ABSTRACT
This work was designed to access reproductive status of Cypermethrin treated rams by measuring the scrotal circumference, live weight, live testicular width and length as well as some physiological parameters to determine its reproductive and general toxicity in Yankasa rams. Sixteen rams aged 18 to 30 months and weighing between 21.5 and 46.5 kg were used for this study. The 16 rams were divided equally into two groups: A (treatment) and B (control). Group A were given Cypermethrin (3%) at the dose rate of 3 mg/kg (0.1 ml/kg) body weight, topically. While group (B) were given distilled water at the same dose rate and route. These treatments were repeated fortnightly for a period of 12 weeks. The animals were weighed weekly using a measuring scale early in the morning before feeding. Their rectal temperature and respiratory rates were taken concurrently. Scrotal circumference, testicular width and length were measured weekly with the use of flexible measuring tape. Results showed no statistically significant difference between the two groups in all the parameters measured (P>0.05). It was concluded that Cypermethrin at the dose rate of 3 mg/kg body weight for twelve weeks increased body weight and scrotal circumference of treated rams, although there was no significant difference between the treated and control rams P>0.05. The treatment did not have any effect on the live testicular width and length, body temperature as well as respiratory rate of Yankasa rams studied. It was recommended that similar studies should be conducted in other domestic ruminants because species differences may play key roles in reproductive and general toxicity of Cypermethrin.
Key words: Scrotal circumference, Cypermethrin, measurements, rams, toxicity.
Pyrethroids have been known as being a potential endocrine disruption compound through human and other mammalian studies. These studies vary in compounds, dosages, routes, exposure durations and evaluated parameters (Martenies and Perry, 2013). Other human research with pesticide endocrine disruption on male reproductive performance consistently focused on concentration, motility and morphology based on World Health Organization (WHO) fertility parameters (Martenies and Perry, 2013). Decreased sperm concentrations were most commonly reported with decreased motility being less frequent and morphology being less clear clinically (Martenies and Perry, 2013).
The absorption and elimination of Cypermethrin is reported as rapid in the different mammalian species tested (WHO, 1989b). The half-life in the fat of rats is about 9 - 12 days for the cis-isomer and 3-4 days for the trans-isomer, the acute toxicity of Cypermethrin for mammals is of moderate order. The oral LD50 for the rat ranged from 200 - 400 mg/kg body weight, short term and long-term toxicity studies on rats, mice, and dogs have shown effects on growth, liver, kidneys, nervous system and blood (WHO, 1989b).
Cypermethrin is toxic not only to insects but also mammals (Barlow et al., 2001; He, 2000). Clinical signs like muscular tremors, ataxia, weakness of limbs, convulsions, coma and death from respiratory depression have been reported in animals after ingesting high doses of Cypermethrin (Sandhu and Brar, 2000). Incoordination, muscular tremors, jerky movements, ataxia, staggering gait and dizziness were observed in dose dependent manner in Cypermethrin treated rabbits (Ullah et al., 2006). Long-term feeding studies with laboratory animals have shown that Cypermethrin causes adverse effects. In rats, it caused reduced growth rate and increased liver weight. In mice, it caused reduced weight gain, mild anemia, and increased liver weight. In dogs, it caused loss of appetite, incoordination and tremors. In rabbits, it caused pathological changes in the thymus, liver, adrenal glands, lungs and skin (Caroline, 1996).
Besides generalized toxic effects of Cypermethrin, decreased number of implantation sites, number of viable fetuses and weight gain of fetuses in rabbits treated with Cypermethrin have been reported (Elbetieha et al., 2001). Histologically, changes were observed in ovary and uterine tissues which were more pronounced at higher doses (EL-Toukhy and Girgis, 1993). The effect of pyrethroid exposure in female animals has been well elaborated by many researchers. (Liu et al., 2011; Petr et al., 2013; Gill et al., 2011; Lemos et al., 2011; Guerra et al., 2011).
Cypermethrin is able to influence some reproductive and fertility parameters as exposure to this chemical can cause significant increase in the production of non-viable or abnormal sperm in mice (Jalal et al., 2010). High oral dosages (30 to 60 mg/kg) of Cypermethrin on adult male rats for 15 days decrease daily sperm output (Yan et al., 2013). High dosages of Cypermethrin also caused histological atrophy and distortion of seminiferous tubules including deformed and disordered arrangement of germ cells. Vacuolization of Sertoli cells and deforming of Leydig nuclei was also noted with subsequent decrease of serum testosterone concentration in oral Cypermethrin treated rats (Yan et al., 2013). Oral treatment of 5 mg/kg deltamethrin for 4 weeks decreased testosterone, LH and FSH concentrations (Ismail and Mohamed, 2012). Male ruminants exposed to pyrethroids have also been clinically observed to have negative effects on reproduction. Observations by Volkmann and Voelkl (2012) implied that pyrethroid exposure to bulls and rams for a short duration could negatively impact sperm concentration, ejaculate volume, progressive sperm motility and sperm morphology. Recently, Cain et al. (2014) showed no differences in sperm motility or morphology throughout an 84-day trial period with exposure to 150% label dose, giving twice (day 0 and 14), of 1% Permethrin dermally to purebred beef bulls. French et al. (2014) found no effect on sperm parameters when crossbred bulls were exposed to Cyfluthrin pour-on, Cyfluthrin fly tags and a combination of Cyfluthrin pour-on and fly tags over a nine week study. Likewise, crossbred bulls exposed to different combinations of pyrethroids 28 and pyrethrins (control – cyfluthrin pour-on and fly tags, treatment – cyfluthrin pour-on and fly tags and pyrethrin premise spray fogger) showed no consistent change in sperm parameters or testosterone in a nine week study (Stewart et al., 2015). Ingestion of Cypermethrin at high doses (18.93 or 39.66 mg per day) resulted in a significant increase in the weights of testes and seminal vesicles of male Sprague Dawley rats, also epididymal and testicular sperm counts as well as daily sperm production were significantly decreased in exposed males (Jalal et al., 2010). Popular press literature has identified potential links between use of pyrethroids and its negative effects on beef bull reproductive health (Ismail and Mohamed, 2012).
The mechanism by which Cypermethrin affects male reproduction is unclear (Wang et al., 2009). Pyrethroids are rapidly metabolized in mammals and several studies have shown that Cypermethrin damages the brain, liver and erythrocytes by causing oxidative stress (Wang et al., 2009). Three doses of β-Cypermethrin decreased body weight gain and weight of testosterone-sensitive organs such as testes, epididymis, seminal vesicles and prostate glands, sperm count, viability and intact acrosome population (Wang et al., 2009). Qualitative analyses revealed that low dose (1 mg/kg) of beta-Cypermethrin decreased the number of interstitial Leydig cells but did not affect the intratubular compartment of seminiferous tubules, as the concentration of beta-Cypermethrin increased, the number of spermatids and cells in the seminiferous tubules decreased (Wang et al., 2009). Low dose of beta-Cypermethrin did not significantly affect sperm concentration, while a high dose (20 mg/kg) significantly reduced the number of sperm cells in the seminiferous tubules, serum testosterone and steroidogenesis acute regulatory protein (StAR) (Wang et al., 2009). Experiment on the effect on biochemical parameters of testes after administration of 250mg/kg, per os of α- Cypermethrin in albino mice was reported to cause histologic changes in spermatogenic cells, like rupture of cell membrane, shrinkage in the nucleus, presence of stages of apoptosis, condensation of chromatin and decrease or absence of cytoplasmic organelles, as revealed by transmission electron microscopy (TEM) (Prakash, 2010). Decrease in food intake, body weight, absolute and relative gonad weights have been observed in rabbits treated with Cypermethrine (Handerson and Parkinson, 1981).
Pesticide exposure is associated with infertility, there is much concern that exposure to environmental contaminants causes decreased sperm counts, impairment of sperm motility, reduced fertilization ability, producing abnormal sperm in men and wildlife (El-betieha et al., 2001). A significant decrease in testicular index weight, sperm mass motility and spermatozoa concentration in the epididymis was also reported following oral administration of Cypermethrin (Assayed et al., 2008). The cytotoxic action has been suggested to be associated with a decrease in testicular index weight of male rats. Such decrease could also be linked to the reduced testosterone synthesis and disruption of normal androgen status (Abd-Allah, 1995). Effects of pyrethroid exposure have been assessed with standard breeding soundness examination (BSE) parameters and steroid (testosterone) concentrations; however, effect of pyrethroids on testicular histopathology has not been addressed in the bovine (Tyler, 2015). Morphology difference after dermal exposure to pyrethroids was not significantly different as compared to controls in all previously published bull data (Cain et al., 2014; French et al., 2014). Given that a BSE is a “snap shot” evaluation of bull reproductive soundness, further research may be warranted to elucidate the long-term effects of chronic pyrethroid exposure. There were no differences noted in average daily gain or body condition scores due to treatment, which was expected due to the short duration of only 19 days between initial and final body weight (BW) and body condition score (BCS) (Tyler, 2015). Scrotal circumference and testicular measurements are component parts of breeding soundness examination in the ram. This work aimed to access reproductive status of treated rams by measuring the scrotal circumference, live weight, live testicular width and length as well as some physiological parameters to determine its reproductive and general toxicity in Yankasa rams.
Study location
The study was carried out at the National Animal Production Research Institute (NAPRI) Shika, Ahmadu Bello University Zaria, which is situated in the Northern Guinea Savannah and lying between latitudes 11° and 12°N and longitude 7° and 8°E, at an elevation of 650 m above sea level. The area has an annual rainfall of 1100 mm (Igono et al., 1982). There are two seasons {rainy season (May-October) and dry season (November-April)}, respectively.
Experimental animals
The animal experiments followed the principles of the laboratory animal care (Canadian Council on Animal Care Guide, 1993). Sixteen sexually-mature, healthy Yankasa rams aged 18 to 30 months, weighing between 21.5 and 46.5 kg with clinically normal genitalia were used in this study. The rams were purchased from the open market in Sabua Local Government Area of Katsina State. They were housed at the Small Ruminant Research Programme Experimental Unit of NAPRI. The house was made of brick concrete pens with concrete floors. The rams were divided into two groups of eight each. They were given concentrate feed ad libitum (cotton seed, maize offal, maize, wheat offal, bone meal and salt) in the morning and later in the evening; hay was made available during the day at intervals. The hay used was Digitaria smutsii, and water was given ad libitum.
Experimental design and treatment
The 16 rams were divided equally into two groups (A and B). Group A served as the treatment group, while group B served as the control. The animals were acclimatized for two weeks during which blood and fecal samples were collected and analyzed for haemoparasites and helminths and treatments where given when necessary.
Administration of 3% cypermethrin
The rams in group A were given Cypermethrin (3%) at the dose rate of 3 mg/kg (0.1 ml/kg) body weight, topically as pour-on. The control group B rams were given distilled water at the same dose rate of 0.1 ml/kg body weight topically as pour-on. These treatments were repeated every two weeks for a period of 12 weeks. Body and testicular measurements, scrotal circumference, body temperature, respiratory rate were collected before the administration of 3% Cypermethrin to establish base line data. This was done by obtaining the average values for the eight animals in each group before the experiment started.
Scrotal circumference and body measurements
The animals were weighed weekly using a measuring scale (Salter suspended weigher, model 235, UK) early in the morning before feeding. Their rectal temperatures (using a clinical thermometer) and respiratory rates (using stop watch) were taken concurrently. Scrotal circumference, testicular width and length were measured weekly with the use of flexible measuring tape.
Statistical analysis of data
Data were expressed as means and standard error of mean (SEM). Data were analyzed using descriptive statistics and paired student’s t-test with SPSS/PC computer program (Version 20.0, SPSS®, Chicago IL, USA). Differences with confidence values of p < 0.05 were considered statistically significant (Daniel, 1991).
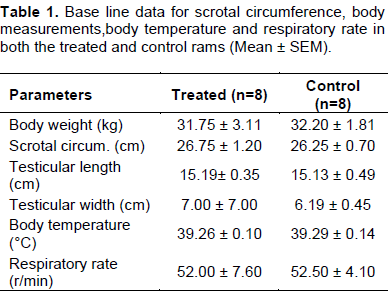
The baseline parameters (Table 1) for the two groups were within normal physiological range for rams.
Body weights and scrotal measurements
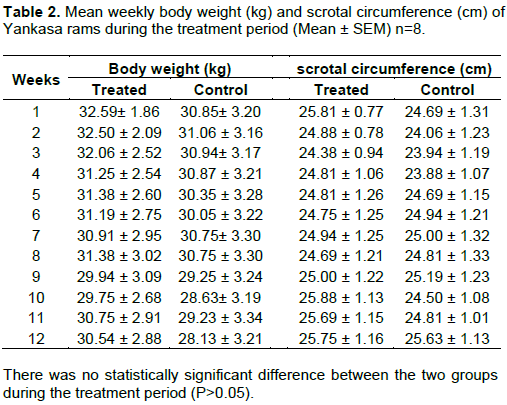
The mean weekly body weights of the treated and the control groups are presented in Table 2. There was no statistically significant difference between the two groups during the treatment period (P>0.05). At week 12 of the experiment, the mean body weight of the treated group was higher than the mean body weight of the control group. The difference was not statistically significant (P>0.05). The mean weekly scrotal circumference of the treated and the control groups are presented in Table 2. There was no statistically significant difference between the two groups during the treatment period (P>0.05). At week 12 of the experiment, the mean scrotal circumference of the treated group was higher than the control group and the difference was statistically not significant (P>0.05).
Testicular measurements
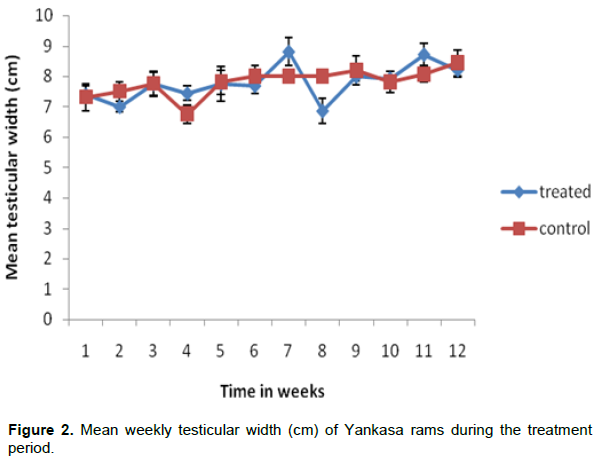
The mean weekly testicular length of the treated and control groups are presented in Figure 1. The difference was statistically not significant during the treatment period (P>0.05). The mean weekly testicular width of the treated and control groups are presented in Figure 2. There was no statistically significant difference between the two groups during the treatment period (P>0.05).
Body temperature and respiratory rate
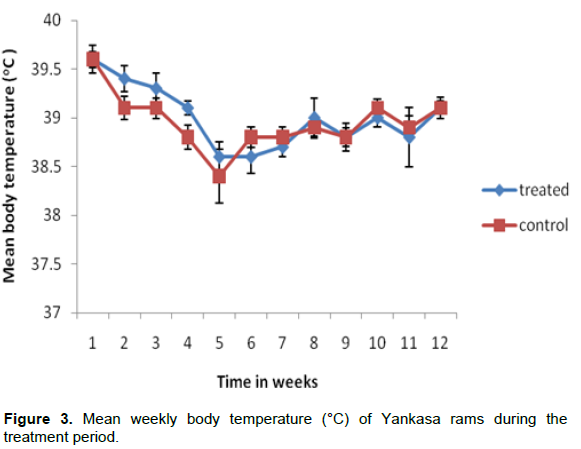
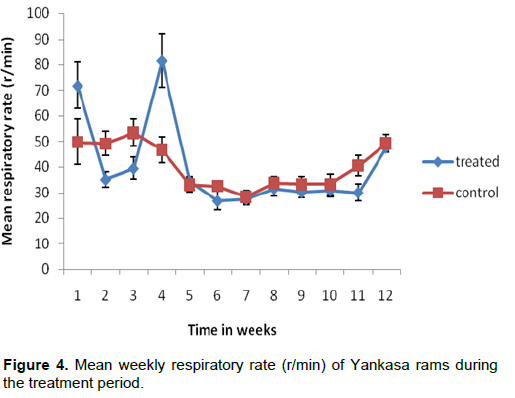
The mean weekly body temperature of the treated and control groups are presented in Figure 3. There was no statistically significant difference between the two groups during the treatment period (P>0.05). The mean weekly respiratory rate of both the treated and control groups are presented in Figure 4. There was no statistically significant difference between the two groups during the treatment period (P>0.05).
The results showed that Cypermethrin treated rams lost weight in the first seven weeks of the study and then re- gained weight as from week eight of the study, while the control rams gradually lost weight till week twelve of the experiment. The difference between the live weight of the treated and control rams was not statistically significant during the treatment period (P>0.05). This finding in the first seven weeks of the experiment agrees with the report in mice on long-term feeding studies where Cypermethrin caused reduced weight gain and increased liver weight (Caroline, 1996). Decrease in food intake, body weight, absolute and relative gonad weights have been observed in rabbits treated with Cypermethrine (Handerson and Parkinson, 1981). Three doses (1, 10 and 20 mg/kg)/os of beta- Cypermethrin caused decrease in body weight in male mice (Wang et al., 2009).
However, the gain in weight of the treated rams from weeks eight to twelve contradicts the above report. The contradiction may have resulted from the route of administration and the species of animals involved in the study. The gain in weight may be due to the effect of Cypermethrin on body fat as Cypermethrin is lipotropic. The highest level of Cypermetherin is found in body fat, which is consistent with lipophilic nature of the compound (WHO, 1989a). There may be more fat depots e.g. the subcutaneous fat in rams where Cypermethrin exerted its effect just like in the liver of rats and mice which culminated into overall weight gain. It is possible that fly repellant property of Cypermethrin made the treated rams to feed better than the control rams, hence gaining weight over time.
The scrotal circumference of both the treated and control rams followed the same trend with the body weight of the rams. This pattern is expected because scrotal circumference is proportional to the body weight and size. However, there was no statistically significant difference between the scrotal circumference of the treated and control rams during the treatment period. The testicular measures showed that there was a slight increase in the testicular length of both the treated and control groups during the treatment period. But there was no statistically significant difference between the testicular length of the treated and control rams. Similarly, the testicular width of both treated and control rams slightly increased during the treatment period, but there was no statistically significant difference between the testicular width of the treated and control animals during the period. These findings showed that Cypermethrin has no effect on the scrotal circumference, testicular length and width of Yankasa rams. In agreement, Cain et al. (2014) who showed no differences in sperm motility or morphology throughout an 84-day trial period with exposure to 150% label dose, giving twice (day 0 and 14) of 1% Permethrin dermally to purebred beef bulls. It is also in accordance with French et al. (2014) who found no effect on sperm parameters when crossbred bulls were exposed to Cyfluthrin pour-on, Cyfluthrin fly tags, and a combination of Cyfluthrin pour-on and fly tags over a nine weeks study. Likewise, crossbred bulls exposed to different combinations of pyrethroids 28 and pyrethrins (control – cyfluthrin pour-on and fly tags, treatment – cyfluthrin pour-on and fly tags and pyrethrin premise spray fogger) showed no consistent change in sperm parameters or testosterone in a nine weeks study (Stewart et al., 2015). In bovines, there were no differences noted in average daily gain or body condition scores due to treatment, which was expected due to the short duration of only 19 days between initial and final body weight (BW) and body condition score (BCS) (Tyler, 2015). Although, observations by Volkmann and Voelkl (2012) implied that pyrethroid exposure to bulls and rams for a short duration could negatively impact sperm concentration, ejaculate volume, progressive sperm motility and sperm morphology. In the present study, Cypermethrin treated group did not show any significant difference from the testicular length and width of the control group (P>0.05). The lack of difference may be due to the dose of 3 mg/kg body weight used in the experiment. The findings showed that Cypermethrin given at the dose of 3 mg/kg body weight to Yankasa rams has no effect on the body temperature of the rams. Again, most reports on the effect of Cypermethrin on health generally were made on laboratory animals. Specific report on the effect of Cypermethrin on the body
temperature of rams has not been elaborated. Instead, emphasis was made on its’ nervous and general effects on the body. This study also revealed that the respiratory rate of both the treated and control groups reduced over time. But the difference between the treated and control groups during the treatment period was not statistically significant. That is to say that Cypermethrin at the dose rate 3 mg/kg body weight used in this experiment did not affect the respiratory rate. However, coma and death from respiratory depression have been reported in animals after ingesting high doses of Cypermethrin, while its dermal contact in facial area may cause a subjective sensation of tingling or numbness (Sandhu and Brar, 2000).
It was concluded that the use of Cypermethrin topically at the dose rate of 3 mg/kg body weight for twelve weeks is safer than other routes and doses. The treatment did not have any effect on the live testicular width and length, body temperature and respiratory rate of Yankasa rams. Gross observations of fertility parameters in Cypermethrin exposed ruminants like scrotal circumferences may be deceptive. It was recommended that similar studies including histopathology and semen evaluation should be conducted in other breeds and ruminants because species differences and several other factors may play key roles in reproductive and general toxicity of Cypermethrin.
The authors have not declared any conflict of interests.
REFERENCES
|
Abd-Allah AEY (1995). Effect of some pesticides on reproduction. Ph.D.Thesis(Toxicology)Faculty of Veterinary Medicine, Cairo University.
|
|
|
|
Assayed ME, Salem HA, Khalaf AA (2008). Protective effects of garlic extract and vitamin C against Cypermethrin reproductive toxicity in male rats. Res. J. Vet. Sc. 1:1-5.
|
|
|
|
|
Barlow SM, Sullivan FM, Lines J (2001). Risk assessment of the use of Deltamethrin on bed nets for the prevention of malaria. Food and Chem. Tox. 39(5): 407-422.
Crossref
|
|
|
|
|
Cain AJ, King H, Wills R, Agee W, Howard S and Hopper R (2014). Evaluation of the effects of topical Permethrin insecticide on bull semen quality. Clin. Therio. 6: 25- 31.
|
|
|
|
|
Canadian Council on Animal Care Guide(1993).
View (second ed.). Accessed 04.11.2015, 10pm.
|
|
|
|
|
Caroline C (1996). Insecticide fact sheet. J. Pest. Ref. Summer. 2(16):1-20.
|
|
|
|
|
Daniel WW (1991). Analysis of variance. In: Daniel, WW. (Ed), Biostatistic: A Foundation for Analysis in the Health Sciences. John Wiley and Sons, Hoboken. pp. 74-320.
|
|
|
|
|
Elbetieha AO, Da'as SI, Khamas W, Darmani H (2001). Evaluation of the toxic Potentials of Cypermethrin pesticide on some reproductive and fertility parameters in the male rats. Arch. Environ. Contamin. Toxicol. 41(4):522-528.
Crossref
|
|
|
|
|
El-Toukhy MA and Girgis RS (1993). In vivo and in vitro studies on the effect of Larvin and Cypermethrin on adenosine triphosphastase activity of male rats. J. Environ. Sci. Health. 28:599-619.
Crossref
|
|
|
|
|
French HM, Shipley CF, Ireland FA, Jarrell VL, Fuselier AJ, Williams DG, ShikeDW (2014). The effect of Cyfluthrin, a commercially available synthetic pyrethroid, on bovine semen quality and pregnancy rates. Clin. Therio. 6:33-39.
|
|
|
|
|
Gill SA, Rizvi F, Khan MZ, Khan A (2011). Toxic effects of Cypermethrin and Methamidophos on bovine corpus luteal cells and progesterone production. Exp. Toxicol. Pathol. 63:131-135.
Crossref
|
|
|
|
|
Guerra MT, de Toledo FC, Kempinas WDG (2011). In utero and lactational exposure to Fenvalerate disrupts reproductive function in female rats. Rep. Toxicol. 32:298-303.
Crossref
|
|
|
|
|
Handerson HK, Parkison FN (1981). Effect of Cypermethrin on haematology, clinical chemistry and gonads of male rabbit. Vet. Med. J. (Giza). 31(1):32-37.
|
|
|
|
|
He F (2000). Neurotoxic effect of insecticides current and future research: A review. Neurotox. 21(5):829-835.
|
|
|
|
|
Igono M, Molokwu ECI, AliuY O (1982). Body temperature responses of Savanah Brown goats to hamattan and hot-dry seasons. Inter. J. Biomet. 26:225-230.
Crossref
|
|
|
|
|
Ismail MF, Mohamed HM (2012). Deltamethrin-induced genotoxicity and testicular injury in rats: Comparison with biopesticide. Food Chem. Toxicol. 50:3421-3425.
Crossref
|
|
|
|
|
Jalal S, Ramin H, Roohollah TZ (2010). Effect of Cypermethrin on sexual behaviour and plasma concentrations of pituitary gonadal hormones. Inter. J. Fertil. Steril. 1(4):23-28.
|
|
|
|
|
Lemos A, Wanderley-Teixeira V, Teixeira AAC, Silva FDA, Oliveira J V, de Siqueira HAA (2011). Response of blastocyst-endometrium interactions in albino rats to sublethal doses of biological and synthetic insecticides. Food Chem. Toxicol. 49:2541-2547.
Crossref
|
|
|
|
|
Li Yan F, Pan C, Hu Jin X, Li J, Xu Li C (2013). Effects of Cypermethrin on Male Reproductive System in Adult Rats. Biomed. Environ. Sci. 26: 201-208.
|
|
|
|
|
Liu J, Yang Y, Zhuang S, Yang Y, Li F, Liu W (2011). Enantioselective endocrine-disrupting effects of Bifenthrin on hormone synthesis in rat ovarian cells. Toxicology 290:42-49.
Crossref
|
|
|
|
|
Martenies S E, Perry M J (2013). Environmental and occupational pesticide exposure and human sperm parameters: A systematic review. Toxicology 307:66-73.
Crossref
|
|
|
|
|
Petr J, Chmelikova E, Zalmanova T, Tumova L, Kheilova K, Kucerova-Chrpova V, Jilek F (2013). Pyrethroids Cypermethrin, Deltamethrin and Fenvalerate have different effects on in vitro maturation of pig oocytes at different stages of growth. Animal 7:134-142.
Crossref
|
|
|
|
|
Prakash N, Kumar VM, Sunichandra U, Pavithra BH, Pawar A (2010). Evaluation of testicular toxicity following short-term exposure to Cypermethrin in albino mice. Toxicol International. Soc. Toxicol. 17:18-21.
|
|
|
|
|
Sandhu HS, Brar RS (2000). Textbook of Veterinary Toxicology. 1st ed. Kalyani Publ., New Dehli, India, pp. 225-235.
|
|
|
|
|
Stewart JL, Shipley CF, Ireland FA (2015). Effects of different applications of pyrethrins and cyfluthrin, a synthetic pyrethroid, on bul reproductive parameters. Clin. Therio. 7:27-34
|
|
|
|
|
Tyler D (2015). Reproductive effects of pyrethroid use in beef cattle, Iowa State University, Graduate theses and dissertation.Graduate college, pp. 2-32.
|
|
|
|
|
Ullah M S, Ahmad M, Ahmad N, Khan M Z and Ahmad 1 (2006). Toxic effects of Cypermethrin in female rabbits. Pak. Vet. J. 26(4):193-196.
|
|
|
|
|
Volkmann DH and Voelkl DL (2012). Clinical observations on the effects of pyrethroidinsecticides on bull semen quality. Proc Acad Vet Consult.
|
|
|
|
|
Wang X-Z, Liu S-S, Sun, Wu J-Y, Zhou Y-L and Zhang J-H (2009). β- Cypermethrin impairs reproductive function in male mice by inducing oxidative stress. Theriogenology 72:599-611.
|
|
|
|
|
World Health Organization (WHO) (1989a). Cypermethrin environmental health criteria 82 WHO/FAO Pest. data sheets No. 58 Cypermethrin.
|
|
|
|
|
World Health Organization (WHO) (1989b). Cypermethrin environmental health criteria 82. Geneva Switzerland; United Nations Env. Prog. Inter. Labor org. and WHO. Health and Safety Guide No 22.
|
|