ABSTRACT
This study evaluated the toxic changes that may accompany treatment of diabetes with Phragmanthera incana, a mistletoe species growing on two plant hosts [Cola nitida (Kolanut; PICN) and Theobroma cacao (Cocoa; PITC)]. The toxic potential of this treatment regimen was evaluated using the effect of the extracts PICN and PITC on the haematology and serum chemistry of the diabetic rats. Alloxan-induced diabetic rats were treated with the extracts at doses of 200, 400 or 800 mg/kg or glibenclamide for 14 days. Blood samples were collected on day 15 for haematology and serum biochemistry. Haematological parameters analyzed were packed cell volume, haemoglobin, red blood cells, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), white blood cell count, platelet count, lymphocytes, neutrophil, monocytes and eosinophil. Serum biochemical parameters analyzed were total protein, albumin, globulin, albumin-globulin ratio, aspartate transaminase, alanine transaminase, alanine phosphatase, blood urea nitrogen, creatinine, cholesterol, bilirubin and glucose. The results showed that P. incana extracts, regardless of the host plant decreased blood glucose and cholesterol levels. Although it depressed packed cell volume (PCV), it also alleviated other complications of diabetes such as liver and kidney injury, and may possess hepatoprotective effect.
Key words: Phragmanthera incana, Cola nitida, Theobroma cacao, diabetes, haematology, serum biochemistry.
Mistletoe, commonly known as bird lime, all heal, devil’s fuge, Iscador is a general term for woody shoot parasites in several plant families, especially in Loranthaceae and Viscaceae families (Polhill and Wiens, 1998; Watson, 2001; Judd et al., 2002). Mistletoe is especially interesting botanically because it is a hemiparasite (Adesina et al., 2013). Mistletoe is also capable of growing on its own like other plants as it can produce its own food by photosynthesis (Williams, 1990; Hoagy, 2008). However, it is more commonly found growing as a parasitic plant (Runyon et al., 2009). Mistletoe is used mainly in Europe as an adjuvant therapy with other drugs and or radiation for treatment of cancer (Maier and Fiebig, 2002; Elluru et al., 2009). While American mistletoe is toxic, European mistletoe is considered to have medicinal properties till today. Mistletoe extracts represent the most unorthodox oncology therapy in Germany (Bock et al., 2004; Mengs et al., 2005).
In Nigeria, the Hausa and Fulani tribes of Northern Nigeria use mistletoe in the treatment of cancers and inflammations (Abubakar et al., 2007). Mistletoe has been used in medicine to prove much of its older frame as “all healer”. In addition to its use for treatment of cancers and as an immune booster, the white-berried mistletoe (Viscum album) has also been documented as a traditional treatment for diabetes and high blood pressure (Orhan et al., 2005). The African mistletoe, Loranthus bengwensis L. (Loranthaceae), has been widely used in Nigeria folk medicine to treat diabetes mellitus (Ibatomi et al., 1994). A recent study on another Nigerian mistletoe Phragmanthera incana (Schum.) Balle from the family Loranthaceae showed it has potent antidiabetic effect (Ogunmefun et al., 2016). Prior to the introduction of insulin in 1922, the treatment of diabetes mellitus relied heavily on dietary measures which included the use of traditional plant therapies.
People in many countries still depend on medicinal plants for the management of diabetes mellitus especially in developing countries where western medical resources are meager (Bnouham et al., 2006). A number of medicinal or culinary herbs have been reported to yield hypoglycaemic effects on diabetic conditions. These include bitter melon, Momordica charantia (Srivastava et al., 1993; Raman and Lau, 1996); onions and garlic, Allium cepa, A. sativum (Koch and Lawson, 1996) and holy basil, Ocimum sanctum (Rai et al., 1997). Some other common botanicals demonstrating in vivo hypoglycaemic activities in animals include juniper berries (Sanchez de Medina et al., 1994) and alfalfa (Gray and Flatt, 1997).
Marles and Farnsworth (1994) however cautioned that one- to two-thirds of the 1123 plants that affect blood glucose may be dangerous and many of their constituents are hypoglycaemic due to metabolic or hepatic toxicity. Initial toxicological evaluation of the extract of P. incana on Wistar rats showed it was safe (Ogunmefun et al., 2013). The caution documented by Marles and Farnsworth (1994) and more recently other researchers such as Eddouks et al. (2002) and Hilmi et al. (2013) informed the study on evaluation of the toxic potentials of P. indica using the effect of the methanol extract on haematology and biochemistry of alloxan- induced diabetic Wistar rats administered with the extract. P. incana growing on kolanut (Cola nitida) and cocoa (Theobroma cacao) were investigated in the study.
Plant sample collection
P. incana (Schum.) Balle, mistletoe growing on Cocoa (T. cacao) and Kolanut (C. nitida) was collected at Alesan Obolode, Owo, Ondo State, Nigeria. Identification and authentication was done at the Forestry Research Institute of Nigeria (FRIN) herbarium. A voucher specimen of P. incana with Forestry Herbarium Index (FHI) 108925 was submitted at the Botany Department herbarium of the University of Ibadan, Nigeria with University of Ibadan herbarium (UIH) number 22332.
Methanol extract preparation
The samples were washed under running water, air dried after which the dried samples were ground to powder and kept dry in an air-tight container. Cold extraction method with methanol for 72 h at room temperature was used (Ogbole et al., 2013). 500 g of powdered mistletoe samples harvested from Cocoa and Kolanut were extracted separately with one litre of methanol each after which concentration of the filtrates were done using rotary evaporator and the extracts were further concentrated on water bath at a low temperature of 40°C to remove all solvents.
Experimental animals
Wistar rats were obtained from and housed at the Experimental Animal House of the Department of Veterinary Physiology, Biochemistry and Pharmacology, University of Ibadan, Ibadan, Nigeria. The animals were fed with commercial pelletized rat ration and portable water ad libitum. The animals were handled humanely in compliance with the Faculty of Veterinary Medicine, University of Ibadan, Ibadan, Nigeria guidelines for the use of laboratory animals.
Induction of diabetes
Blood glucose of rats of average weights 150 g was determined using an AccuChek® active glucometer and only normoglycemic rats were included in the study. Diabetes was induced by intraperitoneal administration of alloxan monohydrate (100 mg/kg). Blood glucose levels were monitored and rats with blood glucose levels of ≥150 mg/dl 48 h after administration of alloxan were included in the groups. Diabetic rats were randomly and equally divided into 8 groups of five rats each. A ninth group of normoglycemic rats were included in the study as non-diabetic untreated control group.
Management of diabetes
Rats in groups 1 to 3 were administered with the extract of P. incana harvested from C. nitida (PICN) at doses of 200, 400 or 800 mg/kg, rats in groups 4 to 6 were administered with the extract of P. incana harvested from T. cacao (PITC) at the same dose rate as above. Group 7 rats were the positive control group and were administered with glibenclamide, a sulfonylurea antidiabetic drug at the dose of 0.07 mg/kg. The rats in group 8 were diabetic but untreated serving as negative controls for the study, while group 9 rats were non-diabetic (normoglycemic) and untreated throughout the course of the study. All treatment groups were administered with the extract or drug for 14 days.
Sample collection and analysis
Blood samples were obtained from the retro-orbital sinus on day 15 to determine haematological and biochemical parameters. Haematological parameters were determined using the method of Jain (1986). The haematological parameters determined were packed cell volume (PCV), red blood cell count (RBC) and other red cell indices such as haemoglobin concentration, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC), white blood cell count (WBC) and its differentials; neutrophils and lymphocytes and platelet count. Biochemical parameters determined include total protein (TP) using the method of Weichselbaum (1946), albumin (Alb) using the method of Doumas et al. (1971), globulin (Glob), albumin-globulin ratio (Alb/Glob), aspartate transaminase (AST) and alanine transaminase (ALT) followed the method described by Reitman and Frankel (1957) following the principle according to Schmidt and Schmidt (1963), alanine phosphatase (ALP) using the assay method first described by King and Armstrong (1934), modified by Ohmori (1937) and later improved by Hausamen et al. (1967), blood urea nitrogen (BUN) using the method of Weatherburn, 1967, creatinine (Crt) assay was based on the principle according to Henry (1974), cholesterol (Chol) was determined according to the procedure of Fiedewald et al. (1972), bilirubin (Bil) using the procedure described by Jendrassik and Grof (1938) and glucose using method of analysis by Trinder (1969).
Statistical analysis
The mean and the standard error of mean (Mean ±SEM) were used in the analysis of the data from this study. The mean ±SEM of five replicates were subjected to DUNCAN multiple range test where the effects of the extracts were compared to those of the three types of control, that is, the control (normoglycemic non-diabetic rats), glibenclamide and the diabetic untreated groups. P<0.05 is considered significant for the parameters examined.
Haematology
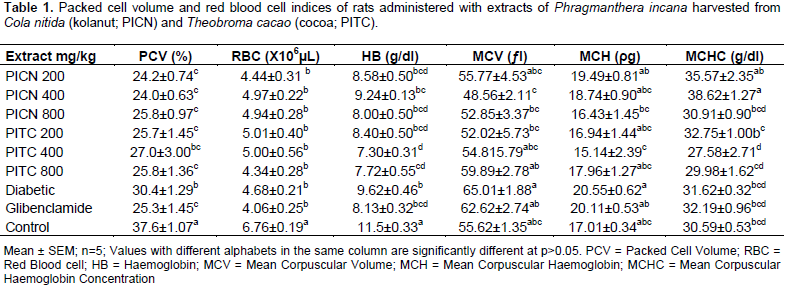
Packed cell volume (PCV)
There was a significant (P<0.05) decrease in the PCV of all diabetic rats compared to the normoglycemic control (37.6±1.07%), with the most marked decrease observed in rats administered with extract of P. incana harvested from C. nitida (PICN) at the dose of 400 mg/kg (24.0±0.63%). The diabetic untreated rats (30.4±1.29%) had the least decrease observed. No significant (P>0.05) difference was observed between rats administered with glibenclamide (25.3±1.45 %) and the other treatment groups (Table 1).
Red blood cells (RBC)
Red blood cell counts (RBC) of all diabetic rats were significantly (P<0.05) decreased compared to the control rats (6.76±0.19×106 µL), with the most significant decrease observed in rats administered with glibenclamide (4.06±0.25×106 µL). For rats administered with the extracts, rats administered with the extract P. incana harvested from T. cacao (PITC) at the dose of 800 mg/kg (4.34±0.28 ×106 µL) had the least RBC. There was no significant (P>0.05) difference in the mean red blood cells of all diabetic treated rats compared to the diabetic untreated rats (4.68±0.21×106 µL) (Table 1).
Haemoglobin (HB)
A significant decrease in haemoglobin concentration (Hb) of diabetic rats was observed when compared to the normoglycemic rats (11.5±0.33 g/dl). Hb of all treatment groups were decreased compared to diabetic but untreated rats (9.62±0.46 g/dl), with significant decreases observed in rats administered with PITC at 400 and 800 mg/kg (7.30±0.31 and 7.72±0.55 g/dl) (Table 1).
Mean corpuscular volume (MCV)
MCV of all rats administered with the extracts were non-significantly (p>0.05) decreased compared to the normoglycemic control rats (55.62±1.35ƒl), except in rats administered with PITC at a dose of 800 mg/kg (59.89±2.78 ƒl). Rats administered with glibenclamide (62.62±2.74 ƒl) and the diabetic but untreated rats (65.01±1.88 ƒl) had increased MCV values (Table 1).
Mean corpuscular haemoglobin (MCH)
The same pattern as with MCV was observed for MCH. MCH of the normoglycemic rats (17.01±0.34 ρg) was higher than that of all rats administered with the extract except for rats administered with PITC at 800 mg/kg (17.96±1.27 ρg). MCH of normoglycemic rats were lower than that of rats administered with glibenclamide (20.11±0.53 ρg) and diabetic untreated rats (20.55±0.62 ρg) (Table 1).
Mean corpuscular haemoglobin concentration (MCHC)
The mean MCHC of rats administered with PICN and 200 mg/kg of PITC were higher than that of normoglycemic rats (30.59±0.53 g/dl) and diabetic untreated rats (31.62±0.32 g/dl). Rats treated with glibenclamide (32.19±0.96 g/dl) also showed a non-signifcantly increased MCHC levels compared to the normoglycemic rats (Table 1).
White blood cells (WBC)
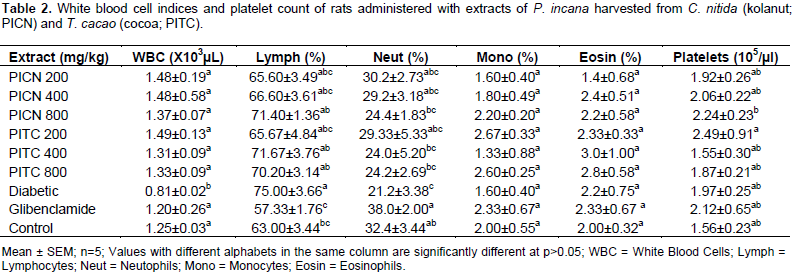
There was a non-significant increase in the mean WBC of rats administered with PICN and PITC compared to the normoglycemic control rats (1.25±0.03×103 µL). Rats administered with glibenclamide (1.20±0.26×103 µL) showed a mild decline in the WBC, but diabetic untreated rats (0.81±0.02×103 µL) showed a significant decrease in WBC compared to all other groups (Table 2).
Lymphocytes
There was a non-significant (P>0.05) increase in the lymphocytes of rats administered the extracts of PICN and PITC compared to the normoglycemic rats (63.00±3.44%). Diabetic untreated rats (75.00±3.66%) however, showed a significant (P<0.05) increase compared to the normoglycemic rats, while rats administered with glibenclamide had a lower percentage of lymphocytes (57.33±1.76%) (Table 2).
Neutrophils
The reverse of our observation for lymphocytes was seen for the neutrophils with rats administered with glibenclamide (38.0±2.00%) having higher neutrophil count compared to all other groups. Diabetic untreated rats (21.2±3.38%) significantly (P<0.05) had the least neutrophil count. Normoglycemic rats had 32.4±3.44% of WBC as neutrophils and this was non-significantly (P>0.05) higher than that observed in PITC at 400 mg/kg which had the least neutrophil count of 24.0±5.20% (Table 2).
Monocytes and eosinophil
There was no significant (P>0.05) difference in the mean monocytes and eosinophil of all extract treated groups compared to that of diabetic untreated, glibenclamide and normoglycemic control groups (Table 2).
Platelets
Platelet counts of all groups non-significantly (P>0.05) increased compared to the normoglycemic rats (1.56±0.23×105/µl), except for rats administered with PITC at 400 mg/kg (1.55±0.30×105/µl) which was slightly decreased (Table 2).
Serum biochemistry
Total protein (TP)
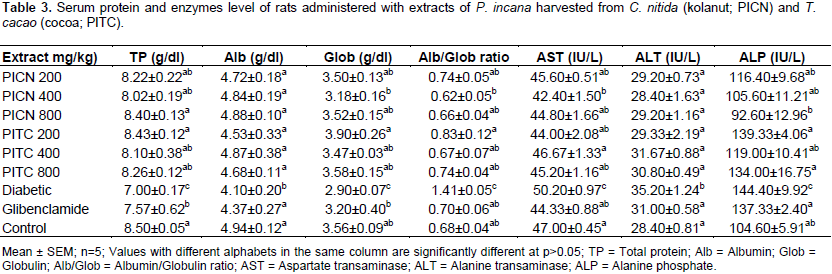
The mean total protein was non-significantly (P>0.05) lowered in all rats administered with the extracts, but there was a significant (P<0.05) decrease in the diabetic untreated rats (7.00±0.17 g/dl) compared to the normoglycemic control rats (8.50±0.05 g/dl). Rats administered with glibenclamide (7.57±0.62 g/dl) also showed a significant (P<0.05) decrease in their total protein compared to the normoglycemic rats (Table 3).
Albumin (ALB)
The mean albumin was non-significantly (P>0.05) lowered
in rats administered with PICN or PITC extract, or even glibenclamide (4.37±0.27 g/dl) compared to the normoglycemic control rats (4.94±0.12 g/dl). Diabetic untreated rats (4.10±0.20 g/dl) had the most marked decline in total protein levels compared to the normoglycemic rats (Table 3).
Globulin (GLB)
The same trend observed for albumin levels was also seen in the globulin levels, except for a non-significant (P>0.05) increase in globulin levels of rats administered with PITC at a dose of 200 mg/kg (3.90±0.26 g/dl) which was increased compared to that of normoglycemic rats (3.56±0.09 g/dl) (Table 3).
Albumin-Globulin ratio (Alb/Glob ratio)
The mean Alb/Glob ratio of rats administered with the extracts (0.62±0.05 - 0.83±0.12) or glibenclamide (0.70±0.06) was non-significantly different compared to normoglycemic control (0.68±0.04).
However, the mean Alb/Glob ratio for diabetic but untreated rats (1.41±0.05) was significantly (P<0.05) lower than all treated and normoglycemic control rats (Table 3).
Aspartate transaminase (AST)
The mean AST value for all rats administered with PICN, PITC or glibenclamide were non-significantly decreased compared to the normoglycemic controls (47.00±0.45 IU/L) except for rats administered with PICN at a dose of 400 mg/kg (42.40±1.50 IU/L), which decreased significantly(P<0.05). On the converse, diabetic untreated rats (50.20±0.97 IU/L) had a significant (P<0.05) increase in AST levels compared to all treatment groups and the normoglycemic group of rats (Table 3).
Alanine transaminase (ALT)
There was no significant (P>0.05) difference in the mean ALT values for all groups except diabetic untreated rats (35.20±1.24 IU/L) which was significantly (p<0.05) higher compared to normoglycemic rats (28.40±0.81 IU/L). ALT level of diabetic untreated group was also significantly (p<0.05) higher than those of all treated groups (Table 3).
Alanine phosphate (ALP)
Rats administered with PICN extract showed a non-significant (P>0.05) change in their ALP levels compared to the normoglycemic rats (104.60±5.91 IU/L), while rats administered with PITC extract showed a significant increase in ALP levels (119.00±10.41 - 139.33±4.06 IU/L). Rats administered with glibenclamide (137.33±2.40 IU/L) and diabetic untreated rats (144.40±9.92 IU/L) also had significantly (P<0.05) increased ALP levels compared to the normoglycemic rats (Table 3).
Blood urea nitrogen (BUN) and creatinine (Crt)
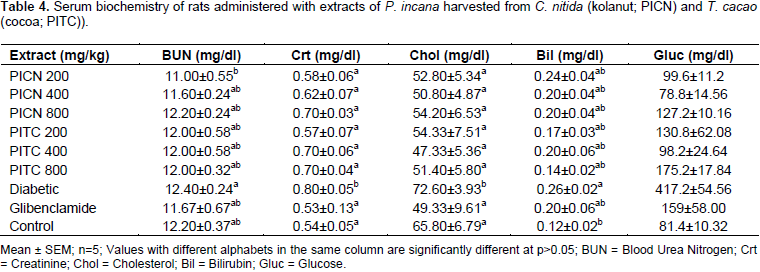
There was no significant (P>0.05) difference between the BUN and creatinine levels of treated rats (extract or glibenclamide) and the normoglycemic rats. However, diabetic untreated rats had significantly increased BUN (12.40±0.24 mg/dl) and creatinine levels (0.80±0.05 mg/dl) compared to normoglycemic controls (12.20±0.37
and 0.54±0.05 mg/dl) (Table 4).
Cholesterol (Chol) and bilirubin (Bil)
Mean cholesterol levels of PICN (50.80±4.87-54.20±6.53 mg/dl), PITC (47.33±5.36 - 54.33±7.51 mg/dl) or glibenclamide (49.33±9.61 mg/dl) treated rats significantly (P>0.05) decreased compared to the diabetic untreated rats (72.60±3.93 mg/dl), but the decline was not significant in comparison to normoglycemic rats (65.80±6.79 mg/dl) (Table 4). Mean bilirubin levels of all treated rats were non-significantly (P>0.05) increased compared to the normoglycemic rats (0.12±0.02 mg/dl), but the diabetic untreated rats (0.26±0.02 mg/dl) had a significantly increased bilirubin level (Table 4).
Glucose level
Rats administered with PICN and PITC extracts showed significantly (P<0.05) lower blood glucose levels compared to the diabetic control (417.2±54.56 mg/kg) and glibenclamide treated rats (159±58.00 mg/dl), but comparable to that observed in the normoglycemic control rats (81.4±10.32 mg/dl) especially at 400 mg/kg (78.8±14.56 and 98.2±24.64 mg/kg) (Table 4).
Findings from this study showed that treatment of diabetes mellitus with extracts of P. incana which is traditionally practiced in South West Nigeria is a safe practice. Judging by the effect of this hemi-parasitic plant on the blood picture of diabetic rats, it was observed that packed cell volume of the diabetic rats decreased which is a typical symptom of diabetes mellitus. Oyedemi et al. (2011) reported that the occurrence of anaemia in diabetes is due to the increased non – enzymatic glycosylation of red blood cell (RBC) membrane proteins. Also, the oxidation of proteins and hyperglycaemia in diabetes mellitus causes an increase in the production of lipid peroxides that lead to haemolysis of RBC (Arun and Ramash, 2002). Treatment of diabetic rats with extract of P. incana (PICN and PITC) improved the RBC count, which glibenclamide a known anti-diabetic agent was unable to reverse, but a further decline in RBC was observed.
An earlier report by Ogunmefun et al. (2013) noted that P. incana does not cause anaemia, but this study has shown that anaemia which results due to the diabetes was essentially not reversed by P. incana. The decline in mean corpuscular volume, haemoglobin and its concentrations are in agreement with the findings of earlier researchers such as Arun and Ramash (2002) that noticed a drastic reduction in the levels of red blood cell (RBC), haemoglobin (Hb), haematocrit (PCV) and mean corpuscular haemoglobin concentration (MCHC) of diabetic animals. This was also observed by Baskar et al. (2006) who reported antihyperglycemic activity of aqueous root extract of Rubia cordifolia in streptozotocin - induced diabetic rats. The alterations in these haematological parameters have also been reported in humans (Balasubraimanian et al., 2009).
Clinically, MCV and MCHC levels are lowered in cases of iron deficiency, sideroblastic anaemia, thalassemia and lead poisoning while they are elevated in liver diseases, megaloblastic anaemia, folic acid and vitamin B12 deficiency (Janz et al., 2013). Findings from our study suggest that the anaemia observed in extract treated rats may clinically be due to iron deficiency while the anaemia in the diabetic untreated and glibenclamide treated rats may tend towards deterioration of liver function. The white blood cell (WBC) population markedly declined in the diabetic untreated rats, but was non-significantly increased in the extract treated rats. Lymphocyte counts were particularly increased which may be indicative of increase immune response such as observed in serum sickness, aplastic anaemia, leukaemia and immune diseases (Scheinberg and Young, 2012).
On the other hand, neutrophil count reduced indicating increased risk of infection which is usually associated with neutropenia (Hsieh, 2007). On the contrary, platelet count increased, but this further supports our findings with red and white cell indices, which point towards the presence of some form of anaemia. Clinically increased platelet (thrombocytosis) is observed in cases of acute blood loss, infection, Iron (Fe) deficiency, haemolytic anaemia or polycythaemia Vera (Skoda, 2009). Serum biochemistry data clearly shows that diabetes is accompanied by impaired hepatic function typified by markedly decreased total protein and its constituent fractions especially albumin, as well as increased expression of liver enzymes. All these were successfully reversed by the treatment of diabetic rats with the extract. Our result shows that the extract had a more profound hepatoprotective effect than even glibenclamide.
Normally, plasma proteins are produced by hepatocytes and hepatic damage is usually indicated by decreased protein synthesis and increased expression of liver specific-enzymes alanine transaminase (ALT) and aspartate transaminase (AST) (Nyblom et al., 2006). Increased expression of alkaline phosphatase (ALP) is more specific for biliary tract damage, obstruction or infection (Aabakken et al., 2007) which was markedly increased in diabetic untreated rats. As earlier mentioned, diabetes is accompanied by increased haemolysis, which consequently will result in increased excretion of heme as a by-product of hemolysis (Woollard et al., 2009). The increase in bilirubin levels in all groups of rats in this study also supports our hypothesis that there was a degree of increased excretion of heme, but was significantly (P<0.05) increased in the diabetic untreated rats. The mean BUN and creatinine levels were significantly increased in diabetic untreated rats which may be indicative of renal injury (Mazze et al., 2000; Waikar and Bonventre, 2006).
The primary metabolite derived from dietary protein and tissue protein turnover is urea while muscle creatinine catabolism results in production of creatinine (Thurman and Parikh, 2008). The extract treated rats however showed a non-significant decline in their BUN levels, while creatinine levels increased. It can be inferred that the extract did try to reverse the renal damage but glibenclamide did a better job at the reversal. The high cholesterol level in diabetic untreated group can be attributed to the diabetic condition which normally lowers the more beneficial cholesterol; high density lipoprotein (HDL) and increases the harmful cholesterols; triglycerides (TG) and low density lipoprotein (LDL), eventually increasing the overall cholesterol level and may result in serious cardiovascular complications (Henry, 2001). The extract showed better glycemic control compared to glibenclamide in lowering blood cholesterol and glucose levels, which is the desired effect of an oral hypoglycemic agent.
A previous toxicological evaluation of the P. incana showed that the hemiparasitic plant had good hypolipidemic properties, particularly by significantly lowering LDL (Ogunmefun et al., 2013). It also has very minimal hypoglycemic property in normoglycemia, but profound antihyperglycemic properties (Ogunmefun et al., 2016). In conclusion, it can be inferred from this study that the extract of P. incana regardless of its host plant, not only decreased blood glucose and cholesterol levels, but also alleviated some complications of diabetes such as liver and kidney injury. The PCV was depressed, but RBC count improved. Traditional therapy with P. incana extract may need to be in combination with a hematinic to prevent development of anaemia. Also, further studies may be warranted to ascertain its effect on cardiovascular complications of diabetes and its potential as a hepatoprotective agent.
The authors hereby disclose that there is no conflict of interest pertaining to this research work.
REFERENCES
|
Aabakken L, Bretthauer M, Line PD (2007). Double-balloon enteroscopy for endoscopic retrograde cholangiography in patients with a Roux-en-Y anastomosis. Endoscopy 39(12):1068-1071.
Crossref
|
|
|
|
Abubakar MS, Musa AM, Ahmed A, Hussaini IM (2007). The Perception and Practice of Traditional Medicine in the Treatment of Cancers and Inflammations by the Hausa and Fulani Tribe of Northern Nigeria. J. Ethnopharmacol. 3(3):625-629.
Crossref
|
|
|
|
|
Adesina SK, Illoh HC, Johnny II, Jacobs IE (2013). African Mistletoes (Loranthaceae); Ethnopharmacology, Chemistry and Medicinal Values: An Update. Afr. J. Tradit. Complement Altern. Med. 10(4):161-170.
Crossref
|
|
|
|
|
Arun GS, Ramash KG (2002). Improvements of Insulin sensitivity by perindopril in spontaneous hypertensive and streptozotocin – diabetic rats. Indian J. Pharmacol. 34:156-164.
|
|
|
|
|
Balasubraimanian T, Lal MS, Mahananda S, Chatterjee TK (2009). Antihyperglycaemia and antioxidant activities of medicinal plant Strereospermum suaveolens in streptozotocin-induced diabetic rats. J. Diet Suppl. 6(3):227-251.
Crossref
|
|
|
|
|
Baskar R, Bhakshu LM, Bharathi GV, Reddy SS, Karuna R, Reddy GK, Saralakumari D (2006). Antihyperglycemic activity of aqueous root extract of Rubia cordifolia in stepzotocin – induced diabetic rats. Pharm. Bio. 44(6):475-479.
Crossref
|
|
|
|
|
Bnouham M, Ziyyat A, Mekhfi H, Tahri A, Legssyer A (2006). Medicinal plants with potential antidiabetic activity - A review of ten years of herbal medicine research (1990-2000). Int. J. Diabetes Metab. 14:1-25.
|
|
|
|
|
Bock PR, Friedel WE, Hanisch J, Karasmann M, Schneider B (2004). Efficacy and safety of long-term Complementary Treatment with Standardized European mistletoe extract (Viscum album L.) in addition to the conventional adjuvant oncologic therapy in Patients with Primary non-metastasized mammary carcinoma. Results of a Multi-center, comparative, Epidemiological cohort Study in Germany and Switzerland [in German]. Arzneimittel forschung 54:456-66.
|
|
|
|
|
Eddouks M, Maghrani M, Lemhadri A, Ouahidi ML, Jouad H (2002). Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet). J. Ethnopharmacol. 82(2-3):97-103.
Crossref
|
|
|
|
|
Elluru SR1, Duong Van Huyen JP, Delignat S, Prost F, Heudes D, Kazatchkine MD, Friboulet A, Kaveri SV (2009). Antiangiogenic properties of viscum album extracts are associated with endothelial cytotoxicity. Anticancer Res. 29(8):2945-2950.
|
|
|
|
|
Gray AM, Flatt, PR (1997). Pancreatic and extra-pancreatic effects of the traditional anti- diabetic plant, Medicago sativa (lucerne). Br. J. Nutr. 78:325-334.
Crossref
|
|
|
|
|
Henry RR (2001). Preventing Cardiovascular Complications of Type 2 Diabetes: Focus on Lipid Management. Clin. Diabetes 19(3):113-120.
Crossref
|
|
|
|
|
Hilmi Y, Abushama MF, Abdalgadir H, Khalid A, Khalid H (2013). A study of antioxidant activity, enzymatic inhibition and in vitro toxicity of selected traditional sudanese plants with anti-diabetic potential. BMC Complement. Altern. Med. 14:149.
Crossref
|
|
|
|
|
Hoagy S (2008). Harvesting Real Mistletoe for Christmas © Hoagy Scoins Dec.
|
|
|
|
|
Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP (2007). Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann. Int. Med. 146 (7):486-492.
Crossref
|
|
|
|
|
Ibatomi DK, Bikomo EO, Temple VJ (1994). Antidiabetic Properties of the African Mistletoe in Streptozotocin-induced Diabetic Rats. J. Ethnopharmacol. 43(1):13-17.
Crossref
|
|
|
|
|
Janz TG, Johnson RL, Rubenstein SD (2013). Anemia in the emergency department: evaluation and treatment. Emerg. Med. Pract. 15(11):1-15.
|
|
|
|
|
Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donaghue MJ (2002). Plant systematics: a phylogenetic approach. Sinauer Associates, Inc., Sunderland Massachusetts, USA. ISBN 0-87893-403-0.
|
|
|
|
|
Koch HP, Lawson LD (1996). Garlic: The Science and Therapeutic Application of Allium sativum L., Related Species, (Vol. 683181475). baltimore, Maryland: Williams & Wilkins xv, 329p. ISBN.
|
|
|
|
|
Maier G, Fiebig HH (2002). Absence of tumor growth stimulation in a panel of 16 human tumor cell lines by mistletoe extracts in vitro. Anticanc. Drugs 13(4):373-9.
Crossref
|
|
|
|
|
Marles RJ, Farnsworth NR (1995). Antidiabetic plants and their active constituents. Phytomedicine 2:137-189.
Crossref
|
|
|
|
|
Mazze RI, Callan CM, Galvez ST (2000). The effect of sevoflurane on serum creatinine and blood urea nitrogen concentrations: a retrospective, twenty-two-center, comparative evaluation of renal function in adult surgical patients. Anaesth. Analg. 90:683-688.
Crossref
|
|
|
|
|
Mengs U, Gothel D, Leng-Peschlow E 2002. Mistletoe extracts standardized to mistletoe lectins in oncology: review on current status of preclinical research. Anticancer Res. 22(3):1399-1407.
|
|
|
|
|
Nyblom H, Björnsson E, Simrén M, Aldenborg F, Almer S, Olsson R (2006). The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 26(7):840-845.
Crossref
|
|
|
|
|
Ogbole OO, Adeniji JA, Ajaiyeoba EO, Adu DF (2013). Anti-polio virus activity of medicinal plants selected from the Nigerian ethno-medicine. Acad. J. 12(24):3878-3883.
|
|
|
|
|
Ogunmefun OT, Fasola TR, Saba AB, Oridupa OA (2013). The toxicity evaluation of Phragmanthera incana (Klotzsch) growing on two plant hosts and its effect on wistar rats' haematology and serum biochemistry. Acad. J. Plant Sci. 6(2):92-98.
|
|
|
|
|
Ogunmefun OT, Saba AB, Fasola TR, Oridupa OA, Adarabioyo MI (2016). Hypoglycemic Effect of Phragmanthera incana (Schum.) Balle on Alloxan-induced diabetic albino rats – Int. J. Med. Plants Res. 5(1):173-177.
|
|
|
|
|
Orhan DD, Aslan M, Sendogdu N, Ergun F, Yesilada E (2005). Evaluation of the Hypoglycemic effect and Antioxidant Activity of three Viscum album subspecies (European mistletoe) in Streptozotocin-Diabetic Rats. J. Ethnopharmacol. 98(1):95-102.
Crossref
|
|
|
|
|
Oyedemi SO, Yakubu MT, Afolayan AJ (2011). Antidiabetic activities of aqueous leaves extract of Leonotis leonurus in streptozotocin – induced diabetic rats. J. Med. Plant Res. 5(1):119-125.
|
|
|
|
|
Polhill R, Wiens D (1998). Mistletoes of Africa. The Royal Botanic Garden, Kew, U.K. 370 p.
|
|
|
|
|
Rai V, Mani UV, Iyer UM (1997). Effect of Ocimum sanctum leaf powder on blood lipoproteins, glycated proteins, and total amino acids in patients with non-insulin dependent diabetes mellitus. J. Nutr. Environ. Med. 7:113-118.
Crossref
|
|
|
|
|
Raman A, Lau C (1996). Antidiabetic properties and Phytochemistry of Momordica charantia L. (Cucurbitaceae) Phytomedicine 2:349-362.
Crossref
|
|
|
|
|
Runyon J, Tooker J, Mescher M, De Moraes C (2009). Parasitic plants in agriculture: Chemical ecology of germination and host-plant location as targets for sustainable control: A review. Sustainable Agric. Rev. 1:123-136.
Crossref
|
|
|
|
|
Sanchez de Medina F, Gamez MJ, Jimenez I, Jimenez J, Osuna JI, Zarzuelo A (1994). Hypoglycemic activity of juniper "berries". Planta Med. 60:197-200.
Crossref
|
|
|
|
|
Scheinberg P, Young NS (2012). How I treat acquired aplastic anemia. Blood 120(6):1185-1196.
Crossref
|
|
|
|
|
Skoda RC (2009). Thrombocytosis. Hematology Am. Soc. Hematol.Educ. Program pp. 159-167.
Crossref
|
|
|
|
|
Srivastava Y, Bhatt HV, Verma Y, Venkaiah K, Ravali BH (1993). Antidiabetic and adaptogenic properties of Momordica charantia extract. An experimental and clinical evaluation. Phytother. Res.7:285-289.
Crossref
|
|
|
|
|
Thurman JM, Parikh CR. (2008). Peeking into the black box: New biomarkers for acute kidney injury. Kidney Int. 73(4):379.
Crossref
|
|
|
|
|
Waikar SS, Bonventre JV (2007). Biomarkers for the diagnosis of acute kidney injury. Curr. Opin. Nephrol. Hypertens. 16:557-564.
Crossref
|
|
|
|
|
Watson D (2001). Mistletoe: a keystone resource in forests and woodlands worldwide. Ann. Rev. Ecol Syst. 32:219-249.
Crossref
|
|
|
|
|
Williams SS (1990). Mistletoe. Garden Line Porpouri Miscellaneous/Mistletoe.
|
|
|
|
|
Woollard KJ, Sturgeon S, Chin-Dusting JPF, Salem HH, Jackson SP (2009). Erythrocyte Hemolysis and Hemoglobin Oxidation Promote Ferric Chloride-induced Vascular Injury. J. Biol. Chem. 284(19):13110-13118.
Crossref
|
|