the 30 days for clinical signs/behavioral changes and/or mortality symptoms before and after dosing.
Plant materials
Leaves of H. rosa-sinesis were procured, based on ethno-pharmacological information, from the Department of Animal Science, and identified in the Department of Plant Science and Biotechnology, The University of Nigeria, Nsukka; by a taxonomist, Cyprian Okafor. A portion of the leaves was deposited in the Departmental herbarium for reference.
Products preparation
Fresh leaves were harvested, washed with distilled water and drain-dried, then divided into nine portions (100 g each). The 1st portion was analyzed as raw leaf (RL) control. The 2nd portion was blended with water, filtered and the filtrate pasteurized at 70°C for 30 min hereafter referred to as raw leaf extract (RLE). The 3rd, 4th and 5th portions were blanched in hot water at 100°C for 2, 4 and 6 min respectively, after which they were blended with water, filtered and the filtrate pasteurized at 70°C for 30 min and respectively called 2 min blanched leaf extract (B2LE), 4 min blanched leaf extract (B4LE) and 6 min blanched leaf extract (B6LE). The 6th portion was dried at 50°C, milled and called raw dried leaf powder (RDLP). The 7th, 8th and 9th portions were blanched in hot water at 100°C for 2, 4 and 6 min respectively, dried at 50°C, milled into powder and called 2 min blanched dried leaf powder (B2DLP), 4 min blanched dried leaf powder (B4DLP) and 6 min blanched dried leaf powder (B6DLP) respectively.
Blood collection for total cholesterol/serum biochemical analysis
At the end of the study, all animals were fasted overnight prior to necropsy, sacrificed and their blood collected by jugular vein puncture. The remaining portion of the blood sample from the euthanized rats was dispensed into plain tubes and allowed to stand for 3 h. Clotted blood samples were centrifuged at 3 x 103 rpm for 10 min. Clear sera were aspirated and stored frozen (at -80°C) for serum biochemical analyses.
Determination of haematological Indices, lipid profile and hepatic parameters
Red and white Blood Cell Counts were determined as described (Schalm et al., 1975). Samples were diluted (1:200) with red blood cell diluting fluid, and loaded into a Neubauer counting chamber. Cells were counted using a light microscope at 40 and 10 magnifications. Packed cell volume (PCV) was determined as described by Coles (1986). A micro capillary tube was nearly filled with the blood sample and sealed at one end. It was centrifuged at 104 rpm for 5 min using a micro haematocrit centrifuge and read with haematocrit reader. Cyanomethaemoglobin method (Dacie and Lewis, 2001) was used for determination of the serum Haemoglobin concentration. Low- and High-density Lipoprotein were determined using the methods described by Assman et al. (1984) and Albers et al. (1978) respectively. Triglycerides were determined using glycerol-phosphate oxidase method (Jacobs and Van Demark, 1960). The serum glucose and cholesterol levels were determined spectrophotometrically after enzymatic oxidation (Sood, 2006). Burtis and Ashwood (1999) method was used for the determination of aspartase and alanine aminotransferase activities. To determine Alkaline Phosphatase, 8 ml of blood was collected from each animal by cardiac puncture, transferred into a centrifuge tube and allowed for 30 min to clot before centrifuging using Wisperfuge Model 1384 centrifuge (Tamson, Holland) for 5 min and the resulting supernatant used for the assessment of liver integrity. Total and conjugated bilirubins, and the alkaline phosphatase activity were assayed using p-nitrophenylphosphate as substrate in a phosphate buffered saline (pH 9.8) using the colorimetric method according to Ojiako and Nwanjo (2006). Total protein was estimated following the method of Lowry et al. (1951).
Ethic statement
All animal use and experimentation were approved by the institutional research committee of the University of Nigeria, Nsukka. Guiding Principles for the Care and Use of Laboratory Animals were strictly followed (NIH Publication No. 85-23, 1985), and with full adherence to the Helsinki Declaration.
Data and statistical analysis
All displayed data are mean of independent triplicate experimental results obtained and statistically analyzed using the analysis of variance (ANOVA) in a completely randomized design (CRD). Differences among means were determined with the least significant difference (LSD) at P < 0.05 (Steele and Torrie, 1980).
Effects of H. rosa-sinensis leaf products on haematological indices of rats
No significant (P > 0.05) difference was observed in both the packed cell volume count and Red Blood Count of the experimental animals both in the period of inducing hyperlipidemia, before and after feeding them with the products (Table 1), suggesting that neither the HFD nor the treatment altered PCV and RBC of the experimented animal. Similarly, after hypercholesterolemia induction (feeding with high fatty diet–group Q), WBC count was found not to be significantly (P > 0.05) different from non-dyslipidemic rats (P) nor was it significantly different from WBC count of those in which cholesterol was induced but later fed normal commercial rat diet (N). However, animals exhibited significant variation in WBC counts post-products administration. Feeding the rats with the two doses of the leaf products (A1 and A2, B1 and B2, C1 and C2, D1 and D2) resulted to lower WBC counts when compared to rats fed diets not containing the leaf products (P, Q and N) as shown in Table 1. A possible explanation could be that the leaf products inhibited WBC production or up-regulated its export from the blood. The normal WBC level in the rats where within the normal range 6 to 18 (×103 ul) according to previous studies. Among the leaf products, the dried leaf products resulted to higher WBC counts compared to their corresponding extracted leaf products.

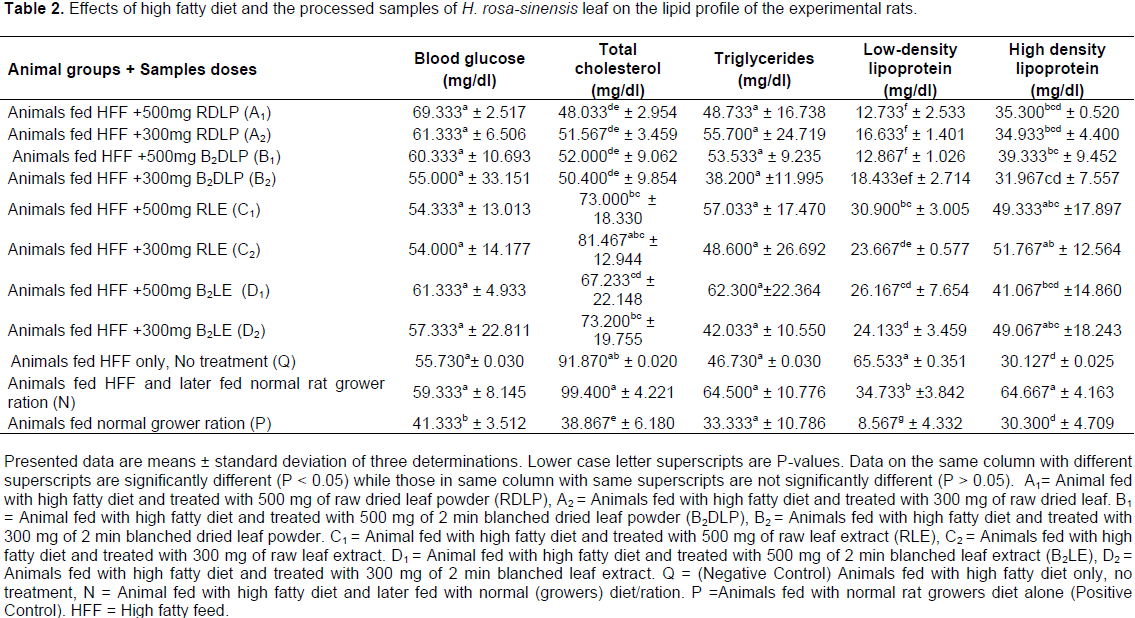
Effect of H. rosa-sinensis leaf products on lipid panel of rats
Blood glucose levels (Table 2) of rats fed normal rat diet (P) was, as expected, significantly (P < 0.05) lower than the blood glucose levels in the rest of the rats. When rats were fed high fat diet without any other treatment, the blood glucose rose significantly (P < 0.05) higher than rats fed normal diet, though no significant (P > 0.05) difference was observed compared to the blood glucose levels of other rats. Interestingly, glycemic levels increased further in high fat diet fed rats fed with normal rat diet. These results suggest that high fat feeds induced a rise in both cholesterol and blood glucose concentrations. Inclusion of the dried leaf products to diet resulted to slightly higher blood glucose compared to corresponding extracts. The differences can be attributed to higher carbohydrate content of the dried leaf products compared to the extracted leaf products, which were between 73 to 79% and 13 to 16% of the products respectively. Cholesterol levels in the animals fed normal ration were low but were increased by high fat diet feeding.
This shows that the high fat diet was effective in inducing hypercholesterolemia in the rats. Remarkably; the products, especially the dried products, significantly (P < 0.05) reduced the cholesterol level in all treatments. However, blanching effects were variable. While in the dried leaf products (A1, A2, B1 and B2), the blanched leaf products (B1 and B2) were slightly less effective in lowering the blood cholesterol compared to the un-blanched products (A1 and A2); but in the extracted leaf products (C1, C2, D1 and D2), the blanched products (D1 and D2) were slightly more effective in lowering blood cholesterol compared to un-blanched products (C1 and C2). Similarly, data of the triglyceride concentration (Table 2) were variable. Low Density Lipoprotein (LDL) and triglyceride levels increased dramatically on feeding with high fat feed. The leaf products slightly reduced the blood triglycerides and LDL in all treatments. When the rats were fed high fat diet, the high-density lipoprotein decreased slightly. Treatment of the rats with normal rat ration after feeding with high fat diet caused an obvious increase in their high density lipoprotein level which also was found to be significantly (P < 0.05) higher than the high density lipoprotein level found in the other animals.
Effect of H. rosa-sinensis leaf products on liver function tests
Generally, blood albumin generally increased (P < 0.05) significantly on administration of the leaf products compared to unfed control, suggesting that the leaf products stimulated the synthesis of albumin. With respect to this present study, comparing the total bilirubin value of animals in group P with those fed with leaf products (A1, A2, B1, B2, C1, C2, D1 and D2), it can be observed that their total bilirubin values were slightly lower than the range stated above and higher than the normal range for rats (0.2 to 0.5 g/dl). The effects of treatment of the rats with the leaf products showed that the rats treated with dried leaf products generally had lower AST than rats treated with corresponding leaf extracts. This may tend to suggest that the aqueous leaf extracts were more hepatotoxic than the dried leaf products. Blanching resulted to higher AST in the rats compared to the corresponding un-blanched counterparts, suggesting that blanching of the leaf products may be more damaging to the liver. Also, it appears that a threshold exists beyond which damage could be more pronounced in the liver. The leaf products lowered the ALT contents significantly (P < 0.05) when compared to rats fed normal rat ration (P) or those induced with cholesterol in a non-dose dependent manner. Again, we find that the dried leaf products gave lower ALP values compared to the un-dried products. This suggests that the dried leaf products may be more effective in preventing liver damage than the leaf extracts (Table 3).

High serum LDL and/or Low serum HDL concentration occasioned by increase in oxidative stress (which can be altered by poor nutrition) are known risk factors of atherosclerosis and other metabolic disorders (Chander et al., 2003; Bhatnagar et al,. 2008). Similarly, rise in blood cholesterol concentration is also a precursor for ischemic heart disease and other cardiovascular disorders (Aparna, 2003; Bhatnagar et al., 2008). The pharmacological and biological roles of the secondary metabolites of H. rosa-sinensis as hypoglycaemic and hypolipidemic agents are well established. The data presented herein are also consistent with the hypocholesterolemic activity previously reported for other plant products. In this study, we employed leaf products with assayed secondary metabolites comprising of saponin (0.06 to 0.19%), tannin (0.05 to 0.2%) and oxalate (0.14 to 0.92%) dry weight ranges. We demonstrated that oral administration of HDF to Wistar rats resulted to surge in blood total cholesterol and LDL as reported previously (Li et al., 2010; Matos et al., 2005). While total glucose (TC) also increased rather slightly, assayed hematological parameters were largely un-affected. Leaf products treatment resulted to increasing concentrations of HDL suggesting that the leaf products possess therapeutic potentials against low HDL engendering disease conditions. Both high fatty diet and the leaf products given to animals at both high and low doses did not reduce its albumin level as obtained data fell within the range of 3 g/dl as reported previously (Thapa and Walia, 2007) and also within the normal albumin level of rats (3.8 to 4.8 g/dl).
Albumin level below 3 g/dl (usually found in hepatitis condition) is a prognostic factor in chronic liver disease caused by decreased albumin synthesis. Neither the high fat diet nor products treatment affected bilirubin concentration. Improper functioning of liver coupled with serum bilirubin levels more than 17 µmol/L are underlying markers of liver disease, while normal total bilirubin level is usually between 0.2 to 0.9 g/dl (2 to 15 µmol/L) in rats.
Furthermore, slight reduction in bilirubin concentration is suggestive that the leaf products have potentials to impart processes leading to haemoglobin breakdown in the experimental rats. Bilirubin is a yellow-orange pigmented molecule and primarily by-product of heme (a component of haemoglobin) degradation. Reduced oxidative stress (due to LDL protection from oxidation) is a promising strategy towards therapeutic interventions against atherosclerosis related cardiovascular problems of coronary heart disease, stroke, peripheral arterial disease and aortic disease (Hayes et al., 2009; Singh et al., 2008; Aviram et al, 2000; Retsky et al, 1993).
The products also seem to significantly lower normal plasma alanine aminotransferase, a key catalyst of the alanine cycle compared to both untreated hyperlipidemic and normal control rats. In conclusion, our findings underscored the fact that sub-chronic exposure of rats to high fat diet, using lard as a source of fat, could induce metabolism conditions, typical of hyperlipidemia, and that the H. rosa-sinensis leaf products administration in the hyperlipidemic rats have promising potentials to improve these conditions.
The authors have not declared any conflict of interests.
Our sincere gratitude to Dr. I. E. Nwaoha for her contributions towards the success of this work.
REFERENCES
|
Abbate SL, Brunzell JD (1990). Pathophysiology of hyperlipidemia in diabetes mellitus. J. Cardiovas. Pharmacol. 16(sup9):S1-7.
|
|
|
|
Alarcon-Aguilar FJ, Zamilpa A, Perez-Garcia MD, Almanza-Perez JC, Romero-Nunez E, Campos-Sepulveda EA, Vazquez-Carrillo LI, Roman-Ramos R (2007). Effect of Hibiscus sabdariffa on obesity in MSG mice. J. Ethnopharmacol. 114 (1): 66-71
Crossref
|
|
|
|
|
Albers JJ, Warnick GR, Cheung MC (1978). Quantification of high-density lipoproteins. Lipids 13:926-932.
Crossref
|
|
|
|
|
Aparna BR (2003). Risk of coronary artery heart disease. Health Screen 1:28-29.
|
|
|
|
|
Assman G, Jab HU, Hohnert U (1984). LDL-Chotesterol determination in blood following precipitation of LDL with Polyvinyl sulfide. J. Clin. Chem. Acta. 140:77- 83.
Crossref
|
|
|
|
|
Aviram M, Dornfeld L, Rosenblat M, Volkova, Kaplan M, Coleman R, Hayek T, Presser D, Fuhrman (2000). Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E–deficient mice. Am. J. Clin. Nutr. 71:1062-1076
|
|
|
|
|
Bangham AD, Horne RW (1962). Action of saponins on biological cell membranes. Nature 196:952-953.
Crossref
|
|
|
|
|
Bhatnagar D, Soran H, Durrington PN (2008). "Hypercholesterolaemia and its management". BMJ 337:993.
Crossref
|
|
|
|
|
Bruneton J (1999). Pharmacognosy phytochemistry medicinal plants, 2nd ed. London: Intercept 24.
|
|
|
|
|
Burtis CA, Ashwood ER (1999). Tietz Textbook of Clinical Chemistry, (3rd ed.); Harcourt Brace and Company PTE Ltd; Forum Singapore.
|
|
|
|
|
Chander R, Kapoorn K, Singh C (2003). Lipid per oxidation of hyperlipidemic rat serum in chronic ethanol and acetaldehyde administration. J. Biosci. 13:289-274.
|
|
|
|
|
Chen CC, Hsu JD, Wang SF, Chiang HC, Yang MY, Kao ES, Ho YC, Wang CJ (2003). Hibiscus sabdariffa Extract Inhibits the Development of Atherosclerosis in Cholesterol-Fed Rabbits. J. Agric. Food Chem. 51:5472-5477.
Crossref
|
|
|
|
|
Coles EH (1986). Determination of packed cell volume. In: Coles EHH. Eds. Veterinary clinical pathology. W.B. Saunders Co. Philadelphia. pp. 17-19.
|
|
|
|
|
Dacie JV, Lewis SM (2001). Practical Haematology. 10th ed, Churchhill Livingstone Edinburg, London.
|
|
|
|
|
Dixit AK, Dey R, Suresh A, Chaudhuri S, Panda AK, Mitra A, Hazra J (2014). The prevalence of dyslipidemia in patients with diabetes mellitus of ayurveda Hospital. J. Diabetes Metab. Disord. 13:58.
Crossref
|
|
|
|
|
El-Saadany SS, Sitohy MZ, Labib SM, El-Massry RA (1991). Biochemical dynamics and hypocholesterolemic action of Hibiscus sabdariffa (Karkade). Nahrung 35(6):567-576.
Crossref
|
|
|
|
|
Glauert AM, Dingle JT, Lucy JA (1962). Action of saponin on biological membranes. Nature. 196:953-955
Crossref
|
|
|
|
|
Hainida E, Ismail A, Hashim N, Mohd-Esa N, Zakiah A (2008). Effects of defatted dried roselle (Hibiscus sabdariffa L.) seed powder on lipid profiles of hypercholesterolemia rats. J. Sci. Food Agric. 88(6):1043-1050.
Crossref
|
|
|
|
|
Hayes JE, Stepanyan V, Allen P, O'Grady MN, O'Brien NM, Kerry JP (2009). The effect of lutein, sesamol, ellagic acid and olive leaf extract on lipid oxidation and oxymyoglobin oxidation in bovine and porcine muscle model systems. Meat Sci. 83:201-208.
Crossref
|
|
|
|
|
Hernández-Pérez F, Herrera-Arellano A (2010). Therapeutic use Hibiscus sabadariffa extract in the treatment of hypercholesterolemia. A randomized clinical trial. Rev. Med. Inst. Mex. Seguro. Soc. 49(5):469-80.
|
|
|
|
|
Hirunpanich V, Utaipat A, Morales NP, Bunyapraphatsara N, Sato H, Herunsale A, Suthisisang (2006). Hypocholesterolemic and antioxidant effects of aqueous extracts from the dried calyx of Hibiscus sabdariffa L. in hypercholesterolemic rats. J. Ethnopharmacol. 103:252-260
Crossref
|
|
|
|
|
Imafidon EK, Okunrobo OL (2009). The effects of aqueous extracts of the leaves of Hibiscus rosa-sinensis Linn. on renal function in hypertensive rats. Afr. J. Biochem. Res. 4(2):43-46.
|
|
|
|
|
Jacobs NJ, Van Denmark PG (1960). Lipids. Arch J. Biochem. Biophys. 88:250-255.
Crossref
|
|
|
|
|
Kim M, Kim J, Kim H, Moon S, Shin B, Park K, Yang H, Kim S, Park R (2003). Hibiscus Extract Inhibits the Lipid Droplet Accumulation and Adipogenic Transcription Factors Expression of 3T3-L1 Preadipocytes. J. Altern Complement. Med. 9(4):499-504.
Crossref
|
|
|
|
|
Li W, Wang D, Song G, Zuo C, Qiao X, Qin S (2010). Effect of Combination Therapy of Allicin and Fenofibrate on High Fat Diet Induced Vascular Endothelium Dysfunction and Liver damage in rats. BioMed. Central J. Chin. 9(131):2-7.
|
|
|
|
|
Lin TL, Lin HH, Chen CC, Lin MC, Chou MC, Wang CJ (2007). Hibiscus sabdariffa extract reduces serum cholesterol in men and women. Nutr. Res. 27:140-145
Crossref
|
|
|
|
|
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951). Protein measurement with the Polin phenol reagent. J. Biol. Chem. 193:265-267.
|
|
|
|
|
Matos SL, Paula HD, Pedrosa ML, Santos RCD, Oliveira ELD, Júnior DAC, Silva ME (2005). Dietary Models for Inducing Hypercholesterolemia in Rats. Br. Arch. Biol Technol. 48(2):203-209
Crossref
|
|
|
|
|
Mohd-Esa N, Hern FS, Ismail A, Yee CL (2010). Antioxidant activity in different parts of roselle (Hibiscus sabdariffa L.) extracts and potential exploitation of the seeds. Food Chem. 122:1055-1060.
Crossref
|
|
|
|
|
NDS (National Diabetes Statistics) (2014). Statistics about Diabetes. Available at:
View
|
|
|
|
|
Ojiako OA, Nwanjo HU (2006). Is Vernonia amygdalina hepatotoxic or hepatoprotective? Response from biochemical and toxicity studies in rats. Afr. J. Biotechnol. 5(18):1648-1651.
|
|
|
|
|
Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N (2010). Oxidized Low-Density Lipoprotein. Methods Mol. Biol. 610:403-417.
Crossref
|
|
|
|
|
Retsky KL, Freeman MW, Frei B (1993). Ascorbic Acid Oxidation Product(s) Protect Human LowDensity Lipoprotein against Atherogenic Modification; Anti- Rather than Prooxidant Activity of Vitamin C in the Presence of Transition Metal Ions. J. Biol. Chem. 268(2):1304-1309.
|
|
|
|
|
Schalm OW, Jain NC, Caroll EJ (1975). Veterinary haematology, 3rd ed., Lea & Febiger, Philadelphia, pp. 19-25.
|
|
|
|
|
Semple RK, Sleigh A, Murgatroyd RP, Adams CA, Bluck L, Jackson S, Vottero A, Kanabar D, Charlton-Menys V, Durrington P, Soos MA, Carpenter TA, Lomas DJ, Cochran EK, Gorden P, O'Rahilly S, Savage DB (2009). Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J. Clin. Investig. 119:315-322.
Crossref
|
|
|
|
|
Singh I, Mok M, Christensen AM, Turner AH, Hawleyl JA (2008). The effects of polyphenols in olive leaves on platelet function. Nutr. Metab. Cardiovasc. Dis. 18:127-132.
Crossref
|
|
|
|
|
Sood R (2006). Text Book of Medical Laboratory. Jaypee Brothers Medical Publisher Ltd, New Delhi. 1286p.
Crossref
|
|
|
|
|
Steele RG, Torrie JH (1980). Principles and Procedures of Statistics (2nd ed.). McGraw-Hill, New York. 672p.
|
|
|
|
|
Thapa BR, Walia A (2007). Liver function tests and their interpretation. Indian J. Pediatr. 74(7):663-671.
Crossref
|
|